Key Points
Only a complete disruption of TP53 function increases the risk for disease progression in previously treated CLL patients.
MiR-34a expression significantly correlates with the predicted TP53 activity in previously treated CLL patients with TP53 abnormalities.
Abstract
In chronic lymphocytic leukemia (CLL) patients, disruptions of the TP53 tumor suppressor pathway by 17p13 deletion (del17p), somatic TP53 mutations, or downregulation of microRNA-34a have been associated with a poor prognosis. So far, the impact of the various TP53 defects has not been evaluated in a large cohort of previously treated and relapsed CLL patients. Here, we present the results of TP53 gene sequencing and fluorescence in situ hybridization for del17p in a phase 3 clinical trial (REACH [Rituximab in the Study of Relapsed Chronic Lymphocytic Leukemia]). Of the 457 patients, 52 had TP53 mutations and 37 had del17p. In 24 (46%) of the TP53 mutated patients, no del17p was found and in 9 of the del17p patients, no TP53 mutation was identified. Based on a predicted proportion of TP53 disruption, a complete disruption of TP53 function, either by a combination of point mutations and/or del17p, was associated with a high risk for disease progression. Progression-free survival of patients with a heterozygous TP53 mutation was not significantly different from patients with a completely intact TP53 locus. In addition, only a complete loss of TP53 function correlated with low microRNA-34a expression levels. This trial was registered at www.clinicaltrials.gov as #NCT00090051.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by a highly variable disease course influenced by genetic risk factors like chromosomal abnormalities, immunoglobulin variable heavy chain gene (IGVH) mutational status, CD38, and ζ-chain associated protein kinase 70 (ZAP70) (reviewed by Zenz et al1 ). Disruption of the tumor suppressor TP53, the guardian of the genome, has been associated with a particularly poor survival and chemotherapy resistance in CLL both in first- and in later-line chemotherapy regimens.2-5 The TP53 gene can be inactivated by point mutations or larger deletions encompassing chromosomal band 17p13 (del17p) where TP53 is located. In ∼80% of CLL patients with a del17p, the remaining TP53 allele is mutated resulting in a complete loss of TP53 tumor suppressor activity and TP53-mediated apoptosis.3-6
The frequency of del17p, which can be detected by fluorescence in situ hybridization (FISH), and TP53 mutations varies according to the stage of the disease.7-9 The highest incidences of TP53 alterations have been reported in fludarabine-refractory CLL.8,9 Despite a high concordance rate between TP53 mutations and del17p, ∼3% to 5% of CLL patients with TP53 mutations at first-line treatment do not have a del17p.3,5,6 There are numerous reports that TP53 mutations without del17p are associated with a poor outcome during first-line treatment.3,5,10,11 However, their independent prognostic value is still controversial and the effect of the different mutations on residual TP53 activity has so far not been taken into account. Certain TP53 mutations encode for mutant p53 proteins which act as dominant-negative inhibitors of the wild-type protein.12
In addition to TP53 mutations/deletions, wild-type TP53 activity is disturbed by enhanced activity of the TP53 inhibitor mouse double minute 2 (Mdm2) protein.13,14 A single nucleotide polymorphism (SNP309, rs2279744, hg19) resulting in a T to G alteration within the first intron of the MDM2 promoter enhances MDM2 protein expression and leads to reduced TP53 activity.15
MicroRNA-34a (miR-34a), located in chromosomal band 1p36, is a microRNA component of the TP53 pathway and a direct transcriptional target of TP53.16,17 MiR-34a mediates some of the effects of TP53 activation including apoptosis, cell-cycle arrest, and senescence.18,19 In CLL cells, miR-34a expression depends on an intact TP53 axis and is downregulated in CLL cells of patients with del17p or mutated TP53.20,21 Low miR-34a expression has been linked to chemotherapy resistance and poor prognosis.21,22 In CLL patients with wild-type TP53, miR-34a expression levels are correlated to the allele status of MDM2 SNP 309 (GG, TG, or TT; rs2279744, hg19).22
So far, the impact of the various TP53 pathway defects including the different types of TP53 mutations on progression-free survival (PFS) has not been evaluated in a large number of previously treated and relapsed CLL patients with detailed clinical and genetic characterization. Therefore, we aimed to (1) evaluate the frequency and impact on PFS of a complete vs a partial disruption of TP53 function by TP53 mutations and/or del17p, (2) correlate TP53 mutations and del17p to miR-34a target gene expression, and (3) analyze the association of the MDM2 SNP309 genotype to miR-34a target gene expression in patients with intact TP53.
These questions were addressed in a large phase 3 clinical study comparing fludarabine and cyclophosphamide with or without rituximab (chimeric monoclonal anti-CD20 antibody [fludarabine and cyclophosphamide alone (FC) vs fludarabine and cyclophosphamide plus rituximab (FCR): REACH (Rituximab in the Study of Relapsed Chronic Lymphocytic Leukemia) trial]).23
Patients and methods
Patients
In this analysis, we included peripheral blood samples from patients with previously treated B-cell CLL who have been enrolled in the REACH trial, an international multicenter randomized trial comparing 6 cycles of FCR with 6 cycles of FC. Patients could be sensitive or refractory to prior alkylating agents but had to be sensitive to fludarabine. All patients were also naive to prior rituximab therapy. Details of trial design and eligibility criteria have been described elsewhere.23 Four hundred fifty-seven patients were selected of a total of 552 patients depending on whether the patients gave their informed consent to further molecular analysis and depending on sample availability. Median PFS did not differ significantly between selected (n = 457, 26 months) and unselected patients (n = 89; 30 months; hazard ratio [HR]: 0.82 [0.62-1.1], P = .18). Available clinical and treatment-associated characteristics were age, sex, Binet stage, Eastern Cooperative Oncology Group (ECOG) performance status, lymphocyte count, β2-microglobulin levels, B symptoms, treatment at first and at second line, and time from last progression. The research project conducted within the NCT00090051 (REACH Trial) was officially approved by our institute. This study was conducted in accordance with the Declaration of Helsinki.
Analysis of TP53 mutations
To reach a maximum purity of the tumor cells, CLL cells in peripheral blood of 75% of analyzed patients were enriched using the MidiMacs system (Miltenyi Biotec) using anti-CD19 magnetic microbeads. The remaining samples had a median of CD19-positive cells of 83% (range, 25%-98%). Genomic DNA was extracted from 1 × 107 CD19-positive B cells/mononuclear cells using the QIamp DNA Mini Kit (Qiagen) following the manufacturer’s instructions. TP53 mutation status was determined using a microarray-based resequencing assay (GEO accession number: GSE45328) as previously described24 (AmpliChip p53-Assay, in development at Roche Molecular Systems). In addition, all samples with TP53 mutations and all patient samples with del17p without detectable TP53 mutations by Amplichip were analyzed by bidirectional DNA sequencing of TP53 exons 2 to 10 and adjacent intronic sequences using exon-spanning primers (kindly provided by Torsten Zenz, University of Heidelberg, Heidelberg, Germany) on an ABI Avant 3100 automated DNA sequence analyzer (Applied Biosystems by Life Technologies). The International Agency for Research on Cancer (IARC) TP53 database (http://www-p53.iarc.fr/StructureAnalysis.html) was used to predict functional consequences of the individual TP53 mutations discovered. In particular, information concerning their transactivation capacity and whether a dominant-negative effect was exerted on both WAF1 and RGC promoters was collated.25,26 The frequency (number of reported mutations in all cancers) and predicted TP53 residual activity was retrieved from the 2007_R1 release of the UMD_TP53 Mutation database (http://p53.free.fr).27 Known polymorphisms in the TP53 gene were treated as being TP53 wild type.
Analysis of IGVH rearrangements
For the analysis of IGVH gene rearrangements and the mutational status, we used complementary DNA (cDNA) and family-specific region primer in the framework region 1 as previously described in Biomed-2 protocols.28 Sequences were aligned to ImMunoGeneTics directories (http://www.imgt.org) and considered mutated if homology to the corresponding germline gene was <98%.
Analysis of ZAP70 expression
Reverse transcription quantitative real-time PCR (RQ-PCR) was applied to monitor ZAP70 gene expression. In brief, CD19-positive B cells were enriched using the MidiMacs system (Miltenyi Biotec) to remove T cells, which have a high ZAP70 content. cDNA was amplified for the target gene ZAP70 and the reference gene ABL1 on a LightCycler 2.0 instrument (Roche Diagnostics GmbH) using the LightCycler ZAP70 mRNA Quantification Kit (prototype kit, not commercially available; Roche Applied Science). ZAP70 expression was calculated relative to ABL1 and normalized to ZAP70/ABL1 ratio of a calibrator cell line (Jurkat). A calibrator normalized ZAP70/ABL1 ratio of 0.3 was found to be the best cutoff to distinguish between ZAP70 positivity and negativity.
Interphase FISH
Probes (Abbott Diagnostics) used for interphase FISH analysis were LSI D13S319 and LSI D13S25 for the detection of del13q, CEP12 for the detection of trisomy 12, LSI p53 for the detection of del17p, and LSI 6q21 for the detection of del11q. For each probe set, at least 200 interphase cells with well-delineated fluorescent spots were evaluated and results were expressed as the percentage of nuclei with an abnormal signal pattern.
MiRNA expression analysis and SNP genotyping
Pretreatment samples of adequate RNA quality for molecular profiling analysis were available for 275 (60%) of 457 patients with known TP53 mutation/deletion status. RNA of CD19-selected B cells was isolated using the Ambion miRVANA total RNA extraction kit and profiled on the Affymetrix miRNA DiscovArray (Asuragen, Inc). In 40 of these patients, miR-34a expression levels were independently evaluated by RQ-PCR. Genotyping was performed using Illumina Infinium BeadChip (Human 1M Duo). More details are given in the supplemental Patients and methods (available on the Blood website).
Statistical analysis
Association of the clinical data with PFS was assessed by Cox proportional hazard models. A Mann-Whitney Wilcoxon (Kruskal-Wallis) test was used to compare distributions across 2 (>2) groups for a continuous variable. A Fisher exact test was used to calculate the association between 2 categorical variables. The correlation between the expression level of miR-34a as measured by the Affymetrix miRNA DiscovArray and RQ-PCR was quantified by calculating the Spearman rank correlation coefficient.
All statistical tests were 2-sided and a P value of .05 was considered statistically significant.
Results
TP53 mutation screening and FISH analysis of 17p13 of relapsed CLL samples
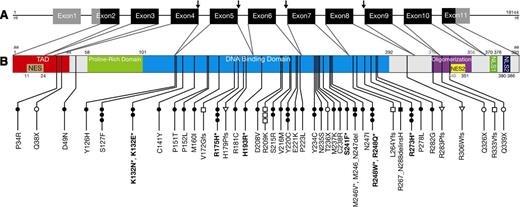
Overall, 61 TP53 mutations were detected in 52 (11.4%) of 457 patients. The type and the location of the different TP53 mutations are described in Figure 1 and supplemental Table 1. Recurrently affected codons in the DNA-binding domain were codons 127, 175, 220, 248, and 273. In addition, 3 patients had a 2-nucleotide deletion in codon 209, a known mutational hotspot.29 All TP53 mutations not associated with a hemizygous del17p appeared heterozygous in Sanger sequencing. However, masked homozygosity due to residual normal cells in patients not selected for CD19-positive B cells cannot be excluded. In patients with hemizygous TP53 mutations associated with hemizygous del17p, some wild-type TP53 allele was detectable in patient cells not selected for CD19-positive B cells.
Locations of 61 TP53 mutations identified in 52 of 457 CLL patients. (A) Organization of the human TP53 gene. Noncoding exons are shown in gray, coding exons in black. TP53 splice mutations are indicated by arrows. (B) TP53 protein with known functional domains. NES, nuclear exclusion domain; NLS, nuclear localization domain; TAD, transactivation domain. Dominant-negative TP53 mutations (according to the IARC database) are shown in bold with an asterisk. ●, missense, *dominant negative; ○, nonsense; ▪, in-frame deletion; □, frameshift deletion; ▼, in-frame insertion; ▿, frameshift insertion; ↓, splice-site mutation.
Locations of 61 TP53 mutations identified in 52 of 457 CLL patients. (A) Organization of the human TP53 gene. Noncoding exons are shown in gray, coding exons in black. TP53 splice mutations are indicated by arrows. (B) TP53 protein with known functional domains. NES, nuclear exclusion domain; NLS, nuclear localization domain; TAD, transactivation domain. Dominant-negative TP53 mutations (according to the IARC database) are shown in bold with an asterisk. ●, missense, *dominant negative; ○, nonsense; ▪, in-frame deletion; □, frameshift deletion; ▼, in-frame insertion; ▿, frameshift insertion; ↓, splice-site mutation.
Hemizygous deletions of 17p13 as assayed by interphase FISH analysis were detected in 37 (8%) of 452 analyzed patients.
Correlation of the TP53 mutation and del17p status with PFS and biological and clinical variables
The median follow-up time and the median PFS for all patients (n = 457) was 43.4 months and 25.8 months, respectively. As expected, the median PFS for patients with TP53 mutations irrespective of the presence of del17p (n = 52, 13 months; HR [95% confidence interval] = 1.9 [1.4-2.7]; P < .001) was significantly shorter than the PFS in patients without TP53 mutations (n = 405, 27 months) (data not shown). Similarly, the median PFS for del17p patients (n = 37) was 12 months (HR = 2.6 [1.8-3.9], P < .001) vs 27 months in patients without del17p (n = 415) (data not shown). The effect of these TP53 abnormalities on disease progression was independent of treatment allocation to FC vs FCR23 (data not shown). Multivariate survival analysis (on 446 patients with complete data) adjusting for treatment arm, Binet stage, age, IGVH mutational status, del17p, and TP53 mutation status confirmed TP53 mutation status (HR = 1.7 [1.1-2.6], P = .009) as a prognostic factor for decreased PFS independent of del17p status (HR = 1.7 [1.1-2.7], P = .024) and with a similar effect size. The other independent prognostic factors were treatment (HR = 0.61 [0.48-0.76], P < .001), Binet stage (HR = 1.64 [1.3-2.1], P < .001), and IGVH mutational status (HR = 2.4 [1.85-3.1], P < .001) (Table 1).
TP53 mutations and 17p13 deletions are independent prognostic factors for PFS in previously treated CLL patients (n = 446)
| Variable . | Comparison (no. of patients) . | HR (95%CI) . | P* . |
|---|---|---|---|
| RND | FC vs FCR | 0.61 (0.48-0.76) | <.001 |
| Binet stage | A/B vs C | 1.64 (1.29-2.09) | <.001 |
| Age | Continuous | 1.01 (1.00-1.02) | .090 |
| IGVH | Mutated vs unmutated | 2.40 (1.85-3.10) | <.001 |
| Del17p | No vs yes (37) | 1.72 (1.07-2.74) | .024 |
| TP53 | Wild type vs mutation (52) | 1.73 (1.14-2.61) | .009 |
| Variable . | Comparison (no. of patients) . | HR (95%CI) . | P* . |
|---|---|---|---|
| RND | FC vs FCR | 0.61 (0.48-0.76) | <.001 |
| Binet stage | A/B vs C | 1.64 (1.29-2.09) | <.001 |
| Age | Continuous | 1.01 (1.00-1.02) | .090 |
| IGVH | Mutated vs unmutated | 2.40 (1.85-3.10) | <.001 |
| Del17p | No vs yes (37) | 1.72 (1.07-2.74) | .024 |
| TP53 | Wild type vs mutation (52) | 1.73 (1.14-2.61) | .009 |
del17p, 17p13 deletion; CI, confidence interval; RND, randomization.
P values were calculated using the Wald test.
Biological and clinical markers did not significantly differ between TP53 mutated and TP53 wild-type patients (supplemental Table 2). Del17p patients had a higher percentage of unmutated IGHV genes (78.4% vs 21.6%, P = .07) and were more likely to be refractory to alkylating agents during previous chemotherapy regimens (52.8% vs 38.9%, P = .002) (supplemental Table 3).
As expected, there was a significant correlation between TP53 mutations and del17p. Twenty-eight of 52 patients with hemizygous TP53 mutations had a hemizygous del17p (concordance rate: 54%, P < .001, Fisher test) predicted to result in a complete disruption of TP53 function. Nevertheless, 24 (46%) of 52 patients carried heterozygous TP53 mutations without del17p, and in 9 (24%) of 37 patients with hemizygous del17p, no TP53 mutations were detected by Amplichip resequencing and Sanger sequencing (supplemental Table 2-3). Three of the TP53mut patients carried >1 mutation in TP53 (>1 TP53mut) and 5 patients had TP53 missense mutations in the DNA-binding domain with a dominant-negative effect according to the IARC database (Figure 1, supplemental Table 1) (TP53DN). These 8 patients with >1 TP53mut or TP53DN without del17p were also predicted to have a complete disruption of TP53 function.
Correlations of the different TP53 mutated/del17p subgroups dependent on a predicted complete vs partial TP53 disruption with biological and clinical parameters and with PFS
Based on a predicted proportion of TP53 disruption, we divided our patient cohort into 5 subgroups: patients with (1) wild-type TP53 and no del17p, a predicted partial TP53 disruption by (2) single heterozygous TP53mut excluding TP53DN (n = 16) or (3) only a detectable hemizygous del17p (n = 9), and a predicted complete TP53 disruption by (4) hemizygous TP53mut and a hemizygous del17p (TP53mut/del17p) (n = 28) or (5) TP53DN or >1TP53mut (n = 8). We assessed whether there were differences in the biological and clinical variables in the whole cohort and among subgroups 2 to 5 (Table 2). The only significant association (P = .03) was found with the IGVH gene mutational status. There was a higher proportion of unmutated IGVH genes in patients with hemizygous del17p with or without a hemizygous TP53 mutation (79% and 78%, respectively) than in patients with a heterozygous TP53 mutation (TP53mut) (56%). In contrast, patients with TP53DN/>1TP53mut (subgroup 5) predominately had IGVH mutated genes (75%). We also compared the predicted residual TP53 activity of TP53 missense/nonsense mutations according to the Universal Mutation Database (UMD) TP53 mutation database between subgroups 2 and 4 (Table 2). In patients with a heterozygous TP53 mutation (subgroup 2), the mean (SD) residual activity of the 15 patients with missense mutations was 24.3 (27.3), whereas it was significantly lower in the 18 TP53mut/del17p patients: 4.1 (5.1), P = .01 (subgroup 4, Table 2).
Clinical and biological variables in previously treated CLL patients with partial or complete TP53 disruption
| . | Total . | Heterozygous TP53mut (subgroup 2) . | Hemizygous del17p (subgroup 3) . | TP53mut/del17p (subgroup 4) . | TP53DN/ >1 TP53mut (subgroup 5) . | P* . |
|---|---|---|---|---|---|---|
| No. of patients, n | 61 | 16 | 9 | 28 | 8 | |
| Sex, n | 61 | 16 | 9 | 28 | 8 | .65 |
| Female | 22 (36%) | 4 (25%) | 3 (33%) | 11 (40%) | 4 (50%) | |
| Male | 39 (64%) | 12 (75%) | 6 (67%) | 17 (60%) | 4 (50%) | |
| Age, n | 61 | 16 | 9 | 28 | 8 | .37 |
| Mean (SD) | 62.5 (8.4) | 65.2 (10.0) | 59.9 (8.1) | 61.5 (7.4) | 63.2 (8.2) | |
| Median (range) | 62 (44-81) | 66.5 (44-81) | 58 (48-73) | 61 (47-76) | 67.5 (51-71) | |
| Binet stage, n | 61 | 16 | 9 | 28 | 8 | .61 |
| A or B | 40 (66%) | 10 (62.5%) | 6 (67%) | 17 (61%) | 7 (87.5%) | |
| C | 21 (34%) | 6 (37.5%) | 3 (33%) | 11 (40%) | 1 (12.5%) | |
| β2 microglobulin, mg/L, n | 60 | 16 | 9 | 27 | 8 | .77 |
| Mean (SD) | 4.6 (2.5) | 4.5 (2.3) | 5.3 (2.9) | 4.6 (2.8) | 3.8 (1.3) | |
| Median (range) | 3.7 (1.5-13.6) | 3.7 (1.9-8.8) | 4.4 (1.5-9.7) | 3.7 (1.6-13.6) | 3.5 (1.7-5.8) | |
| Lymphocytes, 109/L, n | 61 | 16 | 9 | 28 | 8 | .23 |
| Mean (SD) | 68.5 (68.3) | 61.3 (46.7) | 51.8 (78.4) | 83.7 (80.5) | 48.5 (38.1) | |
| Median (range) | 49.2 (0.9-333) | 62.7 (0.9-180) | 17.8 (3.8-244) | 69.0 (8.2-333) | 40.7(6.4-101) | |
| B symptoms, n | 61 | 16 | 9 | 28 | 8 | .92 |
| No | 44 (72%) | 11 (69%) | 6 (67%) | 21 (75%) | 6 (75%) | |
| Yes | 17 (28%) | 5 (31%) | 3 (33%) | 7 (25%) | 2 (25%) | |
| ECOG performance status, n | 61 | 16 | 9 | 28 | 8 | .83 |
| 0 | 31 (51%) | 7 (44%) | 4 (44%) | 15 (54%) | 5 (62.5%) | |
| 1 | 30 (49%) | 9 (56%) | 5 (56%) | 13 (46%) | 3 (37.5%) | |
| IGVH mutational status, n | 61 | 16 | 9 | 28 | 8 | .03 |
| Mutated | 21 (34%) | 7 (44%) | 2 (22%) | 6 (21%) | 6 (75%) | |
| Unmutated | 40 (66%) | 9 (56%) | 7 (78%) | 22 (79%) | 2 (25%) | |
| ZAP70, n | 51 | 12 | 9 | 24 | 6 | .08 |
| Negative | 27 (53%) | 6 (50%) | 5 (56%) | 10 (42%) | 6 (100%) | |
| Positive | 24 (47%) | 6 (50%) | 4 (44%) | 14 (58%) | 0 (0%) | |
| CD38, n | 44 | 11 | 7 | 20 | 6 | .63 |
| Negative | 19 (43%) | 5 (45.5%) | 3 (43%) | 7 (35%) | 4 (67%) | |
| Positive | 25 (57%) | 6 (54.5%) | 4 (57%) | 13 (65%) | 2 (33%) | |
| Del(11q), n | 61 | 16 | 9 | 28 | 8 | .42 |
| No | 55 (90%) | 15 (94%) | 9 (100%) | 23 (82%) | 8 (100%) | |
| Yes | 6 (10%) | 1 (6%) | 0 (0%) | 5 (18%) | 0 (0%) | |
| Del(13q), n | 61 | 16 | 9 | 28 | 8 | .94 |
| No | 31 (51%) | 7 (44%) | 5 (56%) | 15 (54%) | 4 (50%) | |
| Yes | 30 (49%) | 9 (56%) | 4 (44%) | 13 (46%) | 4 (50%) | |
| Trisomy 12, n | 61 | 16 | 9 | 28 | 8 | .96 |
| No | 51 (84%) | 14 (87.5%) | 7 (78%) | 23 (82%) | 7 (87.5%) | |
| Yes | 10 (16%) | 2 (12.5%) | 2 (22%) | 5 (18%) | 1 (12.5%) | |
| Mean residual activity of TP53mut, n (%)† | 33 | 15 | NA | 18 | NA | .01 |
| Mean (SD) | 13.3 (21.0) | 24.3 (27.3) | NA | 4.1 (5.1) | NA | |
| Median (range) | 4.7 (0-73.1) | 12.5 (0-73) | NA | 1.2 (0-12.5) | NA | |
| Previous chemotherapy, n | 60 | 16 | 8 | 28 | 8 | .22 |
| Alkylator refractory | 24 (40%) | 3 (19%) | 4 (50%) | 15 (54%) | 2 (25%) | |
| Alkylator sensitive | 28 (47%) | 9 (56%) | 4 (50%) | 10 (36%) | 5 (62.5%) | |
| Fludarabine | 8 (13%) | 4 (25%) | 0 (0%) | 3 (11%) | 1 (12.5%) | |
| Treatment at second line | 61 | 16 | 9 | 28 | 8 | .3 |
| FCR | 30 (49%) | 9 (56%) | 2 (22%) | 14 (50%) | 5 (62.5%) | |
| FC | 31 (51%) | 7 (44%) | 7 (78%) | 14 (50%) | 3 (37.5%) | |
| Time from last progression (days), n | 61 | 16 | 9 | 28 | 8 | .56 |
| Mean (SD) | 134 (190) | 142 (213) | 186 (289) | 94 (103) | 200 (243) | |
| Median (Range) | 56 (2-929) | 63 (2-845) | 79 (16-929) | 47 (13-378) | 123.5 (10-770) |
| . | Total . | Heterozygous TP53mut (subgroup 2) . | Hemizygous del17p (subgroup 3) . | TP53mut/del17p (subgroup 4) . | TP53DN/ >1 TP53mut (subgroup 5) . | P* . |
|---|---|---|---|---|---|---|
| No. of patients, n | 61 | 16 | 9 | 28 | 8 | |
| Sex, n | 61 | 16 | 9 | 28 | 8 | .65 |
| Female | 22 (36%) | 4 (25%) | 3 (33%) | 11 (40%) | 4 (50%) | |
| Male | 39 (64%) | 12 (75%) | 6 (67%) | 17 (60%) | 4 (50%) | |
| Age, n | 61 | 16 | 9 | 28 | 8 | .37 |
| Mean (SD) | 62.5 (8.4) | 65.2 (10.0) | 59.9 (8.1) | 61.5 (7.4) | 63.2 (8.2) | |
| Median (range) | 62 (44-81) | 66.5 (44-81) | 58 (48-73) | 61 (47-76) | 67.5 (51-71) | |
| Binet stage, n | 61 | 16 | 9 | 28 | 8 | .61 |
| A or B | 40 (66%) | 10 (62.5%) | 6 (67%) | 17 (61%) | 7 (87.5%) | |
| C | 21 (34%) | 6 (37.5%) | 3 (33%) | 11 (40%) | 1 (12.5%) | |
| β2 microglobulin, mg/L, n | 60 | 16 | 9 | 27 | 8 | .77 |
| Mean (SD) | 4.6 (2.5) | 4.5 (2.3) | 5.3 (2.9) | 4.6 (2.8) | 3.8 (1.3) | |
| Median (range) | 3.7 (1.5-13.6) | 3.7 (1.9-8.8) | 4.4 (1.5-9.7) | 3.7 (1.6-13.6) | 3.5 (1.7-5.8) | |
| Lymphocytes, 109/L, n | 61 | 16 | 9 | 28 | 8 | .23 |
| Mean (SD) | 68.5 (68.3) | 61.3 (46.7) | 51.8 (78.4) | 83.7 (80.5) | 48.5 (38.1) | |
| Median (range) | 49.2 (0.9-333) | 62.7 (0.9-180) | 17.8 (3.8-244) | 69.0 (8.2-333) | 40.7(6.4-101) | |
| B symptoms, n | 61 | 16 | 9 | 28 | 8 | .92 |
| No | 44 (72%) | 11 (69%) | 6 (67%) | 21 (75%) | 6 (75%) | |
| Yes | 17 (28%) | 5 (31%) | 3 (33%) | 7 (25%) | 2 (25%) | |
| ECOG performance status, n | 61 | 16 | 9 | 28 | 8 | .83 |
| 0 | 31 (51%) | 7 (44%) | 4 (44%) | 15 (54%) | 5 (62.5%) | |
| 1 | 30 (49%) | 9 (56%) | 5 (56%) | 13 (46%) | 3 (37.5%) | |
| IGVH mutational status, n | 61 | 16 | 9 | 28 | 8 | .03 |
| Mutated | 21 (34%) | 7 (44%) | 2 (22%) | 6 (21%) | 6 (75%) | |
| Unmutated | 40 (66%) | 9 (56%) | 7 (78%) | 22 (79%) | 2 (25%) | |
| ZAP70, n | 51 | 12 | 9 | 24 | 6 | .08 |
| Negative | 27 (53%) | 6 (50%) | 5 (56%) | 10 (42%) | 6 (100%) | |
| Positive | 24 (47%) | 6 (50%) | 4 (44%) | 14 (58%) | 0 (0%) | |
| CD38, n | 44 | 11 | 7 | 20 | 6 | .63 |
| Negative | 19 (43%) | 5 (45.5%) | 3 (43%) | 7 (35%) | 4 (67%) | |
| Positive | 25 (57%) | 6 (54.5%) | 4 (57%) | 13 (65%) | 2 (33%) | |
| Del(11q), n | 61 | 16 | 9 | 28 | 8 | .42 |
| No | 55 (90%) | 15 (94%) | 9 (100%) | 23 (82%) | 8 (100%) | |
| Yes | 6 (10%) | 1 (6%) | 0 (0%) | 5 (18%) | 0 (0%) | |
| Del(13q), n | 61 | 16 | 9 | 28 | 8 | .94 |
| No | 31 (51%) | 7 (44%) | 5 (56%) | 15 (54%) | 4 (50%) | |
| Yes | 30 (49%) | 9 (56%) | 4 (44%) | 13 (46%) | 4 (50%) | |
| Trisomy 12, n | 61 | 16 | 9 | 28 | 8 | .96 |
| No | 51 (84%) | 14 (87.5%) | 7 (78%) | 23 (82%) | 7 (87.5%) | |
| Yes | 10 (16%) | 2 (12.5%) | 2 (22%) | 5 (18%) | 1 (12.5%) | |
| Mean residual activity of TP53mut, n (%)† | 33 | 15 | NA | 18 | NA | .01 |
| Mean (SD) | 13.3 (21.0) | 24.3 (27.3) | NA | 4.1 (5.1) | NA | |
| Median (range) | 4.7 (0-73.1) | 12.5 (0-73) | NA | 1.2 (0-12.5) | NA | |
| Previous chemotherapy, n | 60 | 16 | 8 | 28 | 8 | .22 |
| Alkylator refractory | 24 (40%) | 3 (19%) | 4 (50%) | 15 (54%) | 2 (25%) | |
| Alkylator sensitive | 28 (47%) | 9 (56%) | 4 (50%) | 10 (36%) | 5 (62.5%) | |
| Fludarabine | 8 (13%) | 4 (25%) | 0 (0%) | 3 (11%) | 1 (12.5%) | |
| Treatment at second line | 61 | 16 | 9 | 28 | 8 | .3 |
| FCR | 30 (49%) | 9 (56%) | 2 (22%) | 14 (50%) | 5 (62.5%) | |
| FC | 31 (51%) | 7 (44%) | 7 (78%) | 14 (50%) | 3 (37.5%) | |
| Time from last progression (days), n | 61 | 16 | 9 | 28 | 8 | .56 |
| Mean (SD) | 134 (190) | 142 (213) | 186 (289) | 94 (103) | 200 (243) | |
| Median (Range) | 56 (2-929) | 63 (2-845) | 79 (16-929) | 47 (13-378) | 123.5 (10-770) |
>1 TP53mut, patients with >1 TP53 mutation; NA, not applicable; TP53DN, patients with predicted dominant-negative TP53 mutations according to the IARC database.
*Association between TP53 partial/complete disruption and various clinical and biological variables applying the Kruskal-Wallis test or the Fisher exact test, respectively, for continuous and categorical variables.
†Predicted TP53 residual activity were retrieved from the 2007_R1 release of the UMD_TP53 Mutation database (http://p53.free.fr).
To further dissect the contribution of a predicted partial vs a complete disruption of TP53 on PFS, we added this information into a multivariate survival analysis (on 446 patients with complete data) adjusted for treatment, Binet stage, age, and IGVH mutational status (Table 3). Patients with a predicted complete disruption of TP53 either by having both TP53mut/del17p (HR: 2.8 [1.8-4.2], P ≤ .001) (subgroup 4) or TP53DN or >1 TP53mut (HR = 3.26 [1.5-7.1], P = .003) (subgroup 5) had a similar and relatively high risk for disease progression (the reference to calculate the risk, here and in the following, is the subgroup 1 of patients without TP53 abnormalities) (Table 3). The risk slightly decreased for patients with a hemizygous del17p (HR = 2.2 [1.1-4.3], P = .021) (subgroup 3). Interestingly, heterozygous TP53mut (subgroup 2) showed a much lower risk for disease progression (in this case not even significant) (HR = 1.61 [0.9-2.8], P = .084) especially compared with the risk conferred by a predicted complete TP53 disruption (Table 3).
Multivariable Cox regression analysis for PFS in previously treated CLL patients including predicted partial vs complete TP53 disruption according to the TP53 mutation/deletion status (n = 446)
| Variable . | Comparison (no. of patients) . | HR (95%CI) . | P* . |
|---|---|---|---|
| RND | FC vs FCR | 0.60 (0.48-0.76) | <.001 |
| Binet stage | A/B vs C | 1.68 (1.32-2.14) | <.001 |
| Age | Continuous | 1.01 (1.00-1.02) | .110 |
| IGVH | Mutated vs unmutated | 2.47 (1.90-3.21) | <.001 |
| Heterozygous TP53mut (subgroup 2) | No vs yes (16) | 1.61 (0.94-2.77) | .084 |
| Hemizygous del17p (subgroup 3) | No vs yes (9) | 2.21 (1.13-4.33) | .021 |
| TP53mut/del 17p (subgroup 4) | No vs yes (28) | 2.79 (1.83-4.24) | <.001 |
| TP53DN or > 1TP53mut (subgroup 5) | No vs yes (8) | 3.26 (1.49-7.12) | .003 |
| Variable . | Comparison (no. of patients) . | HR (95%CI) . | P* . |
|---|---|---|---|
| RND | FC vs FCR | 0.60 (0.48-0.76) | <.001 |
| Binet stage | A/B vs C | 1.68 (1.32-2.14) | <.001 |
| Age | Continuous | 1.01 (1.00-1.02) | .110 |
| IGVH | Mutated vs unmutated | 2.47 (1.90-3.21) | <.001 |
| Heterozygous TP53mut (subgroup 2) | No vs yes (16) | 1.61 (0.94-2.77) | .084 |
| Hemizygous del17p (subgroup 3) | No vs yes (9) | 2.21 (1.13-4.33) | .021 |
| TP53mut/del 17p (subgroup 4) | No vs yes (28) | 2.79 (1.83-4.24) | <.001 |
| TP53DN or > 1TP53mut (subgroup 5) | No vs yes (8) | 3.26 (1.49-7.12) | .003 |
P values were calculated using the Wald test.
Correlation of TP53 alterations to miR-34a expression
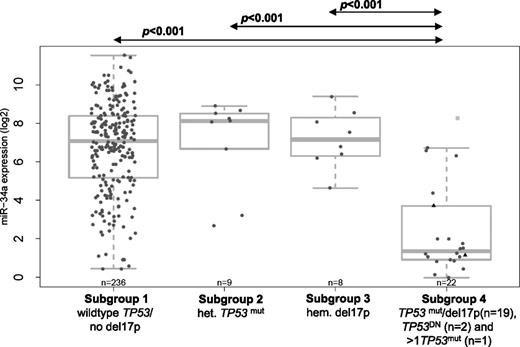
For a subset of 275 patients with miRNA expression data, the TP53 mutation/deletion status according to subgroups 1 to 4 (as described earlier) was correlated to miR-34a expression levels (log 2 transformed) (Figure 2). Due to the low numbers, the 2 patients with TP53DN without del17p (patients 24 and 21, supplementary Table 1) and the only patient with >1 TP53mut without del17p (patient 23, supplementary Table 1) (subgroup 5) were allocated to subgroup 4; they are, however, indicated in Figure 2.
Distribution of miR-34a expression levels from array (log2 transformed) across the different TP53 mutation/del17p subgroups. Black triangles indicate TP53DN; light gray squares, >1TP53mut; het., heterozygous; hem., hemizygous; TP53DN, predicted dominant-negative TP53 mutations.
Distribution of miR-34a expression levels from array (log2 transformed) across the different TP53 mutation/del17p subgroups. Black triangles indicate TP53DN; light gray squares, >1TP53mut; het., heterozygous; hem., hemizygous; TP53DN, predicted dominant-negative TP53 mutations.
Patients with a predicted complete disruption of TP53 function (mean = 2.4, SD = 2.4) (Figure 2, subgroup 4) had significantly lower miR-34a expression levels compared with patients without TP53 aberrations (mean = 6.8, SD = 2.3, P < .001). Very interestingly, patients in subgroup 4 also had lower miR-34a expression levels compared with patients with a predicted partial loss of TP53 function by either a heterozygous TP53mut (mean = 7.0, SD = 2.4, P < .001) or a hemizygous del17p (mean = 7.2, SD = 1.5, P < .001). These patients in subgroups 2 and 3 showed relatively high expression levels of miR-34a and no significant difference in miR-34a expression levels to patients without TP53 aberrations (Figure 2). In a subset of 40 patients, miR-34a expression was analyzed by both microarray and RQ-PCR. We found a highly significant correlation between both methods (Spearman rank correlation coefficient, rs = 0.81, P ≤ .001, supplemental Figure 1), which is also reflected in the equivalent of Figure 2 based on RQ-PCR miR-34a values restricted on those 40 samples (supplemental Figure 2).
There was a high variability of miR-34a expression levels in subgroup 1 (patients without TP53 aberrations), with several patients having low miR-34a expression (Figure 2). A multivariate survival analysis (on 233 patients with complete data) was then performed to further assess whether miR-34a expression levels in subgroup 1 was of prognostic relevance with regards to PFS (Table 4). MiR-34a expression did not predict PFS in patients without TP53 aberrations, neither as a continuous variable (HR: 1.03 [0.95-1.1], P = .5) (Table 4) nor as a binary variable dichotomized by its first quartile (HR: 1.01 [0.7-1.5], P = .95), its median value (HR: 0.92 [0.7-1.3] P = .6), or its third quartile (HR: 1.21 [0.8-1.7], P = .3) (data not shown) after adjustment for treatment, age, Binet stage, and IGVH mutational status.
Multivariate Cox regression analysis for PFS in previously treated CLL patients with wild-type TP53 and no 17p13 deletion including miR-34a expression (n = 233)
| Variable . | Comparison . | HR (95%CI) . | P* . |
|---|---|---|---|
| RND | FC vs FCR | 0.57 (0.42-0.79) | <.001 |
| Binet stage | A/B vs C | 1.88 (1.34-2.63) | <.001 |
| Age | Continuous | 1.02 (1.01-1.04) | .009 |
| IGVH | Mutated vs unmutated | 1.93 (1.37-2.71) | <.001 |
| miR-34a | Continuous | 1.03 (0.95-1.10) | .500 |
| Variable . | Comparison . | HR (95%CI) . | P* . |
|---|---|---|---|
| RND | FC vs FCR | 0.57 (0.42-0.79) | <.001 |
| Binet stage | A/B vs C | 1.88 (1.34-2.63) | <.001 |
| Age | Continuous | 1.02 (1.01-1.04) | .009 |
| IGVH | Mutated vs unmutated | 1.93 (1.37-2.71) | <.001 |
| miR-34a | Continuous | 1.03 (0.95-1.10) | .500 |
P values were calculated using the Wald test.
No correlation of miR-34a expression to MDM2 SNP 309 in TP53 wild-type patients
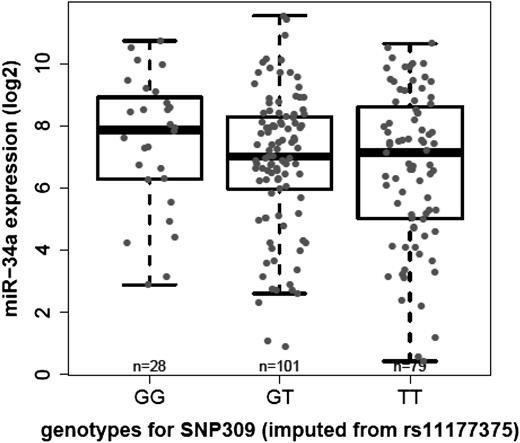
In TP53 wild-type patients with available genotype data (n = 208), miR-34a expression levels were further correlated to the genotype of SNP309 (rs2279744) in the first MDM2 intron. Because SNP309 was not available on the Illumina Human 1M Duo, it was imputed by rs11177375 as a perfect proxy for SNP309 (r2 = 1.0) based on Caucasian public available genotype data from the 1000 Genomes Pilot 1 project.30
There was no significant difference in miR-34a expression levels between groups defined by the SNP309GG genotype (n = 28, mean = 7.5, SD = 2.1), the heterozygous GT variant (n = 101, mean = 6.9, SD = 2.2), and the wild-type TT genotype (n = 79, mean = 6.6, SD = 2.5) (P = .3) (Figure 3). The miR-34a expression levels and the MDM2 SNP 309 genotype for the 452 previously treated CLL patients with TP53 mutation/del17p deletion status are listed in supplemental Table 5.
Correlation of log2 transformed miR-34a array expression across the different MDM2 SNP genotypes (imputed from rs11177375): homozygous GG, heterozygous GT, or wild-type TT.
Correlation of log2 transformed miR-34a array expression across the different MDM2 SNP genotypes (imputed from rs11177375): homozygous GG, heterozygous GT, or wild-type TT.
Discussion
This is, to the best of our knowledge, the first study investigating the frequency and the impact on disease progression of several different TP53 abnormalities in a large and homogeneously treated cohort of previously treated and relapsed CLL patients.
The overall frequency of TP53 mutations (11.7%) and del17p (8%) detected at the time of second-line treatment was similar to the frequencies reported at first-line treatment despite a more unfavorable profile of this patient cohort. This might be explained by the fact that high frequencies of TP53 mutations (up to 40%) and del17p (up to 30%) are found in F-refractory CLL9,31,32 and these patients were excluded from the REACH trial. In accordance to a recent meta-analysis, we show that the TP53 mutational profile identified in F-sensitive previously treated patients (Figure 1) was similar to the mutational profile found at first-line treatment.29
At second-line treatment, however, a substantial proportion of patients either showed only a hemizygous del17p without a TP53 mutation on the remaining allele (2%) or a heterozygous TP53 mutations without a del17p (5.3%). Consequently, only 54% of previously treated CLL patients with any TP53 alteration had both a hemizygous del17p and a heterozygous TP53 mutation. This allowed us to evaluate the effect of the different types of TP53 mutations on disease progression dependent on whether they result in a complete or a partial TP53 disruption. We are the first study to separately analyze the risk for disease progression of heterozygous TP53mut and an intact second TP53 locus, excluding TP53DN or >1 TP53mut. We show that PFS in the heterozygous TP53mut subgroup was not significantly different from patients without TP53 aberrations (Table 3). Previous first-line studies identified a significant adverse effect of heterozygous TP53 mutations on outcome.3-5,11 However, the contribution of TP53DN or >1 TP53mut in these studies is not known. Our data indicate that TP53DN or >1 TP53mut confer a higher risk of disease progression in multivariable analysis, which was similar to the risk seen in patients with hemizygous TP53mut/del17p (Table 3). We further show that patients with a hemizygous del17p only showed a slightly lower risk of disease progression compared with patients with both hemizygous TP53mut/del17p. Due to limited sensitivity of Sanger sequencing or Amplichip technology, we cannot exclude that the small del17p clones in most of our patients carrying a hemizygous del17p (supplemental Table 4) may have precluded the detection of TP53 mutations on the other allele.
Interestingly, the predicted mean residual TP53 activity of the heterozygous TP53 missense mutations according to the UMD database was substantially higher in the group of patients with a heterozygous TP53mut as compared with patients with a predicted complete disruption of TP53 by TP53mut/del17p (Table 2). Dearth et al33 suggest that the residual activity of the TP53 wild-type allele in patients with a heterozygous TP53mut may indicate a good prognosis cancer because the p53 pathway is likely to be somewhat functional whereas complete loss of wild-type p53 activity as in TP53mut/del17p patients might specify a poor prognosis cancer highly resistant to cancer therapy. Interestingly, Malcikova et al found that monoallelic in contrast to biallelic TP53 defects are not clearly associated with high-risk CLL (RaiIII/IV) and lose their association to shorter survival in relation to the IGVH mutational status.11
This also suggests that other prognostic markers like the IGVH mutational status, ZAP70 expression levels and associated chromosomal aberrations, like del11q, may also have an influence on time to progression in the different TP53 mutation/deletion subgroups. Like Zenz et al6 and Gonzales et al,4 we did not observe a correlation between the TP53 mutation status and these biological markers (supplemental Table 2) as described in other publications.11,34,35 However, we observed a higher percentage of unmutated IGVH genes in TP53mut/del17p patients and in patients with a hemizygous del17p (Table 2). Furthermore, a higher percentage of TP53mut/del17p and patients with a hemizygous del17p (54% and 50%, respectively) were refractory to alkylating agents at first-line treatment as compared with patients with a heterozygous TP53mut (19%) (Table 2), which might have had an influence on PFS after second-line treatment with FCR/FC within the REACH trial.36 Unfortunately, we do not know whether the del17p deletion or TP53 mutations detected at relapse have already been present at first-line treatment.
Importantly, our data that the level of predicted residual TP53 function impacts on disease progression was further supported by our findings that in patients with TP53 abnormalities, the predicted TP53 activity significantly correlated with the expression levels of the direct TP53 target miR-34a (Figure 2). Previous studies identified significantly lower miR-34a expression levels in patients with TP53 abnormalities.9,20,22,37,38 However, the patient numbers in these studies are low and there are indications that the size of the TP53 mutated/deleted clone influences miR-34a expression levels.21,39 Only 1 group correlated miR-34a expression levels to single TP53 mutations (including several TP53DN) in a special cohort of fludarabine-refractory CLL patients.9,21 However, in contrast to our study cohort of fludarabine-sensitive previously treated CLL patients, fludarabine-refractory CLL are selected for TP53 abnormalities. In addition, fludarabine refractoriness influences miR-34a expression independent of the TP53 mutation/deletion status.21 Up to now, the impact of TP53DN on miR-34a expression levels has not separately been assessed. Interestingly, we found that the 2 patients with TP53DN had quite low miR-34a expression levels (Figure 2).
Nevertheless, we also identified a few outliers in the subgroup 4 of TP53mut/17pdel patients showing high miR-34a expression (patients 23, 31, 35, and 43, supplemental Table 1) and 2 patients with heterozygous TP53mut but low miR-34a expression (patients 6 and 18, supplemental Table 1) (Figure 2). These results point to alternative mechanisms influencing miR-34a expression levels, like, for example, miR-34a promoter hypermethylation or del1p36.40,41
Furthermore, miR-34a levels were low in a subset of cases without TP53 mutation or deletion (subgroup 1, Figure 2). One group suggested that low miR-34a expression levels in patients without TP53 aberrations might be due to increased tumor suppressor function of TP53 as a result of MDM2 SNP309.22,38 However, we did not find a correlation between miR-34a expression levels and MDM2 SNP309 genotype in patients with wild-type TP53 (Figure 3). Furthermore, in patients with functional TP53 (subgroup 1, Figure 2), miR-34a expression levels were not identified as an independent predictor for PFS in multivariable analysis (Table 4), which has previously been suggested in univariate analysis.22
In summary, at the time of relapse in CLL we observed a prognostic heterogeneity of TP53 abnormalities on PFS depending on whether they are predicted to result in a complete vs a partial disruption of TP53 function. Our data suggest that patients with a partial TP53 disruption at relapse might still benefit from fludarabine-based chemoimmunotherapy. Therefore, in addition to screening for TP53 mutations and del17p at second-line treatment,42 proxies for TP53 activity like miR-34a expression might help to individualize therapeutic strategies for CLL patients in the future. We propose that miR-34a expression quantification by RQ-PCR is an easy implementable biomarker assay to predict TP53 activity in patients with TP53 abnormalities which deserves further evaluation.
Presented at the 53rd annual meeting of the American Society of Hematology, San Diego, CA, December 11, 2011 (Abstracts 2445, 3521).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors highly thank Helen Smith for her efforts with respect to sample logistics and communication.
Authorship
Contribution: A. Dufour and G.P. designed the study and wrote the paper; A. Dufour, E.Z., and G.M. performed the experiments; A. Dufour, G.P., G.D.-N., T.B., S.T., N.P., D.G., and D.D. analyzed the data; G.P., G.D.-N., and R.-F.Y. performed the statistical analyses; S.T., N.P., and L.W. provided support with the Amplichip platform; S.L. provided support with the ZAP70 assay; A. Dufour, S.S., P.M.K., K.S., and J.B. characterized the patient samples; A. Dufour, K.S., S.K.B., and W.H. coordinated the sample analysis within the REACH clinical trial; M.W. and S.K.B. jointly supervised the project and wrote the paper; and G.S., P.S.-C., A. Dmoszynska, T.R., M.M., J.C., and C.H.G. were clinical investigators as part of the REACH trial and submitted samples for the study.
Conflict-of-interest disclosure: G.P., G.D.-N., S.T., N.P., L.W., S.L., and M.W. are employees of Roche and declare competing financial interest. D.D., D.G., and R.-F.Y. are employees of Genentech, Inc. W.H., S.K.B., A. Dufour, K.S., and C.H.G. received research funding from Roche. P.S.-C. received honoraria and research funding from Roche. A. Dmoszynska received honoraria from Roche. The remaining authors declare no competing financial interests.
Correspondence: Annika Dufour, Department of Medicine III, Laboratory for Leukemia Diagnostics, Marchioninistr 25, 81377 Munich, Germany; e-mail: annika.dufour@med.uni-muenchen.de.
References
Author notes
A.D. and G.P. contributed equally.
M.W. and S.K.B. jointly supervised the project.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal