Key Points
Infusion of CMV-specific T cells early posttransplant does not increase acute or chronic graft-versus-host disease.
CMV-specific T cells early posttransplant reduce the need for pharmacotherapy without increased rates of CMV-related organ damage.
Abstract
We investigated the use of adoptively transferred donor-derived cytomegalovirus (CMV) specific cytotoxic T lymphocytes (CTL) as immune reconstitution postallogeneic transplant in a phase 2 study. Fifty patients were infused with a single dose of 2 × 107cells/m2 after day 28 post-transplant. Twenty-six patients reactivated CMV posttransplant (only 5 post-CTL infusion) and 9 required therapy with ganciclovir or foscarnet (only 1 post-CTL infusion). There was 1 case of fatal CMV disease, attributable to high levels of antithymocyte globulin at the time of T cell infusion. We compared the patients in the phase 2 study with a group of contemporaneous controls also treated at the trial centers. There was no increase in acute or chronic graft-versus-host disease attributable to CTL infusion; overall and progression-free survival were similar in both groups. There was a reduction in the percentage of patients who required CMV directed antiviral therapy (17% vs 36%, P = .01) and in the total number of treatment days in the cohort receiving CTL (3.4 days vs 8.9 days, P = .03) without a reduction in CMV reactivation rates. We postulate that adoptively transferred cells are able to expand in response to viral antigen, limit viral replication, and prevent progression to tissue infection. This study was registered on the Australian Clinical Trial Registry as #ACTRN12605000213640 and #ACTRN12607000224426.
Introduction
Cytomegalovirus (CMV) is a β-herpesvirus that causes significant morbidity and mortality in immunosuppressed patients. Despite effective pharmacotherapy with ganciclovir and foscarnet, potentially life threatening tissue disease still occurs in 10% of hemopoietic stem cell transplant (HSCT) recipients.1-6 Monitoring and therapy for CMV incur substantial financial cost and use finite medical resources. Pharmacotherapy also causes morbidity, principally myelosuppression and renal toxicity. Clearly, there is need for improved management of CMV in HSCT recipients. The lack of an adequate immune response to control CMV underlies the limitations of standard therapies.1,7-10
The use of donor-derived antigen-specific T cells for immune reconstitution in transplant recipients has been studied in small phase 1 and 2 clinical trials.11-23 Riddell et al established proof–of-concept for CMV cytotoxic T lymphocytes (CTL) as adoptive therapy in 1994.14,15 Donor-derived CMV CTL can control active CMV reactivation or disease not responding to conventional therapy.11-13 Small trials with limited follow up of CMV CTL administered prophylactically or preemptively posttransplant have delivered encouraging results.14-22,24 However, it remains unclear whether prophylactically administered CMV-specific T cells provide short-term and long-term protection against CMV infection.
The safety of adoptive T-cell therapy delivered early posttransplant remains unclear. There have been safety concerns given that earlier administration of unmanipulated donor lymphocyte infusion increases the risk of graft-versus-host disease (GVHD).25-27 The majority of studies have not reported any induction or worsening of GVHD associated with infusion of antigen-specific CTL, although there have been isolated cases.15,21,22 Long-term follow up of patients treated with Epstein–Barr virus (EBV) CTL did not demonstrate an elevated risk of GVHD,28,29 even with evidence of in vitro alloreactivity.30
We report the long-term follow up of 50 patients treated with CMV-specific donor-derived T cells for prophylaxis of CMV disease. We have compared the outcome of these patients with a contemporaneous cohort of transplant recipients treated at the centers in which the trial was performed.
Methods
Participants’ details
All patients receiving an allogeneic HSCT for hematological malignancy from a fully matched or 1 antigen mismatched related or unrelated donor were eligible for recruitment to the study. The study design is shown in Figure 1. The criteria for inclusion were: (1) transplant recipient with a CMV-seropositive donor, regardless of the recipient’s CMV serostatus; (2) adequate organ function at the time of infusion; and (3) life expectancy of at least 6 months. The criteria for exclusion were: (1) the donor was CMV seronegative; (2) the recipient had used antithymocyte globulin (ATG) within 4 weeks of infusion; (3) acute GVHD of grade II or greater within 1 week of infusion; (4) the recipient had used prednisone >1 mg/kg body weight or equivalent. Participants were recruited from the pool of eligible donor–recipient pairs based on logistic factors including the availability of donors to provide peripheral blood prior to commencing granulocyte colony stimulating factor (CSF) and laboratory capacity for cell generation. Unrelated donors were recruited through the Australian Bone Marrow Donor Registry. International unrelated donors were not recruited due to the logistics of obtaining informed consent and procuring peripheral blood prior to mobilization. The study was approved by the institutional ethics committees of the Sydney West Area Health Service, the University of Sydney, the Children’s Hospital at Westmead and the Australian Bone Marrow Donor Registry. Informed consent was obtained from the donor and the recipient prior to enrollment in accordance with the Declaration of Helsinki. The study was registered on the Australian Clinical Trial Registry (ACTRN12605000213640 and ACTRN1260700022442631 ).
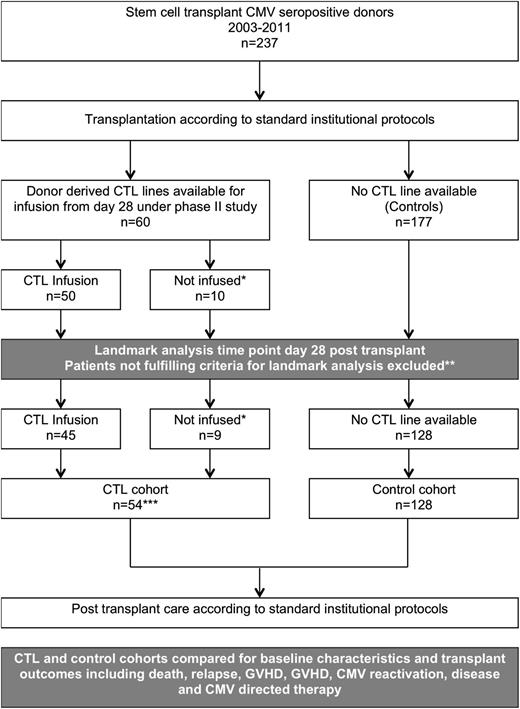
Study design. *Patients who did not receive infusions for clinical reasons. **Exclusion criteria for the landmark analyses were: death, relapse, severe acute GVHD and CMV reactivation occurring prior to day 28. ***CTL trial recruits for whom a CTL product was available but who did not receive an infusion for clinical reasons were included in the CTL cohort; analysis was on an intention-to-treat basis.
Study design. *Patients who did not receive infusions for clinical reasons. **Exclusion criteria for the landmark analyses were: death, relapse, severe acute GVHD and CMV reactivation occurring prior to day 28. ***CTL trial recruits for whom a CTL product was available but who did not receive an infusion for clinical reasons were included in the CTL cohort; analysis was on an intention-to-treat basis.
Descriptive and cohort analyses
A total of 60 patients had CMV-specific T cell products that were suitable for infusion. We present outcome data for the 50 patients who actually received an infusion of CMV-specific T cells after transplantation. In addition, a separate cohort analysis was performed using a contemporaneous group of patients treated at the same hospitals. In this analysis, the treatment cohort included all 60 patients for whom a T-cell product was available, whether it was infused or not (using an intention-to-treat analysis). The control cohort included all patients eligible for inclusion who were not recruited to the study (Figure 1). Data were collected prospectively and entered into a central database, and crosschecked with clinical notes, a pathology database, and pharmacy-dispensing records at various time points to ensure accuracy. To avoid bias in favor of the patients in the CTL cohort, the cohort analysis was restricted to patients eligible for T-cell infusion on day 28. Patients who had reached the primary endpoint of CMV reactivation or had a major event, such as death, relapse or grade III or IV acute GVHD (aGVHD) prior to day 28 were excluded from both the treatment and control cohorts. In total, 6/60 patients were excluded from the treatment cohort and 49/177 from the control cohort. After excluding patients not eligible for T cell infusion on day 28, there were 54 patients in the treatment cohort (45 who received T cells and 9 who did not) and 128 in the control cohort. For the analysis of CTL treatment efficacy, patients who relapsed and underwent reinduction chemotherapy and retransplantation were censored at the time of relapse.
T-cell generation
CMV-specific T-cells were generated from donor peripheral blood and donor stem cell harvest products as previously described.21,22 Briefly, monocyte-derived dendritic cells (mo-DC) were generated from donor mononuclear cells by incubation with granulocyte macrophage-CSF and interleukin (IL) 4 (both 1000 U/mL) in Cellgro-DC serum-free medium for 5 days, followed by maturation with tumor necrosis factor 200 U/mL. Mo-DC were pulsed with the HLA-A*0201 restricted CMV pp65 peptide NLVPMVATV, or transfected with an adenoviral vector genetically modified to express the entire pp65 protein (ad5f35pp65; obtained from the Center for Cell and Gene Therapy, Houston, TX). Irradiated peptide pulsed or transfected mo-DC were used to stimulate donor T cells at an effector–to-stimulator ratio of 10:1. Cells were suspended in AIM-V medium and expanded over 21 days with a second mo-DC stimulation on day 7 and the addition of IL-2 20 U/mL from day 7, and 50 U/mL from day 14. Cell were harvested and cryopreserved for later use. Data presented here were generated as part of a single clinical trial that was amended over time. The NLV peptide was used from 2003 to 2006, during which time the study was limited to donors who were HLA-A*0201 positive. From 2006 onwards, Ad5f35pp65 was used to allow for recruitment of donor–recipient pairs from all HLA types. From 2010, CTL were derived from peripheral blood stem cell harvest product rather than from peripheral blood.
Quality control of the cell product
The fresh cell product was tested for sterility by culture and for mycoplasma contamination by polymerase chain reaction (PCR) (Institute for Clinical Pathology and Medical Research, Westmead, Australia). CTL lines were phenotyped by flow cytometry using monoclonal antibodies directed against CD3, CD4, CD8, CD56, CD14, CD19, CD45RA, CD45RO, CD62L and HLA-DR (BD Biosciences, San Jose, CA). Viability was assessed with 7-amino-actinomycin D (7-AAD) or hydroxystilbamidine (Molecular Probes, Mulgrave, Australia). Where applicable, the proportion of T cells with specificity toward the pp65 epitopes NLVPMVATV (HLA-A*0201), TPRVTGGGAM (HLA-B*0702), QYDPVAALF (HLA-A*2401) and IPSINVHHY (HLA-B*3501) was determined by staining with PE-conjugated MHC tetramers (Beckman Coulter, Fullerton, CA). The flow cytometers used for acquisition were the LSRII (BD Biosciences, Franklin Lakes, NJ), CyanADP (Dako, Glostrup, Denmark), or FACSCanto II (BD Biosciences). Results were analyzed with FlowJo (version 8.8.6; Treestar Inc., Ashland, OR).
The specificity and alloreactivity of CMV–CTL lines was assessed using a standard 4-hour 51Cr-release cytotoxicity assay. Alloreactivity was tested by coincubation of CMV–CTL lines with phytohemagglutinin (PHA) blasts derived from pretransplant recipient blood. CMV specificity was tested using recipient PHA blasts preincubated with an overlapping peptide mix of CMV pp65 (JPT Peptide Technologies GmbH, Berlin, Germany).
IFN-γ EliSpot immunoassay
Pre-CTL and post-CTL infusion immune reconstitution was assessed by interferon (IFN)-γ EliSpot immunoassay. A sample of 0.5 to 1 × 105 cells from each time point were suspended in 200μL AIM-V/10% human AB serum and stimulated with pp65 and adenoviral hexon protein peptide mixes (JPT Peptide Technologies GmbH) for 18 hours in multiscreen MAIPS4510 96-well filter plates (Millipore, Billerica, MA) precoated with catcher antibody (m-AB 1-DIK; BD Biosciences). After washing and incubation with detector antibody (m-AB 7-B6-1-Biotin; BD Biosciences), spots were developed using ExtrAvidin and SigmaFast BCIP/NBT alkaline phosphatase substrate (Sigma-Aldrich, St Louis, MO) following manufacturer’s directions. Spots were counted using AID iSpot (Autoimmun Diagnostika GmBH, Strassburg, Germany), and results were expressed as spot-forming units per 105 cells. Testing was performed in triplicate for each time point.
Quantitative ATG assay
ATG-Fresenius (Fresenius Biotech, Munich, Germany) plasma concentration was determined using a flow cytometry assay; 2 × 105 PHA blasts were incubated with 100μL of recipient plasma for 30 minutes at 4°C. After washing, peripheral blood mononuclear cells (PBMC) were incubated with 5μl of fluorescein isothiocyanate-conjugated swine anti-rabbit immunoglobulins (Dako) for 10 minutes. Fluorescence was acquired on a CyanADP (Dako). Plasma ATG-Fresenius concentration was determined relative to the mean fluorescence intensity of PHA blasts incubated with ATG-Fresenius standards ranging from 0.8 to 100 μg/mL.
Treatment of patients in the study
Transplantation
Patients were treated with standard transplantation protocols at the discretion of their treating physicians. There was no proscribed conditioning or immunosuppressive regimen. However, after patient 5 developed aGVHD in the context of weaning from immunosuppression close to CTL infusion, the study protocol was modified such that for 2 weeks prior and 2 weeks after CTL infusion, corticosteroids and calcineurin inhibitors were maintained at stable doses unless a reduction of calcineurin inhibitor was required due to toxicity or elevated trough levels.
T-cell infusion
On or after day 28 posttransplant, patients were infused with a single dose of 2 × 107 CMV CTLs/m2. Treatment was delayed if the patient had active GVHD, organ dysfunction or active infection.
CMV surveillance and therapy
CMV reactivation was managed according to institutional protocol. CMV pp65 antigenemia (prior to 2004) or CMV PCR (from 2004 onwards) was performed weekly until day 100, and monthly thereafter, until immunosuppression was weaned. PCR quantitation was performed on all positive samples. Ganciclovir 5 mg/kg 2 times daily was commenced when 2 consecutive CMV PCR results showed increasing copy numbers, or if a single result was greater than 2000 copies/mL. Foscarnet 60 mg/kg 3 times daily was used if ganciclovir was contraindicated. A primary course of ganciclovir lasted 14 days, after which further treatment depended on response. For the purposes of data analysis, all positive CMV pp65 and PCR results have been included, regardless of quantitation.
Statistical analyses
Demographics, clinical characteristics and transplantation outcomes were compared between the control and treatment cohorts. For categorical variables, the χ2 or Fisher’s exact tests were used as appropriate. The 2-sample Student t test was used for normally distributed continuous variables, and the Mann–Whitney test for skewed continuous variables. Kaplan–Meier survival curves were used to illustrate the distribution of overall survival and progression-free survival and time to first CMV reactivation by treatment group. The Cox proportional hazard ratio and 95% confidence intervals (95% CI) were used to quantify the difference among treatment groups. Statistical analysis was performed using IBM SPSS for Mac version 20.0.0 (IBM, New York, NY) and Prism 5.0c for Mac (Graphpad Software Inc., La Jolla, CA).
Results
Patients’ characteristics
A total of 60 patients from 2 centers had T-cell products available for infusion between June 2003 and July 2011. The study design is shown in Figure 1. Ten patients did not receive infusions for clinical reasons (early death, n = 2; relapse, n = 1; aGVHD, n = 3; sinusoidal obstruction syndrome, n = 2; graft failure, n = 1; infection, n = 1). The characteristics of the 50 infused patients are shown in Table 1 and Table 2 (see the second column). Over the time that the study was open, 228 transplants were performed from CMV-positive donors at the trial centers. Of these, 182 were eligible for inclusion in the cohort analysis at the landmark time point of day 28 (CTL cohort, n = 54, of whom 9 did not receive CTL infusion; controls, n = 128). Characteristics of the cohort used for the comparative analysis are shown in Table 2 (see the third column and the fourth column). Clinical results are shown in Table 3.
Details of 50 patients who received infusions of CMV specific T-cells
| Patient number . | Transplantation . | T-cell infusion . | CMV . | Graft-versus-host disease . | Long-term follow up . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor . | Indication for transplant . | Conditioning . | T-cell depletion . | Recipient CMV serostatus . | CTL infusion (No. of days posttransplant) . | Reason for infusion delay . | First CMV reactivation (No. of days posttransplant/ relation to CTL infusion) . | Peak CMV titer . | Treatment of CMV (days) . | CMV disease . | aGVHD (relationship to CTL infusion, grade) . | cGVHD . | Relapse (months post-transplant) . | Cause of death . | Days posttransplant at date of death . | Length of follow up (No. of months posttransplant) . | |
| 1* | 5/6 Sib | AML | BU/CY | No | Pos | 69 | Unknown | 33/Prior | Unavailable | Gan+Fosc (12) | Prior, II/ post, III | Yes | TTP | 118 | 4 | ||
| 2 | 6/6 Sib | NHL | FLU/CY | No | Neg | 40 | Yes | Lung ca | 2288 | 76 | |||||||
| 3 | 6/6 Sib | ALL | FLU/BU | ATG | Pos | 33 | No | 80 | Relapse | 2412 | 80 | ||||||
| 4 | 6/6 Sib | MM | FLU/MEL | No | Neg | 29 | Prior, II/ post, III | Yes | 62 | 78 | |||||||
| 5* | 6/6 Sib | AML | FLU/CY | No | Pos | 63 | Mucositits | 50/Prior | 1320 | Gan+Fosc (18) | Prior, II/ post, III | N/A | aGVHD | 86 | 3 | ||
| 6 | 5/6 Sib | ALL | Other | No | Pos | 86 | GVHD, infection | 39/Prior | Colitis (Prior) | Prior, Unknown | Yes | 2207 | 74 | ||||
| 7 | 6/6 Sib | AML | FLU/CY | No | Pos | 29 | Prior, I | No | 36 | Relapse | 1334 | 44 | |||||
| 8 | 6/6 MUD | ALL | Other | CD34+ sel | Neg | 30 | No | 69 | |||||||||
| 9 | 6/6 Sib | NHL | FLU/CY | No | Pos | 115 | GVHD | 59/Prior | <600 | Gan (3) | Prior, I | No | Pulmonary HT, CCF | 222 | 7 | ||
| 10 | 6/6 Sib | SAA | CY/TBI | ATG | Pos | 65 | Infection | 90/Post | <600 | No | 49 | ||||||
| 11 | 6/6 Sib | AML | FLU/MEL | No | Pos | 45 | Renal failure | 46/Day of infusion | <600 | Post, III | Yes | 64 | |||||
| 12 | 5/6 MUD | AML | FLU/MEL | Alem | Pos | 31 | 38/Post | 880 | No | Graft failure, aGVHD after 2nd transplant | 239 | 8 | |||||
| 13 | 5/6 Sib | ALL | CY/TBI | No | Pos | 101 | GVHD | 66/Prior | <600 | Post, II | Yes | 56 | |||||
| 14 | 6/6 Sib | ALL | CY/TBI | No | Neg | 38 | No | 6 | Relapse | 359 | 12 | ||||||
| 15 | 6/6 Sib | AML | FLU/MEL | No | Pos | 38 | 45/Post | <600 | Post, II | Yes | 60 | ||||||
| 16 | 6/6 MUD | AML | FLU/MEL | No | Neg | 59 | GVHD | Prior, II | Yes | 6 | Relapse | 349 | 12 | ||||
| 17 | 6/6 Sib | AML | BU/CY | No | Pos | 47 | Nausea and vomiting | Yes | 55 | ||||||||
| 18 | 6/6 Sib | AML | BU/CY | No | Neg | 68 | Immune mediated red cell aplasia | No | 50 | ||||||||
| 19 | 6/6 Sib | AML | BU/CY | No | Neg | 52 | Patient preference | No | 15 | 53 | |||||||
| 20* | 6/6 MUD | AML | FLU/MEL | Alem | Pos | 47 | Infection | 10/Prior | 2810 | Fosc (30) | Yes | 52 | |||||
| 21 | 6/6 MUD | AML | CY/TBI | No | Pos | 73 | aGVHD | 52/Prior | 965 | Yes | 4 | 53 | |||||
| 22 | 6/6 Sib | AML | FLU/MEL | No | Pos | 61 | Renal failure | No | 7 | Relapse | 590 | 20 | |||||
| 23 | 6/6 Sib | AML | FLU/BU | ATG | Neg | 32 | No | 44 | |||||||||
| 24 | 6/6 Sib | ALL | CY/TBI | No | Pos | 104 | aGVHD | 31/Prior | <600 | Gan (21) | Prior, I | Yes | 45 | ||||
| 25 | 6/6 MUD | AML | CY/TBI | ATG | Pos | 74 | aGVHD | 74/Day of infusion | <600 | Prior, II | No | 39 | |||||
| 26 | 6/6 Sib | MM | FLU/MEL | ATG | Pos | 46 | Unknown | Yes | 15 | 37 | |||||||
| 27 | 5/6 MUD | AML | Flu/Mel/BCNU | ATG | Pos | 31 | 31/Day of infusion | <600 | No | 33 | |||||||
| 28 | 6/6 Sib | NHL | FLU/MEL | ATG | Pos | 38 | 45/Post | 57400 | Gan+Fosc (22) | Post, I | N/A | Pneumonia | 67 | 2 | |||
| 29 | 6/6 Sib | AML | Flu/Mel/BCNU | ATG | Pos | 31 | No | 13 | 30 | ||||||||
| 30 | 6/6 Sib | AML | CY/TBI | No | Pos | 34 | Post, II | Yes | 31 | ||||||||
| 31 | 6/6 MUD | Non-haem | FLU/MEL | Alem | Pos | 73 | Infection | 31/Prior | 16900 | Gan (18) | Prior, I | Yes | 31 | ||||
| 32 | 6/6 MUD | MDS | Flu/Mel/BCNU | ATG | Pos | 31 | 31/Day of infusion | 42900 | Gan+Fosc (66) | Pneumonitis/colitis | N/A | CMV pneumonitis | 88 | 3 | |||
| 33 | 6/6 Sib | NHL | Other | No | Pos | 31 | 31/Day of infusion | <600 | No | 28 | |||||||
| 34 | 6/6 Sib | ALL | CY/TBI | No | Pos | 59 | Unknown | Post, I | Yes | 25 | |||||||
| 35* | 6/6 Sib | HD | FLU/MEL | ATG | Neg | 31 | 19/Prior | <600 | No | 5 | Progressive disease | 459 | 15 | ||||
| 36* | 6/6 Sib | AML | Flu/Mel/BCNU | ATG | Pos | 77 | Infection | 42/Prior | 974 | No | 27 | 27 | |||||
| 37 | 6/6 MUD | NHL | FLU/MEL | ATG | Neg | 31 | Post, IV | No | aGVHD | 140 | 5 | ||||||
| 38 | 6/6 Sib | AML | BU/CY | No | Pos | 31 | Yes | 23 | |||||||||
| 39 | 6/6 MUD | AML | BU/CY | ATG | Neg | 34 | Yes | 13 | 22 | ||||||||
| 40 | 6/6 MUD | AML | Flu/Mel/BCNU | ATG | Pos | 31 | 31/Day of infusion | 3160 | Gan (14) | No | 21 | ||||||
| 41 | 6/6 Sib | AML | Other | No | Pos | 34 | 63/Post | <600 | No | 20 | |||||||
| 42 | 6/6 Sib | AML | Flu/Mel/BCNU | ATG | Pos | 38 | 33/Prior | <600 | Yes | 21 | |||||||
| 43 | 6/6 MUD | AML | Other | ATG | Neg | 62 | aGVHD, infection | Prior, I | No | 22 | |||||||
| 44 | 6/6 Sib | AML | BU/CY | No | Neg | 45 | Laboratory delay | No | 18 | ||||||||
| 45 | 6/6 Sib | MDS/MPD | Flu/Mel/BCNU | ATG | Pos | 45 | Laboratory delay | Yes | 17 | ||||||||
| 46 | 6/6 Sib | AML | Flu/Mel/BCNU | ATG | Pos | 52 | Laboratory delay | 38/Prior | <600 | No | Lung ca | 116 | 4 | ||||
| 47 | 6/6 Sib | AML | BU/CY | No | Pos | 45 | Laboratory delay | 45/Day of infusion | 4010 | Yes | 17 | ||||||
| 48 | 6/6 Sib | MDS | Flu/Mel/BCNU | ATG | Neg | 38 | Post, II | No | 17 | ||||||||
| 49 | 6/6 Sib | AML | Flu/Mel/BCNU | ATG | Pos | 90 | aGVHD | Prior, II | No | 14 | |||||||
| 50 | 6/6 MUD | AML | Flu/Mel/BCNU | ATG | Pos | 52 | Reducing immunosuppression | 31/Prior | 5320 | No | 10 | ||||||
| Patient number . | Transplantation . | T-cell infusion . | CMV . | Graft-versus-host disease . | Long-term follow up . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor . | Indication for transplant . | Conditioning . | T-cell depletion . | Recipient CMV serostatus . | CTL infusion (No. of days posttransplant) . | Reason for infusion delay . | First CMV reactivation (No. of days posttransplant/ relation to CTL infusion) . | Peak CMV titer . | Treatment of CMV (days) . | CMV disease . | aGVHD (relationship to CTL infusion, grade) . | cGVHD . | Relapse (months post-transplant) . | Cause of death . | Days posttransplant at date of death . | Length of follow up (No. of months posttransplant) . | |
| 1* | 5/6 Sib | AML | BU/CY | No | Pos | 69 | Unknown | 33/Prior | Unavailable | Gan+Fosc (12) | Prior, II/ post, III | Yes | TTP | 118 | 4 | ||
| 2 | 6/6 Sib | NHL | FLU/CY | No | Neg | 40 | Yes | Lung ca | 2288 | 76 | |||||||
| 3 | 6/6 Sib | ALL | FLU/BU | ATG | Pos | 33 | No | 80 | Relapse | 2412 | 80 | ||||||
| 4 | 6/6 Sib | MM | FLU/MEL | No | Neg | 29 | Prior, II/ post, III | Yes | 62 | 78 | |||||||
| 5* | 6/6 Sib | AML | FLU/CY | No | Pos | 63 | Mucositits | 50/Prior | 1320 | Gan+Fosc (18) | Prior, II/ post, III | N/A | aGVHD | 86 | 3 | ||
| 6 | 5/6 Sib | ALL | Other | No | Pos | 86 | GVHD, infection | 39/Prior | Colitis (Prior) | Prior, Unknown | Yes | 2207 | 74 | ||||
| 7 | 6/6 Sib | AML | FLU/CY | No | Pos | 29 | Prior, I | No | 36 | Relapse | 1334 | 44 | |||||
| 8 | 6/6 MUD | ALL | Other | CD34+ sel | Neg | 30 | No | 69 | |||||||||
| 9 | 6/6 Sib | NHL | FLU/CY | No | Pos | 115 | GVHD | 59/Prior | <600 | Gan (3) | Prior, I | No | Pulmonary HT, CCF | 222 | 7 | ||
| 10 | 6/6 Sib | SAA | CY/TBI | ATG | Pos | 65 | Infection | 90/Post | <600 | No | 49 | ||||||
| 11 | 6/6 Sib | AML | FLU/MEL | No | Pos | 45 | Renal failure | 46/Day of infusion | <600 | Post, III | Yes | 64 | |||||
| 12 | 5/6 MUD | AML | FLU/MEL | Alem | Pos | 31 | 38/Post | 880 | No | Graft failure, aGVHD after 2nd transplant | 239 | 8 | |||||
| 13 | 5/6 Sib | ALL | CY/TBI | No | Pos | 101 | GVHD | 66/Prior | <600 | Post, II | Yes | 56 | |||||
| 14 | 6/6 Sib | ALL | CY/TBI | No | Neg | 38 | No | 6 | Relapse | 359 | 12 | ||||||
| 15 | 6/6 Sib | AML | FLU/MEL | No | Pos | 38 | 45/Post | <600 | Post, II | Yes | 60 | ||||||
| 16 | 6/6 MUD | AML | FLU/MEL | No | Neg | 59 | GVHD | Prior, II | Yes | 6 | Relapse | 349 | 12 | ||||
| 17 | 6/6 Sib | AML | BU/CY | No | Pos | 47 | Nausea and vomiting | Yes | 55 | ||||||||
| 18 | 6/6 Sib | AML | BU/CY | No | Neg | 68 | Immune mediated red cell aplasia | No | 50 | ||||||||
| 19 | 6/6 Sib | AML | BU/CY | No | Neg | 52 | Patient preference | No | 15 | 53 | |||||||
| 20* | 6/6 MUD | AML | FLU/MEL | Alem | Pos | 47 | Infection | 10/Prior | 2810 | Fosc (30) | Yes | 52 | |||||
| 21 | 6/6 MUD | AML | CY/TBI | No | Pos | 73 | aGVHD | 52/Prior | 965 | Yes | 4 | 53 | |||||
| 22 | 6/6 Sib | AML | FLU/MEL | No | Pos | 61 | Renal failure | No | 7 | Relapse | 590 | 20 | |||||
| 23 | 6/6 Sib | AML | FLU/BU | ATG | Neg | 32 | No | 44 | |||||||||
| 24 | 6/6 Sib | ALL | CY/TBI | No | Pos | 104 | aGVHD | 31/Prior | <600 | Gan (21) | Prior, I | Yes | 45 | ||||
| 25 | 6/6 MUD | AML | CY/TBI | ATG | Pos | 74 | aGVHD | 74/Day of infusion | <600 | Prior, II | No | 39 | |||||
| 26 | 6/6 Sib | MM | FLU/MEL | ATG | Pos | 46 | Unknown | Yes | 15 | 37 | |||||||
| 27 | 5/6 MUD | AML | Flu/Mel/BCNU | ATG | Pos | 31 | 31/Day of infusion | <600 | No | 33 | |||||||
| 28 | 6/6 Sib | NHL | FLU/MEL | ATG | Pos | 38 | 45/Post | 57400 | Gan+Fosc (22) | Post, I | N/A | Pneumonia | 67 | 2 | |||
| 29 | 6/6 Sib | AML | Flu/Mel/BCNU | ATG | Pos | 31 | No | 13 | 30 | ||||||||
| 30 | 6/6 Sib | AML | CY/TBI | No | Pos | 34 | Post, II | Yes | 31 | ||||||||
| 31 | 6/6 MUD | Non-haem | FLU/MEL | Alem | Pos | 73 | Infection | 31/Prior | 16900 | Gan (18) | Prior, I | Yes | 31 | ||||
| 32 | 6/6 MUD | MDS | Flu/Mel/BCNU | ATG | Pos | 31 | 31/Day of infusion | 42900 | Gan+Fosc (66) | Pneumonitis/colitis | N/A | CMV pneumonitis | 88 | 3 | |||
| 33 | 6/6 Sib | NHL | Other | No | Pos | 31 | 31/Day of infusion | <600 | No | 28 | |||||||
| 34 | 6/6 Sib | ALL | CY/TBI | No | Pos | 59 | Unknown | Post, I | Yes | 25 | |||||||
| 35* | 6/6 Sib | HD | FLU/MEL | ATG | Neg | 31 | 19/Prior | <600 | No | 5 | Progressive disease | 459 | 15 | ||||
| 36* | 6/6 Sib | AML | Flu/Mel/BCNU | ATG | Pos | 77 | Infection | 42/Prior | 974 | No | 27 | 27 | |||||
| 37 | 6/6 MUD | NHL | FLU/MEL | ATG | Neg | 31 | Post, IV | No | aGVHD | 140 | 5 | ||||||
| 38 | 6/6 Sib | AML | BU/CY | No | Pos | 31 | Yes | 23 | |||||||||
| 39 | 6/6 MUD | AML | BU/CY | ATG | Neg | 34 | Yes | 13 | 22 | ||||||||
| 40 | 6/6 MUD | AML | Flu/Mel/BCNU | ATG | Pos | 31 | 31/Day of infusion | 3160 | Gan (14) | No | 21 | ||||||
| 41 | 6/6 Sib | AML | Other | No | Pos | 34 | 63/Post | <600 | No | 20 | |||||||
| 42 | 6/6 Sib | AML | Flu/Mel/BCNU | ATG | Pos | 38 | 33/Prior | <600 | Yes | 21 | |||||||
| 43 | 6/6 MUD | AML | Other | ATG | Neg | 62 | aGVHD, infection | Prior, I | No | 22 | |||||||
| 44 | 6/6 Sib | AML | BU/CY | No | Neg | 45 | Laboratory delay | No | 18 | ||||||||
| 45 | 6/6 Sib | MDS/MPD | Flu/Mel/BCNU | ATG | Pos | 45 | Laboratory delay | Yes | 17 | ||||||||
| 46 | 6/6 Sib | AML | Flu/Mel/BCNU | ATG | Pos | 52 | Laboratory delay | 38/Prior | <600 | No | Lung ca | 116 | 4 | ||||
| 47 | 6/6 Sib | AML | BU/CY | No | Pos | 45 | Laboratory delay | 45/Day of infusion | 4010 | Yes | 17 | ||||||
| 48 | 6/6 Sib | MDS | Flu/Mel/BCNU | ATG | Neg | 38 | Post, II | No | 17 | ||||||||
| 49 | 6/6 Sib | AML | Flu/Mel/BCNU | ATG | Pos | 90 | aGVHD | Prior, II | No | 14 | |||||||
| 50 | 6/6 MUD | AML | Flu/Mel/BCNU | ATG | Pos | 52 | Reducing immunosuppression | 31/Prior | 5320 | No | 10 | ||||||
Alem, alemtuzumab; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; BCNU, carmustine; Bu, busulphan; Ca, carcinoma; CD34+ sel, in vitro CD34+ selection; Cy, cyclophosphamide; Flu, fludarabine; Fos, foscarnet; Gan, ganciclovir; HD, Hodgkin’s disease; MDS, myelodysplastic syndrome; Mel, melphalan; MM, multiple myeloma; MPD, myeloproliferative disorder; MUD, matched unrelated donor; N/A, not applicable; Neg, Negative; NHL, non-Hodgkin lymphoma; Nonhaem, nonhematological (Krabbe’s disease); PBSC, peripheral blood stem cells; PBSCH, peripheral blood stem cell harvest product; Pos, Positive; SAA, severe aplastic anemia; Sib, sibling; TAM, transplant-associated microangiopathy; TBI, total body irradiation.
Indicates that patient did not fulfill criteria for comparison with controls.
Characteristics of participants
| Characteristics . | All CTL infused patients (n = 50) . | Cohorts used for comparative analysis . | P* . | |
|---|---|---|---|---|
| CTL (n = 54) . | Control (n = 128) . | |||
| Age (median, range) | 48 (4-68) | 48 (4-68) | 46 (5-69) | .73 |
| Sex (M:F) (%) | 58:42 | 54:46 | 60:40 | .42 |
| Indication for transplant | ||||
| AML | 30 (60) | 31 (57) | 64 (50) | .52 |
| ALL | 7 (14) | 8 (14) | 24 (19) | |
| CML | — | — | 5 (4) | |
| MDS | 2 (4) | 3 (6) | 4 (3) | |
| NHL | 5 (10) | 6 (11) | 15 (12) | |
| HD | 1 (2) | — | 1 (1) | |
| MM | 2 (4) | 2 (4) | 3 (2) | |
| Other | 3 (6) | 4 (7) | 12 (9) | |
| Stage of disease | ||||
| CR1 | 18 (36) | 19 (35) | 50 (39) | .72 |
| >CR1 | 24 (48) | 25 (46) | 50 (39) | |
| N/A or unknown | 8 (16) | 10 (19) | 28 (22) | |
| CMV serostatus (recipient) | .38 | |||
| Positive | 36 (72) | 38 (70) | 98 (77) | |
| Negative | 14 (28) | 16 (30) | 30 (23) | |
| Donor | ||||
| Sib | 36 (72) | 40 (74) | 85 (66) | .57 |
| MUD | 14 (28) | 14 (26) | 41 (32) | |
| Matched other family | — | — | 2 (2) | |
| HLA matching | ||||
| 6/6 | 45 (90) | 49 (91) | 126 (98) | .03 |
| 5/6 | 5 (10) | 5 (9) | 2 (2) | |
| Stem-cell source | ||||
| Bone marrow | 8 (16) | 8 (15) | 18 (14) | .90 |
| PBSC | 42 (84) | 46 (85) | 110 (86) | |
| Conditioning | ||||
| Busulphan/Cyclophosphamide | 8 (16) | 11 (20) | 32 (25) | .13 |
| Cyclophosphamide/TBI | 8 (16) | 10 (19) | 37 (29) | |
| Fludarabine/Melphalan/BCNU | 11 (22) | 11 (20) | 18 (14) | |
| Fludarabine/Melphalan | 12 (24) | 10 (19) | 8 (6) | |
| Fludarabine/Busulphan | 2 (4) | 2 (4) | 7 (6) | |
| Fludarabine/Cyclophosphamide | 4 (8) | 4 (7) | 16 (13) | |
| Other/None | 5 (10) | 6 (11) | 10 (8) | |
| T-cell depletion | 26 (52) | 26 (48) | 53 (41) | .41 |
| Antithymocyte globulin | 22 | 23 | 46 | |
| Alemtuzumab | 3 | 2 | 4 | |
| CD34+ selection | 1 | 1 | 3 | |
| Characteristics . | All CTL infused patients (n = 50) . | Cohorts used for comparative analysis . | P* . | |
|---|---|---|---|---|
| CTL (n = 54) . | Control (n = 128) . | |||
| Age (median, range) | 48 (4-68) | 48 (4-68) | 46 (5-69) | .73 |
| Sex (M:F) (%) | 58:42 | 54:46 | 60:40 | .42 |
| Indication for transplant | ||||
| AML | 30 (60) | 31 (57) | 64 (50) | .52 |
| ALL | 7 (14) | 8 (14) | 24 (19) | |
| CML | — | — | 5 (4) | |
| MDS | 2 (4) | 3 (6) | 4 (3) | |
| NHL | 5 (10) | 6 (11) | 15 (12) | |
| HD | 1 (2) | — | 1 (1) | |
| MM | 2 (4) | 2 (4) | 3 (2) | |
| Other | 3 (6) | 4 (7) | 12 (9) | |
| Stage of disease | ||||
| CR1 | 18 (36) | 19 (35) | 50 (39) | .72 |
| >CR1 | 24 (48) | 25 (46) | 50 (39) | |
| N/A or unknown | 8 (16) | 10 (19) | 28 (22) | |
| CMV serostatus (recipient) | .38 | |||
| Positive | 36 (72) | 38 (70) | 98 (77) | |
| Negative | 14 (28) | 16 (30) | 30 (23) | |
| Donor | ||||
| Sib | 36 (72) | 40 (74) | 85 (66) | .57 |
| MUD | 14 (28) | 14 (26) | 41 (32) | |
| Matched other family | — | — | 2 (2) | |
| HLA matching | ||||
| 6/6 | 45 (90) | 49 (91) | 126 (98) | .03 |
| 5/6 | 5 (10) | 5 (9) | 2 (2) | |
| Stem-cell source | ||||
| Bone marrow | 8 (16) | 8 (15) | 18 (14) | .90 |
| PBSC | 42 (84) | 46 (85) | 110 (86) | |
| Conditioning | ||||
| Busulphan/Cyclophosphamide | 8 (16) | 11 (20) | 32 (25) | .13 |
| Cyclophosphamide/TBI | 8 (16) | 10 (19) | 37 (29) | |
| Fludarabine/Melphalan/BCNU | 11 (22) | 11 (20) | 18 (14) | |
| Fludarabine/Melphalan | 12 (24) | 10 (19) | 8 (6) | |
| Fludarabine/Busulphan | 2 (4) | 2 (4) | 7 (6) | |
| Fludarabine/Cyclophosphamide | 4 (8) | 4 (7) | 16 (13) | |
| Other/None | 5 (10) | 6 (11) | 10 (8) | |
| T-cell depletion | 26 (52) | 26 (48) | 53 (41) | .41 |
| Antithymocyte globulin | 22 | 23 | 46 | |
| Alemtuzumab | 3 | 2 | 4 | |
| CD34+ selection | 1 | 1 | 3 | |
The table presents characteristics for all 50 patients who received CTL infusion (column: All CTL infused patients); patients for whom a CTL product was available and who fulfilled criteria for comparative analysis at day 28, including 45 patients who received CTL and 9 who did not receive infusions for clinical reasons (column: CTL); and control patients who fulfilled eligibility criteria for the trial and for the comparative analysis but who did not have a CTL product made (column: Control). (See text for inclusion and exclusion criteria for comparative analysis of CTL trial recruits and controls.) Values are numbers (percentages) unless otherwise indicated. Abbreviations are explained in Table 1.
P values refer to comparisons between columns 3 and 4; all are 2-sided; and values < .05 are considered significant. Percentages may not add to 100% due to rounding. CR1, first complete remission.
Patient transplantation outcomes
| Characteristics . | All CTL-infused patients (n=50) . | Cohorts used for comparative analysis . | P* . | |
|---|---|---|---|---|
| CTL (n = 54) . | Control (n = 128) . | |||
| Median length of follow-up, months (range) | 26 (2-80) | 26 (2-80) | 25 (1-98) | .79 |
| Number of patients treated with CTL | 50 | 45 | — | |
| Median day of infusion | 45 (29-115) | 45 (29-115) | — | |
| No. of days posttransplant (range) | ||||
| CMV reactivation | ||||
| Total | 26 (52) | 25 (46) | 77 (60) | .17 |
| Preinfusion | 14 (28) | 9 (2) | — | |
| Day of infusion | 7 (14) | 4 (8) | — | |
| Postinfusion | 5 (10) | 6 (11) | — | |
| CMV in uninfused patients | — | 6 (11) | 77 (60) | |
| Peak titer (median) | Positive below linear limit of assay | 0 | 600 | .04 |
| IV treatment | 9 (18) | 9 (17) | 46 (36) | .01 |
| Days of IV treatment (days per patient in cohort) | 4.08 | 3.4 | 8.9 | .03 |
| CMV disease | 1 (2) | 3 (6) | 11 (9) | .76 |
| Death from CMV disease | 1 (2) | 1 (2) | 1 (1) | .51 |
| aGVHD | ||||
| 2-4 | 12 (24)† | 13 (24) | 24 (18) | .42 |
| 3-4 | 4 (8)‡ | 4 (7) | 9 (7) | 1.0 |
| cGVHD | 21 (42) | 23 (45) | 68 (57) | .15 |
| Limited | 3 | 5 | 17 | |
| Extensive | 17 | 18 | 49 | |
| Not recorded | 1 | 2 | ||
| Mortality day 1-100 | 3 (6) | 3 (6) | 9 (7) | 1.0 |
| Relapse | 13 (26) | 11 (20) | 23 (18) | .70 |
| Death | ||||
| Total | 15 (30) | 18 (33) | 37 (29) | .55 |
| Relapse/progressive disease | 6 (12) | 6 (11) | 13 (10) | |
| aGVHD | 2 (4) | 1 (2) | 1 (1) | |
| cGVHD | — | — | 3 (2) | |
| Infection | 2 (4) | 4 (7) | 10 (8) | |
| Graft failure | 1 (2) | 1 (2) | — | |
| TTP | 1 (2) | — | — | |
| Second malignancy | 2 (4) | 2 (4) | — | |
| Other | 1§ (2) | 4‖ (7) | 10¶ (9) | |
| Characteristics . | All CTL-infused patients (n=50) . | Cohorts used for comparative analysis . | P* . | |
|---|---|---|---|---|
| CTL (n = 54) . | Control (n = 128) . | |||
| Median length of follow-up, months (range) | 26 (2-80) | 26 (2-80) | 25 (1-98) | .79 |
| Number of patients treated with CTL | 50 | 45 | — | |
| Median day of infusion | 45 (29-115) | 45 (29-115) | — | |
| No. of days posttransplant (range) | ||||
| CMV reactivation | ||||
| Total | 26 (52) | 25 (46) | 77 (60) | .17 |
| Preinfusion | 14 (28) | 9 (2) | — | |
| Day of infusion | 7 (14) | 4 (8) | — | |
| Postinfusion | 5 (10) | 6 (11) | — | |
| CMV in uninfused patients | — | 6 (11) | 77 (60) | |
| Peak titer (median) | Positive below linear limit of assay | 0 | 600 | .04 |
| IV treatment | 9 (18) | 9 (17) | 46 (36) | .01 |
| Days of IV treatment (days per patient in cohort) | 4.08 | 3.4 | 8.9 | .03 |
| CMV disease | 1 (2) | 3 (6) | 11 (9) | .76 |
| Death from CMV disease | 1 (2) | 1 (2) | 1 (1) | .51 |
| aGVHD | ||||
| 2-4 | 12 (24)† | 13 (24) | 24 (18) | .42 |
| 3-4 | 4 (8)‡ | 4 (7) | 9 (7) | 1.0 |
| cGVHD | 21 (42) | 23 (45) | 68 (57) | .15 |
| Limited | 3 | 5 | 17 | |
| Extensive | 17 | 18 | 49 | |
| Not recorded | 1 | 2 | ||
| Mortality day 1-100 | 3 (6) | 3 (6) | 9 (7) | 1.0 |
| Relapse | 13 (26) | 11 (20) | 23 (18) | .70 |
| Death | ||||
| Total | 15 (30) | 18 (33) | 37 (29) | .55 |
| Relapse/progressive disease | 6 (12) | 6 (11) | 13 (10) | |
| aGVHD | 2 (4) | 1 (2) | 1 (1) | |
| cGVHD | — | — | 3 (2) | |
| Infection | 2 (4) | 4 (7) | 10 (8) | |
| Graft failure | 1 (2) | 1 (2) | — | |
| TTP | 1 (2) | — | — | |
| Second malignancy | 2 (4) | 2 (4) | — | |
| Other | 1§ (2) | 4‖ (7) | 10¶ (9) | |
The table presents characteristics of all 50 patients who received CTL infusion (column: All CTL-infused patients); patients for whom a CTL product was available and who fulfilled criteria for comparative analysis at day 28, including 45 patients who received CTL and 9 who did not receive infusions for clinical reasons (column: CTL); and control patients who fulfilled eligibility criteria for the trial and for the comparative analysis but who did not have a CTL product made (column: Control) (See text for inclusion and exclusion criteria for comparative analysis of CTL trial recruits and controls). Values are numbers (percentages) unless otherwise indicated. Abbreviations are explained in Table 1.
P values refer to comparisons between columns 3 and 4; all are 2-sided; and values < .05 are considered significant.
Altogether 7 of 12 cases developed post-CTL infusion.
Altogether 2 of 4 cases developed post-CTL infusion
Pulmonary hypertension.
One case of pulmonary hypertension and 3 cases of organ failure (not specified).
Two cases of hemorrhage, 1 case of hepatic failure, 5 cases of organ failure (not specified), and 2 cases in which the cause was not recorded.
Characteristics of administered CTL
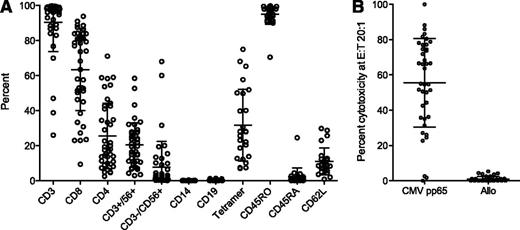
Characteristics of infused CTL products are shown in Figure 2A. The majority of CTL lines comprised CD3+ T cells (91%, range 26 to 100%). CD8+ cells accounted for a mean of 63% of CD3+ cells (range 9 to 94%), and CD4+ cells accounted for a mean 26% (range 3 to 71%). The mean of tetramer-positive cells in 25 assessable cases was 32% of CD3+ cells (range 7 to 75%). T-cell subset markers as a percentage of CD3+ were: CD62L+, 11.5% (range 0.8 to 29.8%), CD45RO+, 95% (range 71 to 100%), and CD45RA+, 2% (range 0 to 25%). Other cell types were present in small numbers: CD19+, mean 0.4% (range 0 to 1.3%) and CD14+, mean 0.1% (range 0 to 0.4%). True natural killer (NK) cells showed variation between individuals (CD3−CD56+ 8%, range 0.1 to 68%). The two cases with high NK cell levels (patient 14, NK = 60%; patient 41, NK = 68%) did not develop significant CMV infection. Patient 14 had no episodes of reactivation; patient 41 developed CMV reactivation post-CTL infusion but it resolved without intervention. Cytotoxicity data were available for 40/50 patients (Figure 2B). Mean specific lysis of pp65-pulsed targets at an effector-to-target ratio of 20:1 was 56% (range 0 to 100%). There were 3 CTL lines in which CMV-specific cytotoxic activity was low or undetectable by 51Cr assay. Patients 35 and 41 (pp65-specific lysis 0% and 2.6%, respectively) had low-level CMV reactivation that resolved without therapy. Patient 38 (pp65-specific lysis 1.7%) did not develop CMV reactivation. None of the 50 cases showed significant alloreactivity in vitro, with a mean percentage killing of recipient PHA blasts of 0.9% (range 0 to 5%).
Characteristics of CTL product for 50 patients who received CTL infusion. (A) Cell-surface phenotype of CTL lines infused (n = 50). CD3+, CD19+, CD14+, CD3−/CD56+ and CD3+/CD56+ are shown as a percentage of total cells; CD4+, CD8+, CD45RO+, CD45RA+, CD62L+ and tetramer are shown as a percentage of CD3+. Tetramer represents the total percentage of tetramer-positive cells. For participants with a tetramer for more than one HLA allele, the sum of all available tetramers is shown. Bars and whiskers represent means and standard deviations. (B) Cytotoxic capacity and alloreactivity of CMV-specific CTL using the 51Cr assay. CMV-specific cytotoxic activity was measured by specific lysis of pretransplant recipient-derived PHA blasts pulsed with an overlapping peptide mix spanning the pp65 protein; alloreactivity was measured by specific lysis of unpulsed recipient PHA blasts. Values shown are specific lysis at an effector-to-target ratio of 20:1. Bars and whiskers represent means and standard deviations.
Characteristics of CTL product for 50 patients who received CTL infusion. (A) Cell-surface phenotype of CTL lines infused (n = 50). CD3+, CD19+, CD14+, CD3−/CD56+ and CD3+/CD56+ are shown as a percentage of total cells; CD4+, CD8+, CD45RO+, CD45RA+, CD62L+ and tetramer are shown as a percentage of CD3+. Tetramer represents the total percentage of tetramer-positive cells. For participants with a tetramer for more than one HLA allele, the sum of all available tetramers is shown. Bars and whiskers represent means and standard deviations. (B) Cytotoxic capacity and alloreactivity of CMV-specific CTL using the 51Cr assay. CMV-specific cytotoxic activity was measured by specific lysis of pretransplant recipient-derived PHA blasts pulsed with an overlapping peptide mix spanning the pp65 protein; alloreactivity was measured by specific lysis of unpulsed recipient PHA blasts. Values shown are specific lysis at an effector-to-target ratio of 20:1. Bars and whiskers represent means and standard deviations.
CTL infusion
A total of 50 patients received CMV CTL between day 29 and day 115 posttransplant (median, day 45). Participants’ characteristics are shown in Table 1. Ten patients received CTL generated with NLV peptide pulsed mo-DC (patients 1 to 10 in Table 1); 33 patients received pp65-specific CTL generated with ad5f35pp65 transfected mo-DC derived from unprimed donor peripheral blood (patients 11 to 43); and 7 patients received infusions of CMV CTL generated with ad5f35pp65 transfected mo-DC and PBMC derived from a granulocyte-CSF stimulated peripheral blood stem cell harvest (patients 44 to 50). Characterization of CTL products and early postinfusion follow up for cohorts 1 and 2 have previously been published.21,22 The outcome for the combined group of 50 is presented here.
There were no serious adverse events related to infusion.
Cell dose
The target cell dose was 2 × 107 cells/m2. If cell expansion was insufficient for this target dose and the CTL product fulfilled all other acceptability criteria, a lower cell dose was infused. This occurred in 9 cases (patients 2, 7, 10, 11, 12, 14, 23, 35, 42). The median cell dose in these 9 patients was 1.2 × 107 cells/m2 (range, 0.06 to 1.9). Five of these patients developed CMV reactivation; in only 2 cases did this occur after T-cell infusion (patient 10 cell dose 1.2 × 107/m2 and patient 12 cell dose 0.06 × 107/m2). None of the patients who received low cell doses required CMV directed IV pharmacotherapy.
GVHD
Overall, 12 of the 50 patients who received CTL developed aGVHD grade 2 to 4 (24%), of which 4 (8%) were grade 3 to 4. A total of 5 of the 12 cases developed prior to CTL infusion; 7 occurred postinfusion. One patient with grade 1 skin GVHD prior to infusion developed grade 2 gastrointestinal GVHD post-CTL infusion. There were 4 cases of grade 3 to 4 GVHD (8%) observed post-CTL infusion. Of these, 2 had preexisting grade 2 GVHD, and worsening was associated with weaning from corticosteroids. The protocol was subsequently amended to ensure that doses of corticosteroids remained stable during the 2 weeks before and after T-cell infusion, and that patients were subsequently weaned by no more than 10% weekly. Two patients developed de novo grade 3 to 4 GVHD post-CTL infusion. One developed GVHD of the skin and liver that resolved with corticosteroids. The second patient developed GVHD of the gastrointestinal tract that was unresponsive to corticosteroids, ATG and etanercept; the patient died 108 days after T-cell infusion and 140 days posttransplant.
A landmark analysis was performed to assess the rate of chronic GVHD. Patients who were alive at day 100 were included (n = 47); 21 (42%) developed chronic GVHD.
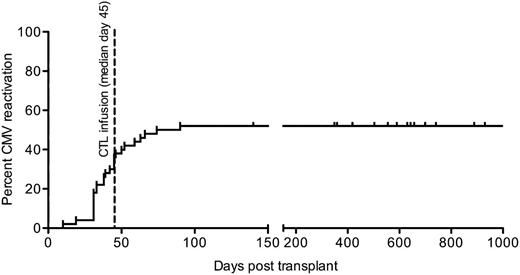
CMV reactivation, treatment and disease
CMV outcomes are described in Table 3. The incidence of CMV reactivation in the 50 patients treated with CTL was 26/50 (52%). All patients had their first reactivation of CMV prior to day 100; there were no cases of late CMV reactivation (after day 100 posttransplant, Figure 3). A total of 14 of 26 patients developed CMV reactivation prior to CTL infusion, 7 of the 26 had PCR positive for CMV on the day of infusion (results became available only after the infusion had been administered), and 5 of the 26 had CMV reactivation post-CTL infusion. Of the 26 patients with CMV reactivation, 9 required therapy with antiviral medications. Of the 5 patients who reactivated CMV after CTL infusion, only 1 required IV pharmacotherapy (patient 28). This occurred after initiation of methylprednisolone for aGVHD. CMV reactivation in the remainder of these 5 patients resolved without intervention. The majority of IV therapy was administered to those who developed CMV reactivation prior to CTL infusion (total days of therapy for 8 patients whose first episode of CMV reactivation occurred prior to T-cell transfusion was 187 days vs 22 days of therapy for 1 patient whose first episode of CMV reactivation occurred after T-cell infusion).
CMV reactivation in 50 patients who received CMV CTL infusions. Cumulative incidence of first CMV reactivation in 50 patients treated with CMV CTL. Median day of infusion is shown (day 45; range 29 to 115 days).
CMV reactivation in 50 patients who received CMV CTL infusions. Cumulative incidence of first CMV reactivation in 50 patients treated with CMV CTL. Median day of infusion is shown (day 45; range 29 to 115 days).
There was one case in which CMV stains were positive on colonic biopsy at the same time as the patient had aGVHD prior to CTL infusion (patient 6). No specific anti-CMV therapy was instituted, and CMV stains were negative on subsequent biopsy.
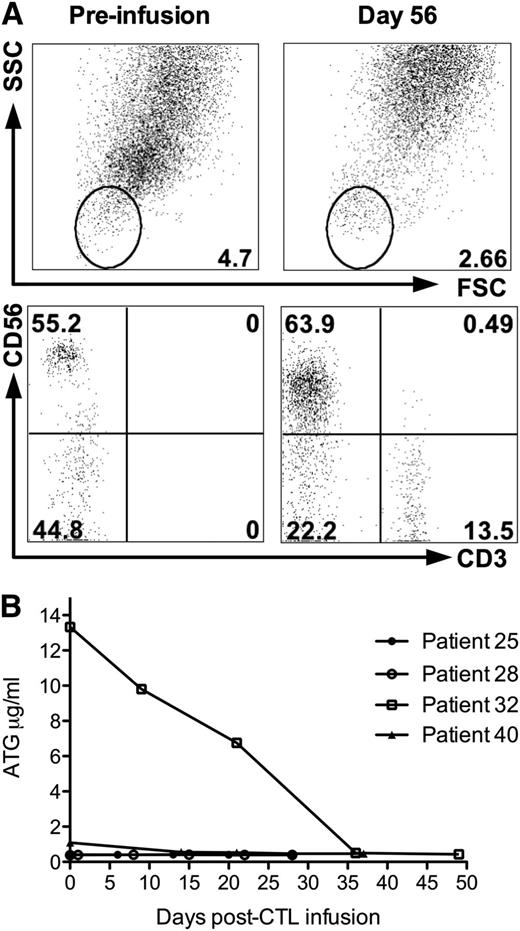
There was 1 death attributable to CMV disease. Patient 32 developed CMV reactivation on the day of CTL infusion (day 31 posttransplant). Despite treatment with ganciclovir, he developed CMV colitis and pneumonitis; he died of respiratory failure 88 days posttransplant and 57 days post-CTL infusion. In addition to CMV reactivation, he was positive for EBV by PCR. This patient had no detectable CD3+ cells in peripheral blood at the time of infusion (Figure 4A). Since this was the only case in which this phenomenon was observed, we performed a quantitative assay of ATG levels on stored plasma from patient 32 and on 3 patients treated with CMV CTL who also received ATG during conditioning; the results are shown in Figure 4B. Serum ATG levels were elevated at the time of infusion and fell steadily until prior to patient 32’s death. In contrast, the other patients had low or undetectable levels of ATG at the time of infusion.
Correlative studies of peripheral blood immunophenotype and ATG levels in patient 32; this patient died of CMV pneumonitis post-CTL infusion. (A) Peripheral blood immunophenotype of lysed whole blood of patient 32 pre-CTL infusion (day 31 posttransplant) and day 56 postinfusion (day 87 posttransplant). (B) Plasma ATG levels in 4 patients who received CTL infusions following conditioning containing ATG. Patient 32 ( ) developed CMV pneumonitis and died 88 days posttransplant (57 days post-CTL infusion). Total doses of ATG administered were: patient 25, 30 mg/kg; patient 28, 15 mg/kg; patient 32, 30 mg/kg; and patient 40, 20 mg/kg.
) developed CMV pneumonitis and died 88 days posttransplant (57 days post-CTL infusion). Total doses of ATG administered were: patient 25, 30 mg/kg; patient 28, 15 mg/kg; patient 32, 30 mg/kg; and patient 40, 20 mg/kg.
Correlative studies of peripheral blood immunophenotype and ATG levels in patient 32; this patient died of CMV pneumonitis post-CTL infusion. (A) Peripheral blood immunophenotype of lysed whole blood of patient 32 pre-CTL infusion (day 31 posttransplant) and day 56 postinfusion (day 87 posttransplant). (B) Plasma ATG levels in 4 patients who received CTL infusions following conditioning containing ATG. Patient 32 ( ) developed CMV pneumonitis and died 88 days posttransplant (57 days post-CTL infusion). Total doses of ATG administered were: patient 25, 30 mg/kg; patient 28, 15 mg/kg; patient 32, 30 mg/kg; and patient 40, 20 mg/kg.
) developed CMV pneumonitis and died 88 days posttransplant (57 days post-CTL infusion). Total doses of ATG administered were: patient 25, 30 mg/kg; patient 28, 15 mg/kg; patient 32, 30 mg/kg; and patient 40, 20 mg/kg.
Correlative studies of viral immunity
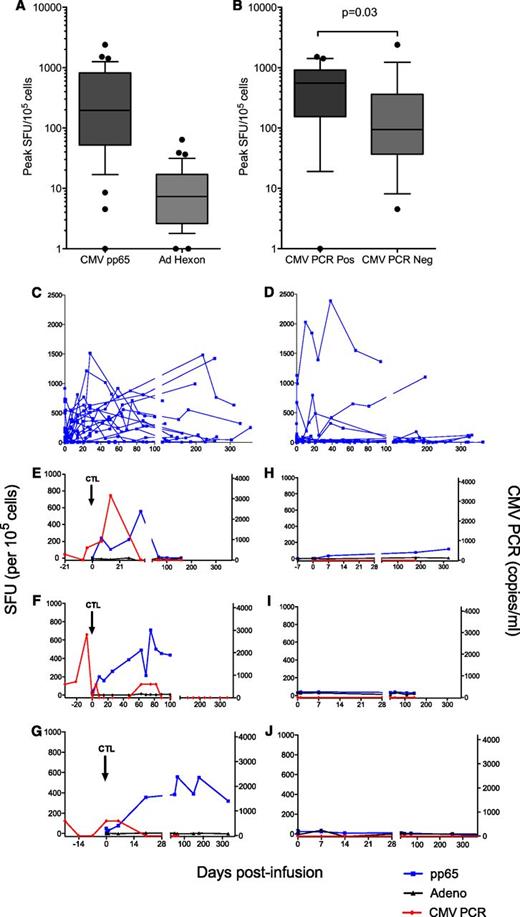
Immune monitoring data were available for 37 of 50 patients. Functional pp65 and adenoviral hexon-specific immunity were measured by EliSpot immunoassay. The median peak pp65-specific immunity measured 219 SFU/105 cells (range 0 to 2387; Figure 5A). Adenovirus-specific immunity was detectable at low levels (median peak 7 SFU/105 cells, range 0 to 64). No patient taking part in the study experienced clinically significant adenovirus infection. The peak of pp65-specific immunity correlated with the presence of CMV antigen (Figure 5B). Patients who had measurable CMV antigen by CMV PCR or pp65 antigenemia were more likely to have a greater increase in measurable anti-CMV immunity than those who did not (Figure 5C-D). There was a statistically significant difference in the peak CMV-specific immunity in those who had CMV reactivation (median peak 556SFU/105 cells, range 0 to 1515) compared with those who did not (median peak 94SFU/105 cells, range 5 to 2387; P = .03. Figure 5B). In some cases, pp65-specific immunity was seen to rise at the time of CMV antigenemia as measured by quantitative PCR; and in many cases no increase was seen in the absence of CMV antigen. While there were some examples in which there was a detectable rise in immunity without detectable CMV antigenemia, the only case in which there was no detectable CMV immunity in response to CMV antigenemia was in patient 32, who had elevated ATG. Representative examples are shown in Figure 5E-J.
Correlative studies of immune function in patients who received CTL infusion. Data for 37 patients for whom results were available. (A) Peak IFN-γ EliSpot for pp65 (CMV) and adenovirus hexon protein postinfusion of T cells. Counts displayed are the maximum measured for each patient above negative control. Bars represent medians, boxes represent 25th to 75th percentiles, and whiskers represent the 10th and 90th percentiles. Individual results outside these parameters are shown (●). (B) Peak pp65-specific immunity as measured by IFN-γ EliSpot in recipients of CTL who did and did not develop CMV reactivation. Bars represent medians, boxes represent 25th to 75th percentiles, and whiskers represent the 10th and 90th percentiles. Individual results outside these parameters are shown (●). (C-D) CMV-specific immunity over time in recipients of CTL who did (C, n = 19) and did not (D, n = 18) develop CMV reactivation at any time posttransplant. PBMC collected at preinfusion and follow-up time points were incubated with pp65 peptide mix in an IFN-γ EliSpot immunoassay. Results are expressed as spot-forming units/105 cells above negative control. (E-G) Representative examples of CMV pp65-specific immunity and adenovirus hexon-specific immunity as measured by IFN-γ EliSpot in 3 recipients of CMV CTL who developed CMV reactivation. (E, patient 40; F, patient 20; G, patient 25). (H-J) Representative examples of CMV pp65-specific immunity and adenovirus hexon-specific immunity as measured by IFN-γ EliSpot in 3 recipients of CMV CTL who did not develop CMV reactivation. (H, patient 26; I, patient 17; J, patient 44).
Correlative studies of immune function in patients who received CTL infusion. Data for 37 patients for whom results were available. (A) Peak IFN-γ EliSpot for pp65 (CMV) and adenovirus hexon protein postinfusion of T cells. Counts displayed are the maximum measured for each patient above negative control. Bars represent medians, boxes represent 25th to 75th percentiles, and whiskers represent the 10th and 90th percentiles. Individual results outside these parameters are shown (●). (B) Peak pp65-specific immunity as measured by IFN-γ EliSpot in recipients of CTL who did and did not develop CMV reactivation. Bars represent medians, boxes represent 25th to 75th percentiles, and whiskers represent the 10th and 90th percentiles. Individual results outside these parameters are shown (●). (C-D) CMV-specific immunity over time in recipients of CTL who did (C, n = 19) and did not (D, n = 18) develop CMV reactivation at any time posttransplant. PBMC collected at preinfusion and follow-up time points were incubated with pp65 peptide mix in an IFN-γ EliSpot immunoassay. Results are expressed as spot-forming units/105 cells above negative control. (E-G) Representative examples of CMV pp65-specific immunity and adenovirus hexon-specific immunity as measured by IFN-γ EliSpot in 3 recipients of CMV CTL who developed CMV reactivation. (E, patient 40; F, patient 20; G, patient 25). (H-J) Representative examples of CMV pp65-specific immunity and adenovirus hexon-specific immunity as measured by IFN-γ EliSpot in 3 recipients of CMV CTL who did not develop CMV reactivation. (H, patient 26; I, patient 17; J, patient 44).
Long-term outcomes and major adverse events
Treatment outcomes are seen in Table 3. The median follow-up time for patients treated with CTL was 26 months posttransplant (range, 2 to 80 months). A total of 13 deaths occurred in treated patients during the study period. Causes of death are shown in Table 3. The median overall length of survival for the 50 patients was 76 months (95% CI, 56 to 96 months). Thirteen of 50 (26%) patients treated with CTL relapsed during the follow-up period. Median progression-free survival was not reached. Progression-free survival at 1 year was 89%, and at 5 years was 66%.
Other grade 3 or 4 adverse events occurring post-CTL infusion included transplant associated microangiopathy (n = 1) and graft failure (n = 1). Other adverse events included second malignancy (adenocarcinoma of the lung, n = 2), pulmonary hypertension (n = 1), bacterial infection (n = 6), yeast and fungal infection (n = 6), and nonfatal intracerebral hemorrhage (n = 1).
Comparison with control cohort
Of 228 transplants performed from CMV-positive donors, 182 were eligible for inclusion in the cohort analysis at the landmark time point of day 28 (CTL cohort, n = 54; controls, n = 128). The 2 groups were similar in terms of demographics and all treatment factors except HLA matching. There were more patients with mismatched donors in the CTL cohort (9% vs 2%; P = .03; Table 3). This factor could increase the risk of GVHD and bias in favor of the control group.
Given that the study design was for early administration of CTL at a time when patients were at risk of aGVHD, rates of aGVHD were of particular interest. Grade 2 to 4 aGVHD occurred in 13/54 (24%) of the CTL cohort vs 24/128 (18%) of the controls (P = .42). Grade 3 to IV aGVHD occurred in 4/54 (7%) of the CTL cohort vs 9/128 (7%) of the controls (P = 1.0). For the cGVHD landmark analysis, 170 patients were alive at day 100 (51 in the CTL cohort and 119 in the control cohort); 91 (54%) developed cGVHD (CTL cohort, n = 23 [45%] vs control, n = 68 [57%]; P = .15).
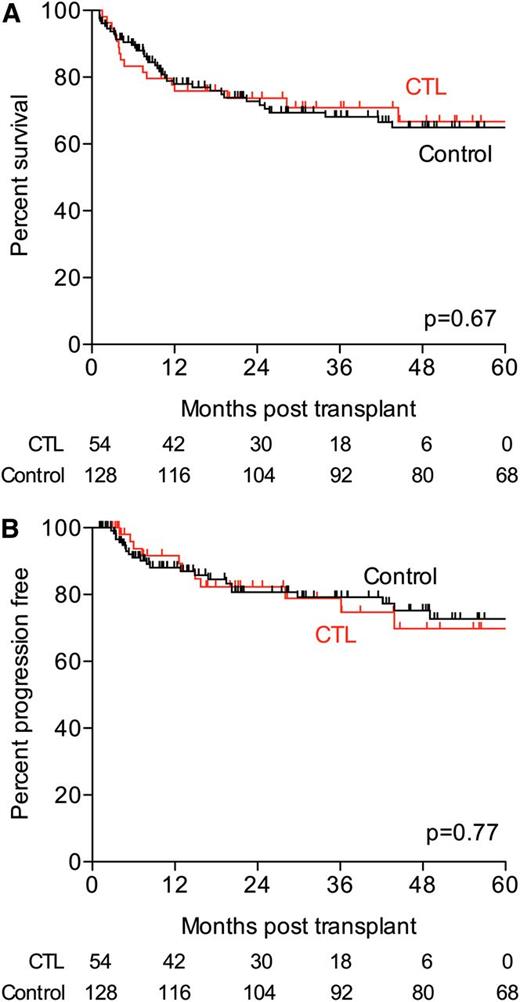
Median overall survival was 76 months for the CTL cohort and not reached for controls (P = .67; hazard ratio, 1.13; 95% CI, 0.64 to 1.99; Figure 6A). Overall survival at 1 year and at 5 years was similar (76 vs 78% at 1 year for CTL vs controls; 67 vs 65% at 5 years for CTL vs controls).
Survival in CTL (red) and control (black) cohorts. (A) Overall survival in CTL and control groups. (B) Progression-free survival in CTL and control groups.
Survival in CTL (red) and control (black) cohorts. (A) Overall survival in CTL and control groups. (B) Progression-free survival in CTL and control groups.
The median progression-free survival was not reached in either group (P = .77; hazard ratio, 1.11; 95% CI, 0.54 to 2.3). The percentage of patients free from progression was similar in both groups at the 1 year and 5 year time points (88% vs 91% at 1 year; 73% for both groups at 5 years; Figure 6B).
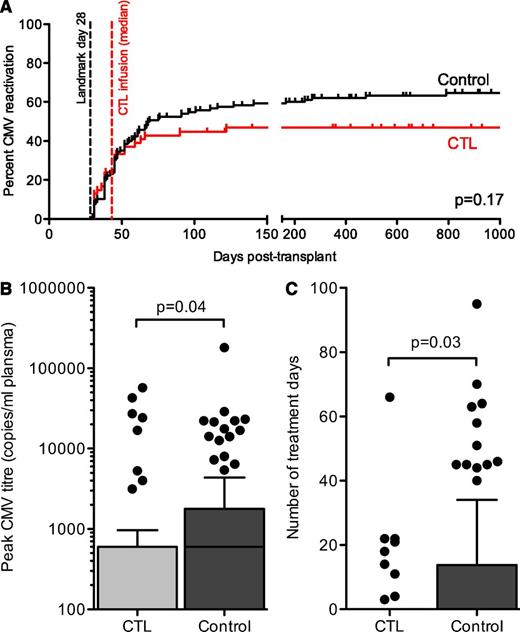
Regarding CMV outcomes, there was no statistically significant reduction in the cumulative incidence of CMV reactivation (as defined by positive CMV antigenemia or PCR of any copy number) (46% in the CTL cohort vs 60% in the control cohort; P = .17; hazard ratio, 0.73; 95% CI, 0.46 to 1.14; Figure 7A).
CMV outcomes in CTL and control groups. (A) Cumulative incidence of first CMV reactivation in the subgroups used for comparative analysis (CTL group, n = 54; control group, n = 128). The day posttransplant of the landmark day (day 28) and the median day of infusion for the CTL group (day 45, range 29 to 115 days) are shown. The difference was not statistically significant (P = .10). (B) Peak CMV titer in CTL and control cohorts by quantitative PCR. Bars represent medians, boxes represent 25th to 75th percentiles, and whiskers represent 1.5 times the interquartile distance. Individual results outside these parameters are shown (●). (C) Number of days of treatment with intravenous anti-CMV therapy (ganciclovir, foscarnet, or both) for all patients in the CTL and control cohorts. Bars represent medians, boxes represent 25th to 75th percentiles, and whiskers represent 1.5 times the interquartile distance. Individual results outside these parameters are shown (●).
CMV outcomes in CTL and control groups. (A) Cumulative incidence of first CMV reactivation in the subgroups used for comparative analysis (CTL group, n = 54; control group, n = 128). The day posttransplant of the landmark day (day 28) and the median day of infusion for the CTL group (day 45, range 29 to 115 days) are shown. The difference was not statistically significant (P = .10). (B) Peak CMV titer in CTL and control cohorts by quantitative PCR. Bars represent medians, boxes represent 25th to 75th percentiles, and whiskers represent 1.5 times the interquartile distance. Individual results outside these parameters are shown (●). (C) Number of days of treatment with intravenous anti-CMV therapy (ganciclovir, foscarnet, or both) for all patients in the CTL and control cohorts. Bars represent medians, boxes represent 25th to 75th percentiles, and whiskers represent 1.5 times the interquartile distance. Individual results outside these parameters are shown (●).
We compared CMV peak titer as a measure of the ability of transferred T cells to control CMV replication. There was a statistically significant difference in the distribution of peak CMV titer between the groups (median, 0 for the CTL group vs 600 for the controls; P = .04; Figure 7B).
The total number of treatment days of IV pharmacotherapy was assessed as a measure of the CMV burden over time. There was a reduction in the number of patients requiring pharmacotherapy in the CTL cohort (17% vs 36%; P = .01) as well as in the total number of treatment days per patient in each cohort (3.4 vs 8.9 days; P = .03; Figure 7C). The rate of CMV disease was 6% in the CTL cohort (3 cases: 1 of CMV myelosuppression who did not receive a CTL infusion, 1 of CMV colitis prior to CTL infusion, and 1 who had elevated ATG as described and died of CMV pneumonitis). In the control cohort the rate of CMV disease was 9% (11 cases: 7 of colitis, 1 of pneumonitis, 2 of myelosuppression, 1 of retinitis). The study was not powered to detect a difference in event rates at this low magnitude (for this comparison, P = .76). There was 1 death attributable to CMV in each group: the CTL recipient with elevated ATG and a control patient who died of CMV pneumonitis.
CMV reactivation occurred primarily within the first 100 days in both groups. There was 1 case of late CMV reactivation in the CTL cohort (at day 122). This patient did not receive CTL due to the presence of grade 3 GVHD. The peak CMV copy number was 600 copies/mL, and this patient did not require intravenous antiviral therapy. There were 9 patients in the control cohort who developed late CMV reactivation (13% of the 77 patients who reactivated CMV in the control cohort). Of these 9 patients, 8 required IV pharmacotherapy and 4 developed CMV disease (3 developed colitis and 1 developed myelosuppression). This group of late CMV reactivations required a disproportionate amount of therapy (281 of the total 1143 days [25%] for the control cohort for 8/78 [10%] of CMV reactivations).
Discussion
We report the first data examining the long-term safety and efficacy of CMV-specific T cells administered early postallogeneic HSCT, and the first cohort study to assess the risks and benefits of CMV-specific T-cell therapy. Our data provide robust support for the safety of CTL when given in this way. There were no serious, immediate infusion-related toxicities. Although a small number of patients developed severe GVHD after infusion, this occurred during the time when aGVHD is known to occur. Compared with a control cohort, there was no increase in either aGVHD or cGVHD attributable to CMV-specific T-cell infusion. Overall survival and progression-free survival rates were similar in the trial cohort and the control cohort.
A reduction in the incidence of CMV reactivation in patients receiving CMV-specific T cells was noted but did not reach statistical significance. Like others32,33 we observed that antigen stimulation in the form of reactivation was associated with more easily detectable CMV-specific immune responses. We used EliSpot because this allowed us to measure specific CD4+ and CD8+ immune responses. We speculate that the expansion of adoptively transferred cells at low CMV copy numbers was able to control viral replication. Consistent with this, we found a significant reduction in the percentage of patients receiving CMV-specific T cells who required intravenous antiviral pharmacotherapy. There was also a reduction in the total number of days of treatment required for patients whose CMV burden reached the threshold for treatment with ganciclovir or foscarnet according to institutional guidelines. This is a measure of the ability of transferred cells to impact the burden of CMV over time because patients with recurrent or persistent CMV reactivation were treated with prolonged courses of IV therapy. There were no trial or institutional policies for cessation of antiviral medicines beyond having the initial duration of therapy last 2 weeks. These decisions were left to the discretion of the treating clinicians, most of whom were not involved with the study. Defining rules for stopping antiviral treatment in future studies would render data measuring the duration of antiviral treatment more robust.
The peak titer of CMV was used as a measure of CMV CTL ability to control viral replication. The median peak titer was significantly reduced in the treatment cohort. Although the study design allowed for infusion of CTL any time from day 28, the median time to infusion was 45 days posttransplant. A rapid increase in the cumulative incidence of CMV reactivation occurred prior to this time, at around day 21 posttransplant (Figure 3A). Patients in whom reactivation occurred prior to cell infusion required the most anti-CMV pharmacotherapy. These data suggest that earlier infusion of CMV-specific T cells may be even more successful in preventing the need for posttransplant CMV pharmacotherapy, but this will require more rapid manufacture and exclusion of T cell-recognizing antibodies from conditioning regimens.
Among the patients who received CTL infusion, there were no late CMV reactivations (that is, none occurred after day 100) compared with the control cohort in which 13% had late CMV reactivations. We did not detect any reduction in the use of corticosteroids or calcineurin inhibitors in the infusion group that might explain this finding (data not shown). These results are encouraging, and suggest that early infusion of CMV-specific T cells reconstitutes both immediate and longer term CMV immunity, or at least bridges the period of posttransplant immunosuppression that exists until the development of stem cell-derived immunity. Preventing late CMV reactivation was of particular benefit, since late reactivations in the control cohort resulted in a disproportionately high requirement for treatment with ganciclovir or foscarnet. CMV disease in both the treated and control cohorts was low. The 1 case of CMV disease and death attributable to CMV occurred in a patient in whom we documented high ATG levels at the time of CTL infusion, which resulted in profound lymphopenia.
In addition to safety and efficacy, our data confirm the feasibility of routine therapy with CMV-specific T cells following allogeneic transplant. Although 10/60 patients for whom a CTL product was manufactured did not eventually have therapy for clinical reasons, a CTL product was available for and delivered to the majority of study participants. This study used a standard target dose of CTL of 2 × 107/m2, but there were 9 cases in which this dose was not reached. We did not observe any obvious association between decreased cell dose and lack of efficacy. Although we used 3 different techniques to generate T cells, we were unable to identify any differences in the rates of CMV reactivation or requirements for therapy associated with the different methods. Larger studies may yet show that such differences exist. Logistic or laboratory delays in CTL administration could be reduced in future studies by using the more rapid manufacturing techniques that have been described12,18-20,34,35 and by streamlining product clearance.
Reducing the use of medicines may result in clinical improvements by avoiding drug-related morbidity, such as myelosuppression and renal impairment; it may also reduce the burden of costly pharmacotherapy, which often requires inpatient administration. Aside from the direct benefit in terms of CMV, if confirmed to be of benefit this approach may also be applied to a number of other common posttransplant opportunistic infections, including adenovirus, human herpesvirus type 6, and others.
Care must be taken when interpreting the results of nonrandomized studies. A contemporaneously treated control cohort was used to allow comparison with similarly treated patients. While posttransplant care was delivered according to institutional protocol and not dictated by the study, physicians’ preferences may have altered care in this nonblinded study. Participation in a trial may have led to the selection of a more favorable patient group in the CTL cohort. Nevertheless, we believe that this study contributes significant information on the long-term outcomes of patients treated with ex vivo manipulated antigen-specific T cells, and suggests that randomized studies are needed to confirm the benefits we describe.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Angela Bayley, Gillian Huang, Kylee Donaldson, Lisa Fisher and Jiin Fung for assistance with data collection.
E.B. was a Leukaemia Foundation of Australia Clinical Fellow, a Cancer Institute NSW Research Scholar (RSA07-1-02), and a recipient of the Royal College of Pathologists of Australasia Research Award. K.P.M. was a Leukaemia Foundation of Australia Clinical Fellow. C.K.K.M. is a current Leukaemia Foundation of Australia Clinical Scholar. This research was supported by funding from the Cancer Council NSW (CCNSW 07-13) and Research Infrastructure Support Services (RANSW403).
Authorship
Contribution: E.B. recruited, treated and followed up with patients taking part in the study, collected data for the control cohort, analyzed the data, and wrote the manuscript; L.C. supervised the running of the clean-room facilities, manufactured T-cell lines for therapeutic use, performed assays for cell-line release, and assisted with preparation of the manuscript; R.S. and J.B. manufactured cell lines for therapeutic use and assisted with maintenance of clean-room facilities; C.K.K.M. recruited, treated and followed up with patients taking part in the study; S.D. assisted with postinfusion immune-monitoring experiments; K.B. assisted with statistical analysis; M.-C.D. collected data on transplantation outcomes; P.J.S. supervised the recruitment, treatment and follow up of patients at the second treatment center, and collected data on patients in the control cohort; K.P.M. recruited, treated and followed up with patients taking part in the study, and assisted with preparation of the manuscript; D.J.G. provided overall academic leadership, treated and followed up on patients, and assisted with preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emily Blyth, Westmead Hospital Department of Haematology, PO Box 533, Wentworthville NSW 2145, Australia; e-mail: emily.blyth@sydney.edu.au.