Key Points
A sizable fraction of CLL patients is characterized by the expansion of clonal B cells with anergic features.
The constitutive biochemical signature of B-cell anergy can be efficiently targeted in CLL for therapeutic purposes.
B-cell receptor (BCR) triggering and responsiveness have a crucial role in the survival and expansion of chronic lymphocytic leukemia (CLL) clones. Analysis of in vitro response of CLL cells to BCR triggering allowed the definition of 2 main subsets of patients and lack of signaling capacity was associated with constitutive activation of extracellular-regulated kinases 1/2 (ERK1/2) and nuclear factor of activated T cells c1 (NF-ATc1), consistent with the idea that at least one group of CLL patients derives from the abnormal expansion of anergic B cells. In the present work, we further investigated the anergic subset of CLL (defined as the one with constitutive ERK1/2 phosphorylation) and found that it is characterized by low levels of surface immunoglobulin M and impairment of calcium mobilization after BCR engagement in vitro. Chronic BCR triggering promoted CLL cell survival selectively in phosphorylated ERK1/2 samples and the use of mitogen-activated protein kinase and NF-AT signaling inhibitors specifically induced apoptosis in this group of patients. Apoptosis induction was preceded by an initial phase of anergy reversal consisting in the loss of ERK phosphorylation and NF-AT nuclear translocation and by the restoration of BCR responsiveness, reinforcing the idea that the anergic program favors the survival of leukemic lymphocytes.
Introduction
Chronic lymphocytic leukemia (CLL), a chronic lymphoid malignancy characterized by the accumulation of monoclonal, CD5+ B lymphocytes in peripheral blood, bone marrow, and secondary lymphoid organs, is clinically heterogeneous. Patients may present with an indolent disease and a life expectancy similar to healthy individuals or with an aggressive tumor, shorter survival, and early treatment requirements.1 Biological predictors of clinical outcome, such as the mutational status of the immunoglobulin (Ig) heavy chain variable genes (IGHV) and the expression of signaling molecule ZAP70,2,3 suggest a key role for the CLL cell membrane Ig in the pathogenesis of the disease. In addition, molecular and functional analyses of the B-cell receptor (BCR) strongly support the hypothesis that a stimulation through the BCR is involved in the selection and expansion of the malignant CLL clone.4,-6 In line with this, several groups (reviewed by Stevenson et al7 ) have found that a number of molecules along the signaling pathways originating from the BCR are constitutively activated in all patients with CLL, providing the basis for effective therapeutic targeting, which currently is being tested in prospective clinical trials (reviewed by Woyach et al8 ).
We previously reported that in approximately one-half of CLL cases leukemic cells constitutively express phosphorylated extracellular-regulated kinases 1/2 (ERK1/2) and mitogen-activated protein kinase (MAPK)/ERK kinases 1/2 (MEK1/2) together with activated nuclear factor of activated T cells c1 (NF-ATc1) transcription factor. Such cases also are characterized by cellular unresponsiveness to surface Ig M (sIgM) ligation in terms of phosphotyrosine induction.9 In addition, Mockridge et al10 demonstrated that cells from patients with CLL that express low levels of sIgM fail to mobilize intracellular calcium upon BCR triggering. Taken together, these features recapitulate the biochemical program observed in mouse models of B-cell anergy.11 Anergy12 is one of the mechanisms that the immune system adopts to silence autoreactive B lymphocytes upon low-affinity recognition of self antigens (Ag).13 The state of BCR desensitization induced by chronic Ag binding in vivo results in cell unresponsiveness when B lymphocytes are further stimulated in vitro by an Ag-like activation.14 In mouse models, anergized B cells share a common biochemical signature that includes low levels of sIgM (as the result of constant BCR internalization and recycling15 ), elevated basal intracellular calcium concentrations, and subsequent constitutive activation of ERK1/2 and NF-ATc1.11,16 This biochemical program is not permanent, but it is reversible and lasts as long as B lymphocytes are exposed to the Ag.14
Together, these observations led us to propose that the constitutive activation of the MAPK signaling pathway along with NF-ATc1 transactivation also represents the molecular signature of human anergic B lymphocytes and thus that CLL cases presenting this signature may be taken as a human model of leukemic expansions of B cells with anergic properties.9
In this work, we investigated the molecular features of the CLL anergic subset (defined as the one that presents constitutive phosphorylated ERK [pERK]), and we also took advantage of the use of small chemical compounds that specifically block the activation of both ERK1/2 and NF-ATc1. We concluded that anergy induction and maintenance favors the survival of leukemic lymphocytes and may be effectively targeted for therapeutic purposes.
Materials and methods
Tissue samples and cell purification
Leukemic lymphocytes were obtained from the peripheral blood of patients with CLL who were diagnosed according to the International Workshop on CLL/National Cancer Institute Working Group guidelines.17 All patients were either untreated or off therapy for at least 6 months before the beginning of the study. The following parameters were analyzed for each patient: age, gender, disease stage at diagnosis according to Binet18 or modified Rai19 criteria, CD38 expression, IGHV gene mutational status,20 ZAP70 expression,2 history of treatment, characterization of disease as progressive or stable as defined by the International Workshop on CLL/National Cancer Institute Working Group, and survival time.
Leukemic cells were purified immediately after blood withdrawal, by negative depletion, with the use of a B-lymphocyte enrichment kit (RosetteSep; StemCell Technologies). The purity of all preparations was always more than 99%, and the cells coexpressed CD19 and CD5 on their cell surfaces as checked by flow cytometry (FC500; Beckman Coulter); preparations were virtually devoid of natural killer cells, T lymphocytes, and monocytes.
All tissue samples were obtained with approval by the institutional review board of San Raffaele University Hospital (Milan, Italy). Informed consent was obtained in accordance with the Declaration of Helsinki. The MEC1 CLL cell line21 was purchased from DSMZ.
Antibodies and reagents
Antibodies.
For flow cytometry, anti-CD19 and anti-CD5 were purchased from Beckman Coulter and IgM from Southern Biotechnology. Alexafluo488-pERK1/2 antibody was from BD Biosciences Pharmingen. Cells were analyzed with a FC500 flow cytometer (Beckman-Coulter).
For western blotting, antiphospho-ERK1/2 (Y202/Y204) and antiphospho-RSK1/2 (T359/S363) were purchased from Cell Signaling Technology. Anti-ERK1/2 and anti-RSK1 were from Santa Cruz Biotechnology, Inc.
For western blot analysis, specific secondary antibodies horseradish peroxidase (HRP)-conjugated, (goat antirabbit Ig and goat antimouse Ig) were purchased from Upstate Biotechnology. For BCR triggering, goat F(ab′)2 antihuman IgM antibodies were purchased from CALTAG Laboratories.
Inhibition and stimulation.
U0126 was purchased from Calbiochem and CI1040 and AZD6244 were from Selleck Chemicals. For in vitro studies, 11R-VIVIT peptide was purchased from Calbiochem; for in vivo experiments, the peptide was chemically synthesized by Genescript (purity >98%) and resuspended in water at the concentration of 5 mg/mL. NMS6E was provided by Nerviano Medical Sciences. As a positive control for phospho-flow staining, processed murine splenocytes were stimulated with phorbol 12-myristate 13-acetate (100 ng/mL; Sigma-Aldrich).
Cell culture and stimuli
Purified leukemic cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 2mM l-glutamine, and 15 μg/mL gentamicin (complete RPMI 1640; Invitrogen). Lymphocytes were cultured at the concentration of 3 × 106 cells/mL in the presence or the absence of different stimuli or inhibitors as indicated.
Western blot analysis
Cells were lysed with ice-cold lysis buffer (NaCl 0.15M; 1% v/v NP40; 1mM EDTA, pH 8; 50mM Tris-HCl, pH 7) with fresh protease and phosphatase inhibitors cocktail (Roche).
Whole protein extracts (30 µg) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and proteins from the gel were electron-transferred onto nitrocellulose membranes and incubated with the indicated antibodies. Antibodies were diluted in a solution of phosphate-buffered saline (PBS) + 0.1% Tween containing 5% of nonfat dry milk or 5% of bovine serum albumin according to the manufacturers’ instructions. Immunoreactivity was revealed by incubation with specific secondary antibodies conjugated with HRP and was followed by enhanced chemiluminescence reaction (Pierce) and film exposures. Densitometric analysis of ERK-specific bands was performed with ImageQuant Software (GE Healthcare). The values of individual patients were calculated as percentage of the positive control (MEC1 cell line) and determined as the ratio of the optical density of phospho-ERK and optical density of total ERK.
NF-ATc1 activation assay
Nuclear extraction.
Nuclear and cytoplasmic extracts were obtained by the use of a Nuclear Extract Kit from Active Motif. To summarize, 8.8 × 106 cells (left untreated or treated with the indicated stimuli/inhibitors) were collected in ice-cold PBS in the presence of phosphatase inhibitors (provided with the kit). The cells were then resuspended in hypotonic buffer (20mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid, pH 7.5; 5mM NaF; 10μM Na2MoO4; 0.1mM EDTA) and incubated on ice for 15 minutes. The addition of detergent caused the cytoplasmic proteins to leak into the supernatant. After collection of the cytoplasmic fraction, the nuclei were lysed, and the nuclear proteins were solubilized in lysis buffer in the presence of the protease inhibitor cocktail (all solutions were provided with the kit). Nuclear extracts were quantified (by using the bicinchoninic acid assay) and used for the evaluation of NF-ATc1 activation.
NF-ATc1 enzyme-linked immunosorbent assay−based assay.
The level of NF-ATc1 activation was determined by use of the TransAM transcription factor kit from Active Motif. Nuclear extracts, purified as described in the previous section, were incubated with oligonucleotides containing the NF-AT−specific consensus sequence immobilized on a 96-well plate. Active NF-AT contained in nuclear extracts bound specifically to this oligonucleotide and was detected with a specific antibody against NF-AT. The addition of a secondary antibody conjugated to HRP provided colorimetric readout that we quantified by measuring the absorbance at 450 nm. To confirm specificity, we also performed competition experiments by adding 2 μL of free oligonucleotide to the wells before nuclear extract (data not shown).
Analysis of cell viability and apoptosis
After each different treatment at indicated doses and time points, the percentage of viable cells was determined by use of the CellTiter-Glo Luminescent Cell Viability Assay kit, according to the manufacturer's instructions (Promega). All measurements were performed in duplicate, and the viability of treated cells was normalized to the relative untreated control.
Induction of apoptosis was evaluated by double staining with fluorescein isothiocyanate−conjugated Annexin V and propidium iodide with the Annexin V-fluorescein isothiocyanate Apoptosis Detection Kit (eBioscence) according to the manufacturer’s instructions. Samples were analyzed on a FC500 flow cytometer (Beckman Coulter).
Intracellular calcium flux
Intracellular calcium flux was measured by use of the fluorogenic probe Fluo3AM (Invitrogen) as previously described.10 To summarize, purified leukemic cells (at concentration of 107 cells/mL in complete RPMI 1640) were incubated with 4μM Fluo3-AM and 0.02% (v/v) of Pluronic F-127 (Sigma-Aldrich) for 30 minutes at 37°C. Cells were then washed and resuspended at 5 × 106 cells/mL in complete RPMI 1640 at room temperature. Cells (250 μL) were warmed to 37°C for 5 minutes before the acquisition of background fluorescence (ie, of unstimulated cells), followed by addition of 20 μg/mL goat F(ab′)2 antihuman IgM (Southern Biotechnology) and data acquisition for a further 5 minutes. Data were acquired on a BD FACScalibur and analyzed with the use of Flowjo software (Tree Star).
To calculate the percentage of cells responding to BCR triggering, we first established a baseline fluorescence threshold (T) for each sample (by acquiring samples for 40 seconds without any stimulus) at the fluorescence intensity of the 85th percentile of unstimulated cells. We then calculated the peak percentage of cells that exhibited an increase in fluorescence intensity above the threshold after treatment with anti-IgM. As previously described,10 we classified patient as signalers when the percentage of cell responding to anti-IgM stimulation was greater than 5%.
In vivo experiments
Rag2−/− γc−/− mice were kindly provided by CIEA and Taconic. All mice were housed and bred in a specific pathogen-free animal facility, treated in accordance with the European Union guidelines and approval of the San Raffaele Scientific Institute Institutional Ethical Committee.
Eight-week-old Rag2−/−γc−/− female mice were challenged subcutaneously in the left flank with 10 × 106 MEC1 cells in 0.1 mL of saline through a 27-gauge needle, as previously described.22 Ten days after cells injection, mice bearing subcutaneous tumor were intraperitoneally injected with a specific schedule (5 days on, 2 days off) with 11R-VIVIT (10 mg/kg, dissolved in PBS) or with phosphate buffer solution (PBS-saline) as a control.
Animals were monitored twice a week for weight and tumor growth (measuring 3 perpendicular diameters) and sacrificed when the mean tumor volume reached 1000 mm3 or when animals experienced clinical signs and symptoms.
Peripheral blood and/or tissue (spleen, lymph nodes, and femoral bone marrow) single-cell suspensions were depleted of red blood cells by incubation in an ammonium chloride solution (ACK) lysis buffer (NH4Cl 0.15M, KHCO3 10mM, Na2EDTA 0.1mM, pH 7.2-7.4). After blocking fragment-crystallizable receptors with Fc block (BD Biosciences Pharmingen) for 10 minutes at room temperature to avoid nonspecific binding of antibodies, cells from peripheral blood, bone marrow, peritoneal exudates, lymph nodes (when present), and spleen were stained with antihuman CD19 antibody to investigate the presence of MEC1 cells in the different compartments and analyzed with a FC500 flow cytometer (Beckman-Coulter).
Phospho-flow cytometry
ERK1/2 phosphorylation status was analyzed by flow cytometry on processed murine spleens. In summary, single-cell suspensions were fixed in Lyse/Fix Solution (BD Biosciences Pharmingen) for 10 minutes at 37°C. Cells were then washed and permeabilized with a Perm Buffer (0.5% saponin, 5% fetal calf serum, 10mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid) for 20 minutes at room temperature. Cells were washed and incubated with antihuman CD19 and antihuman pERK1/2 for 30 minutes at 4°C and analyzed with a FC500 flow cytometer. Phorbol 12-myristate 13-acetate cells stimulated for 10 minutes at 37°C were used as positive control (data not shown).
Statistical analysis
Data were compared with the use of either the paired or unpaired Student t tests or the nonparametric Mann-Whitney U test. Comparison of survival curves was performed with the use of the log-rank test. Analyses were performed by the use of GraphPad Prism 4 software (GraphPad Software, San Diego, CA). A P < .05 was considered as statistically significant.
Results
Identification of the molecular features of anergy in CLL
On the basis of our previous observation of the existence of anergic features (ie, constitutive ERK1/2 phosphorylation and NF-ATc1 activation) in a subset of patients with CLL, we started by characterizing an unselected cohort of 52 patients with CLL. CLL cases with constitutive phosphorylated ERK1/2 (pERK1/2+) were 23 of 52, and they preferentially showed an absence of CD38 expression (P = .01), low ZAP70 expression (P = .02), and a more stable clinical course (P = .007). They also showed an association trend with mutated status of IGHV genes even if this correlation did not reach statistical significance (P > .05; Table 1 and supplemental Figure 1; see the Blood Web site). As expected, at biochemical levels, constitutive ERK1/2 phosphorylation correlated with greater NF-ATc1 nuclear translocation (Figure 1A), as leukemic cells from the pERK(+) subset showed a mean NF-ATc1 activation of 80.7 ± 8.2 (n = 23) whereas those from the pERK(−) group had a mean NF-ATc1 activation of 48.4 ± 5.9 (n = 29) (P = .004).
Clinical and biological features of CLL patients
| . | pERK1/2(+) . | pERK1/2(−) . | P . |
|---|---|---|---|
| CD38 positive (≥30%) | 10.5% (2/19) | 44.4% (12/27) | .01 |
| Unmutated IGHV genes | 42.1% (8/19) | 57.7% (15/26) | .4 |
| ZAP70 positive (≥20%) | 35.3% (6/17) | 66.7% (18/27) | .02 |
| Disease progression | 26.7% (4/15) | 76.5% (13/17) | .007 |
| . | pERK1/2(+) . | pERK1/2(−) . | P . |
|---|---|---|---|
| CD38 positive (≥30%) | 10.5% (2/19) | 44.4% (12/27) | .01 |
| Unmutated IGHV genes | 42.1% (8/19) | 57.7% (15/26) | .4 |
| ZAP70 positive (≥20%) | 35.3% (6/17) | 66.7% (18/27) | .02 |
| Disease progression | 26.7% (4/15) | 76.5% (13/17) | .007 |
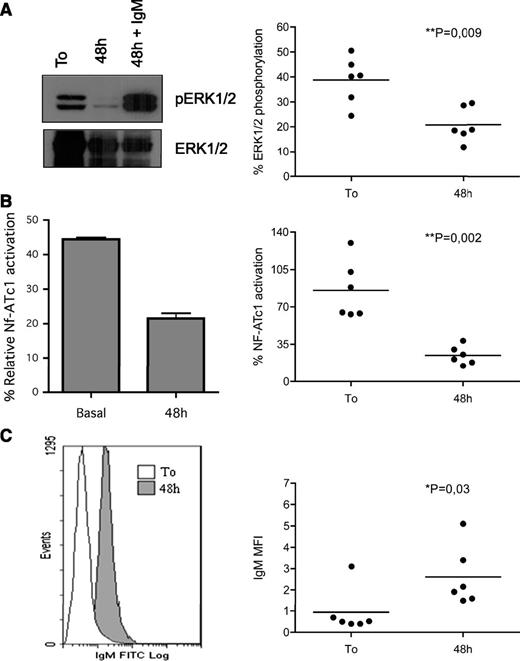
Molecular determinants of B-cell anergy. (A) ERK1/2 constitutive phosphorylation significantly correlated with NF-ATc1 nuclear translocation (P = .004). Constitutive ERK1/2 and NF-ATc1 activation (each normalized to specific positive controls) were evaluated in a cohort of 52 freshly purified samples. Samples were grouped on the basis of the ERK1/2 phosphorylation status [23 pERK(+), with relative % pERK >10%, and 29 pERK(−), with relative % p-ERK <10%], and the percentage of NF-ATc1 activation was reported on the y-axis. (B) ERK1/2 activation and calcium flux. Leukemic cells from 32 patients were labeled with the calcium-sensitive dye Fluo3-AM and analyzed by flow cytometry before and after addition of F(ab′)2 anti-IgM. Samples were grouped on the basis of the ERK1/2 activation status. Graph shows individual data point and means (horizontal line). Data were analyzed by using Mann-Whitney U test (P value is indicated). (C) ERK1/2 activation and IgM expression. Leukemic cells from 24 patients were analyzed for surface IgM expression (mean fluorescence intensity) and grouped on the basis of pERK1/2 status. The graph shows individual data point and means (horizontal line). Data were analyzed with the Mann-Whitney U test (P value is indicated). (D) Anergic cells displayed an increasing survival in vitro. Anergic signature correlated with increased in vitro survival of CLL cells. Freshly purified leukemic cells (10 pERK(+) and 9 pERK(−) samples) were cultured for 24 or 48 hours, and cell viability was analyzed with Annexin/PI staining. Samples were grouped on the basis of the ERK1/2 activation status, and data were analyzed with the Mann-Whitney U test (P value is indicated).
Molecular determinants of B-cell anergy. (A) ERK1/2 constitutive phosphorylation significantly correlated with NF-ATc1 nuclear translocation (P = .004). Constitutive ERK1/2 and NF-ATc1 activation (each normalized to specific positive controls) were evaluated in a cohort of 52 freshly purified samples. Samples were grouped on the basis of the ERK1/2 phosphorylation status [23 pERK(+), with relative % pERK >10%, and 29 pERK(−), with relative % p-ERK <10%], and the percentage of NF-ATc1 activation was reported on the y-axis. (B) ERK1/2 activation and calcium flux. Leukemic cells from 32 patients were labeled with the calcium-sensitive dye Fluo3-AM and analyzed by flow cytometry before and after addition of F(ab′)2 anti-IgM. Samples were grouped on the basis of the ERK1/2 activation status. Graph shows individual data point and means (horizontal line). Data were analyzed by using Mann-Whitney U test (P value is indicated). (C) ERK1/2 activation and IgM expression. Leukemic cells from 24 patients were analyzed for surface IgM expression (mean fluorescence intensity) and grouped on the basis of pERK1/2 status. The graph shows individual data point and means (horizontal line). Data were analyzed with the Mann-Whitney U test (P value is indicated). (D) Anergic cells displayed an increasing survival in vitro. Anergic signature correlated with increased in vitro survival of CLL cells. Freshly purified leukemic cells (10 pERK(+) and 9 pERK(−) samples) were cultured for 24 or 48 hours, and cell viability was analyzed with Annexin/PI staining. Samples were grouped on the basis of the ERK1/2 activation status, and data were analyzed with the Mann-Whitney U test (P value is indicated).
We next evaluated the ability of CLL cells from the anergic subset to mobilize calcium after stimulation with 20 μg/mL of soluble goat F(ab′)2 antihuman IgM, as anergic B cells are characterized by BCR desensitization with consequent unresponsiveness to Ag triggering.
As previously reported10 and as described in the “Materials and methods” section, patients were considered as able to signal (so-called “signalers”) through the BCR when >5% of cells were able to increase the calcium-associated fluorescence. In detail, 13 of 16 pERK(+) samples were unable to increase intracellular Ca2+ upon BCR stimulation and were considered “nonsignalers” (Figure 1B).10 In contrast, 12 of 16 pERK(−) samples fully responded by mobilizing calcium (P = .0005). Interestingly, ERK1/2 phosphorylation was associated with a lower expression of sIgM (Figure 1C; P = .03), recalling normal B cells chronically triggered by Ag.15
We next evaluated whether the previously detected features of CLL cells might translate into a greater resistance to apoptosis. To this end, we cultured in vitro leukemic cells from 10 pERK(+) and 9 pERK(−) samples and analyzed spontaneous apoptosis after 24 and 48 hours. As shown in Figure 1D, pERK(+) B cells were more protected from spontaneous apoptosis than pERK(−) cells both at 24 (P = .001) and at 48 hours (P = .004). This finding might possibly indicate that persistent in vivo BCR engagement may be responsible for maintaining survival in pERK(+) cells.
Functional anergy, as shown by BCR desensitization, is a reversible process that can be reverted by simply culturing cells in vitro long enough to allow the removal of BCR occupancy.10,14,23 Thus, we next evaluated whether the biochemical features associated with anergy might also be reversible and might correlate with the changes in the response through the BCR. To this end, we cultured CLL cells from 6 pERK(+) cases and from 4 pERK(−) cases for 48 hours, and we started measuring ERK1/2 phosphorylation, NF-ATc1, and sIgM changes over time. Both ERK (Figure 2A) and NF-ATc1 (Figure 2B) activation were lost upon culture, and this effect was accompanied by the re-expression of sIgM (Figure 2C). As expected, cultured leukemic cells that had lost the biochemical features of anergy regained the ability to fully respond to BCR triggering (Figure 2A, left). On the other hand, no changes were observed in the case of pERK(−) samples (supplemental Figure 2).
Biochemical signature of B-cell anergy is a reversible event. Cells from 6 different anergic samples were cultured for 48 hours. ERK1/2 phosphorylation (A), NF-ATc1 nuclear translocation (B), and IgM expression levels (C) were analyzed both immediately after purification and after 48 hours of cell culture. Each figure shows one representative case (left) and the dot plot analysis of all the analyzed samples (right).
Biochemical signature of B-cell anergy is a reversible event. Cells from 6 different anergic samples were cultured for 48 hours. ERK1/2 phosphorylation (A), NF-ATc1 nuclear translocation (B), and IgM expression levels (C) were analyzed both immediately after purification and after 48 hours of cell culture. Each figure shows one representative case (left) and the dot plot analysis of all the analyzed samples (right).
Targeting anergic BCR signaling pathways: how to reprogram functional response
On the basis of this evidence (Figure 2), we then asked whether the direct inhibition of the signaling molecules active in the biochemical program of anergy could also have an effect on the reversal of BCR responsiveness.
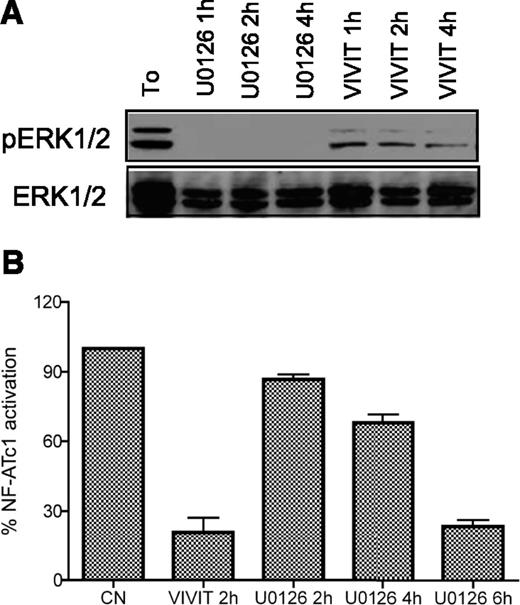
To this end, we used different small chemical inhibitors including: (1) U0126 and CI1040 that specifically target the activation of MEK1/2, the direct upstream kinases of ERK; (2) NMS6E, a new-generation ERK1/2 inhibitor; and (3) a cell permeable version of VIVIT, an inhibitory peptide that blocks NF-AT nuclear translocation. As shown in supplemental Figure 3A-C, treatment with the different compounds was able to block the activation of each specific target after short-term incubation (maximal inhibition observed after 1 hour).
In addition, time−course experiments revealed that exposure to VIVIT not only inhibited NF-AT nuclear translocation but also blocked ERK1/2 phosphorylation (maximal inhibition observed after 4 hours of treatment; Figure 3A). Conversely, treatment with U0126 induced inhibition of NF-AT activation (maximal inhibition at 6 hours; Figure 3B) besides pERK blockade.
Small chemical compounds: analysis of target inhibition. CLL cells were treated with 10μM U0126 or VIVIT for increasing time points (as indicated). ERK1/2 activation was measured by western blot (A) and NF-ATc1 nuclear translocation was quantified by specific enzyme-linked immunosorbent assay (B). NF-ATc1 values are normalized to the untreated control.
Small chemical compounds: analysis of target inhibition. CLL cells were treated with 10μM U0126 or VIVIT for increasing time points (as indicated). ERK1/2 activation was measured by western blot (A) and NF-ATc1 nuclear translocation was quantified by specific enzyme-linked immunosorbent assay (B). NF-ATc1 values are normalized to the untreated control.
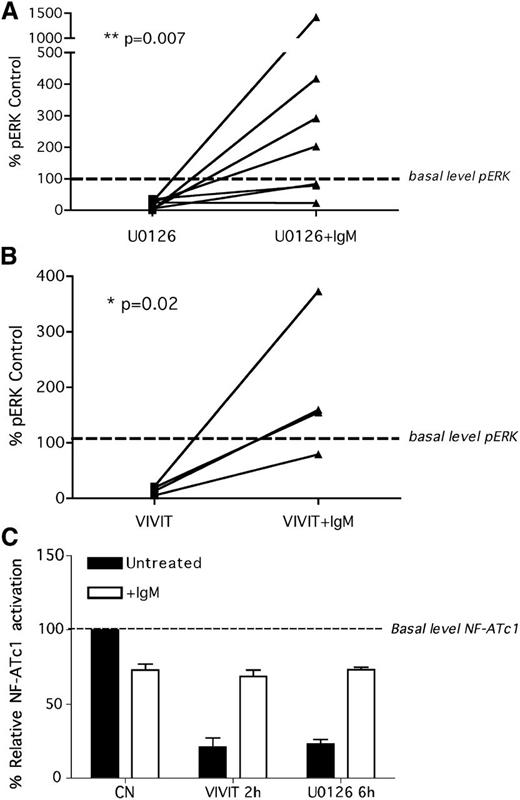
To verify whether the direct blockade of ERK and NF-AT might restore the ability of anergic cells to fully respond to antigen-like stimulation, we pretreated pERK(+) cells with 10µM U0126 (1 hour, 7 patient samples) and with 10µM VIVIT (4 hours, 4 patient samples), washed out the inhibitors at the indicated time point, left cell untreated or stimulated with soluble anti-IgM for an additional 5 minutes, and measured the activation levels of ERK1/2. Both U0126 (Figure 4A) and VIVIT (Figure 4B) decreased the levels of pERK1/2 and restored the cell ability to respond to anti-IgM stimulation. The same evidence was observed with NF-ATc1 (Figure 4C) as both U0126 and VIVIT inhibited its activation and restored the responsiveness to BCR triggering in the 6 patients tested. Because no modification of sIgM was observed during the inhibition time period (data not shown), these results suggest that CLL cells restore their signaling capacity independent of the levels of sIgM expression.
Signaling inhibitors: how to reprogram antigen response. Inhibition of both ERK1/2 and NF-ATc1 by treatment with U0126 and VIVIT, respectively, reverse the anergic phenotype and restore the ability of CLL cells to respond to anti-IgM stimulation in vitro. CLL cells were treated with U0126 (1 hour; A) or with VIVIT (4 hours; B), washed, and left untreated or stimulated for an additional 5 minutes with anti-IgM antibodies. The phosphorylation status of ERK1/2 was analyzed by western blotting, and band intensity was quantified by densitometry. pERK1/2 values are normalized to the control-untreated sample. (C) CLL cells were treated with U0126 (6 hours) or with VIVIT (2 hours), washed, and stimulated for additional 20 minutes with anti-IgM antibodies. NF-ATc1 activation was measured by specific ELISA assay. NF-ATc1 values are normalized to the untreated control.
Signaling inhibitors: how to reprogram antigen response. Inhibition of both ERK1/2 and NF-ATc1 by treatment with U0126 and VIVIT, respectively, reverse the anergic phenotype and restore the ability of CLL cells to respond to anti-IgM stimulation in vitro. CLL cells were treated with U0126 (1 hour; A) or with VIVIT (4 hours; B), washed, and left untreated or stimulated for an additional 5 minutes with anti-IgM antibodies. The phosphorylation status of ERK1/2 was analyzed by western blotting, and band intensity was quantified by densitometry. pERK1/2 values are normalized to the control-untreated sample. (C) CLL cells were treated with U0126 (6 hours) or with VIVIT (2 hours), washed, and stimulated for additional 20 minutes with anti-IgM antibodies. NF-ATc1 activation was measured by specific ELISA assay. NF-ATc1 values are normalized to the untreated control.
Prolonged blockade of anergic signaling pathways induces apoptosis
To verify whether prolonged blockade of anergic signaling might affect leukemic cell survival especially of the pERK(+) subset, we treated freshly purified leukemic cells for 48 hours with increasing concentrations of MEK1/2 inhibitors (U0126, CI1040), ERK1/2 inhibitor (NMS6E), or NF-AT inhibitor (VIVIT); evaluated cell viability by using a luminescence-based assay; and performed dose−response curves (supplemental Figure 4, 2 representative samples). To analyze the response to treatment and to verify potential differences among patients on the basis of ERK1/2 phosphorylation status, we analyzed the level of cell viability after 48 hours of treatment with 10μM each compound.
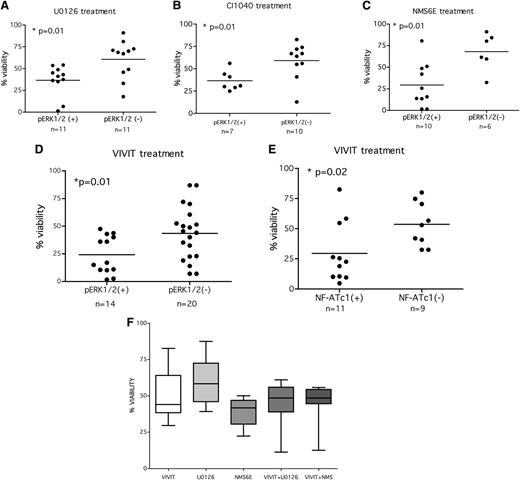
The use of MEK inhibitors induced a significant decrease of cell viability in pERK(+) patients (Figure 5A: U0126 and Figure 5B: CI1040). In detail, pERK(+) samples treated with U0126 had a mean percentage of survival of 36.6% ± 5.2 compared with 60.6% ± 6.6 survival of the pERK(−) subset (P = .01; n = 22). Similarly, pERK(+) samples treated with CI1040 had a mean percentage of survival of 36.6 ± 4.1% compared with 59.2 ± 6.3% survival of the pERK(−) subset (P = .01; n = 17). A smaller cohort of patients [5 pERK(+) and 4 pERK(−)] was treated with an additional MEK1/2 inhibitor, AZD6244 (supplemental Figure 5), that was shown to be very effective in pERK inhibition in clinical trials for solid tumors.24 This inhibitor induced cell death in pERK(+) samples (60.0 ± 4.9% of cell viability in pERK(+) vs 81.5 ± 3.5% in pERK(−), P = .03).
Longer blockade of anergic signaling induces apoptosis in the pERK(+) subset. (A) Leukemic cells were left untreated or treated for 48 hours with 10μM U0126, and cell viability was measured with the use of a luminescent-based cell viability assay. Each value from treated samples was normalized to the untreated control, and samples were grouped on the basis of the ERK1/2 activation status. Data were analyzed by using Mann-Whitney U test (P value is indicated). (B) Leukemic cells were left untreated or treated for 48 hours with 10μM CI1040, and cell viability was measured with a luminescent-based cell viability assay. Each value from treated samples was normalized to the untreated control, and samples were grouped on the basis of the ERK1/2 activation status. Data were analyzed by using Mann-Whitney U test (P value is indicated). (C) Leukemic cells were left untreated or treated for 48 hours with 10μM NMS6E and cell viability was measured by the use of a luminescent-based cell viability assay. Each value from treated samples was normalized to the untreated control, and samples were grouped on the basis of ERK1/2 activation status. Data were analyzed by using Mann-Whitney U test (P value is indicated). (D-E) Leukemic cells were left untreated or treated for 48 hours with 10μM 11R-VIVIT, and cell viability was measured by the use of luminescent-based cell viability assay. Each value from treated samples was normalized to the untreated control, and samples were grouped on the basis of ERK1/2 (D) or NF-ATc1 (E) activation status. Data were analyzed with the Mann-Whitney U test (P value is indicated). (F) ERK1/2 and NF-ATc1 inhibitors do not exert any synergistic effect. Samples from 9 anergic CLL patients were treated with 10μM each inhibitor (U0126, NMS6E, VIVIT) or with a MAPK inhibitor in combination with VIVIT for 48 hours, and cell viability was measured. Viability is expressed as percentage of the untreated control.
Longer blockade of anergic signaling induces apoptosis in the pERK(+) subset. (A) Leukemic cells were left untreated or treated for 48 hours with 10μM U0126, and cell viability was measured with the use of a luminescent-based cell viability assay. Each value from treated samples was normalized to the untreated control, and samples were grouped on the basis of the ERK1/2 activation status. Data were analyzed by using Mann-Whitney U test (P value is indicated). (B) Leukemic cells were left untreated or treated for 48 hours with 10μM CI1040, and cell viability was measured with a luminescent-based cell viability assay. Each value from treated samples was normalized to the untreated control, and samples were grouped on the basis of the ERK1/2 activation status. Data were analyzed by using Mann-Whitney U test (P value is indicated). (C) Leukemic cells were left untreated or treated for 48 hours with 10μM NMS6E and cell viability was measured by the use of a luminescent-based cell viability assay. Each value from treated samples was normalized to the untreated control, and samples were grouped on the basis of ERK1/2 activation status. Data were analyzed by using Mann-Whitney U test (P value is indicated). (D-E) Leukemic cells were left untreated or treated for 48 hours with 10μM 11R-VIVIT, and cell viability was measured by the use of luminescent-based cell viability assay. Each value from treated samples was normalized to the untreated control, and samples were grouped on the basis of ERK1/2 (D) or NF-ATc1 (E) activation status. Data were analyzed with the Mann-Whitney U test (P value is indicated). (F) ERK1/2 and NF-ATc1 inhibitors do not exert any synergistic effect. Samples from 9 anergic CLL patients were treated with 10μM each inhibitor (U0126, NMS6E, VIVIT) or with a MAPK inhibitor in combination with VIVIT for 48 hours, and cell viability was measured. Viability is expressed as percentage of the untreated control.
Likewise, the direct inhibition of ERK1/2 with 10µM NMS6E induced a strong decrease in viability (29.4 ± 8.1% mean survival) compared with pERK(−) samples (68.1 ± 8.8%; Figure 5C; P = .01; n = 16).
In parallel, NF-AT inhibition with VIVIT reduced cell viability in the cells from 14 pERK1/2(+) patients (21.3 ± 4.2% mean survival) compared with those from the pERK(−) group (20 cases, 42.9 ± 5.5 mean survival, P = .01; Figure 5D). The effect correlated also with NF-AT activation status (29.5 ± 7.5% a mean survival for NF-AT(+) group of and 53.6 ± 6.1% mean survival for the NF-AT(−), P = .02; n = 20; Figure 5E).
Taken together, our results demonstrate that the presence of anergic features is associated with the maintenance of cell survival in CLL and that the specific inhibition of the signaling pathways involved in this biochemical program may selectively induce cell death among pERK(+) patients.
As our results (Figure 3A-B) suggested that MAPK and NF-AT are interconnected, we tested whether the concomitant inhibition of both pathways could modify the proapoptotic effect on CLL cells. To this end, we treated pERK(+)/NF-AT(+) cells (n = 6) with 10µM each inhibitor (NMS6E, U0126, VIVIT) alone or in combination and we analyzed cell viability after 48 hours. The simultaneous inhibition of both MAPK and NF-AT signaling did not enhance apoptosis (Figure 5F), thus confirming that both molecules lay on the same signaling pathway of anergy that is effectively blocked by single inhibitors.
NF-ATc1 inhibition slows leukemia progression in vivo
To strengthen our in vitro data, we evaluated the effect of NF-AT inhibition in vivo after VIVIT administration in the xenograft Rag2−/−γc−/− mouse model subcutaneously transplanted with the CLL cell line MEC1,22 which shows specific features of anergy (constitutive pERK1/2, constitutive NF-ATc1 nuclear translocation9 ).
Mice were subcutaneously injected with 10 × 106 cells and then challenged with VIVIT (10 mg/kg)25 or with vehicle alone starting from day 10 after tumor injection. The effect of the inhibitor was monitored by tumor volume growth. Mice were sacrificed when the tumor volume reached 1000 mm3 or when the animals showed clinical signs and symptoms.
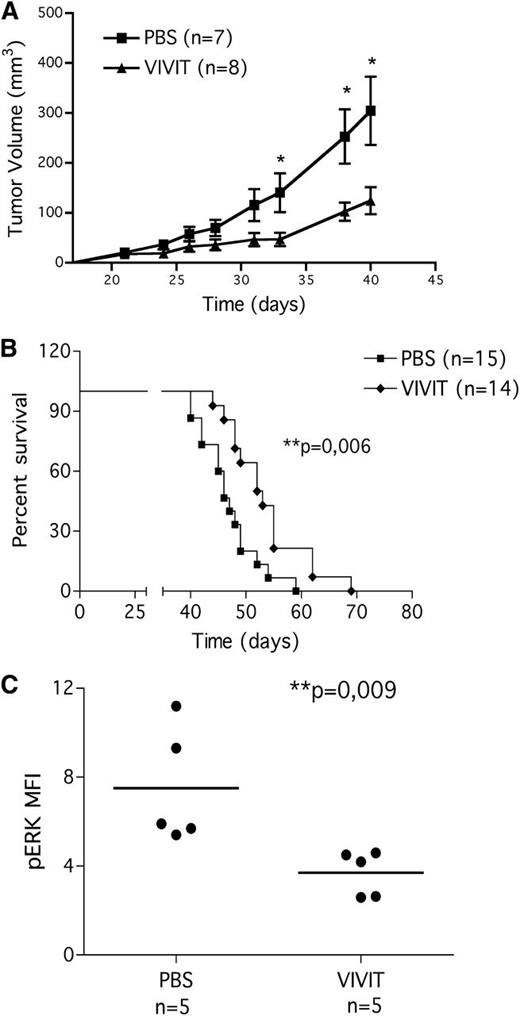
VIVIT administration delayed tumor growth (Figure 6A; day 33, day 38, day 40; P < .05) and improved the survival of the mice (Figure 6B); the median survival of VIVIT-treated group was 52.5 days compared with the untreated group (46 days; P = .006).
11R-VIVIT slows leukemia progression in CLL xenograft model. MEC1 CLL cell line was injected subcutaneously into Rag2−/−γc−/− mice. Mice were challenged with 11R-VIVIT (daily intraperitoneal administration of 10 mg/kg) or with vehicle alone starting from day 10 after tumor injection. (A) In vivo VIVIT administration reduces tumor growth. Tumor growth curves obtained in Rag2−/−γc−/− mice that received a transplant subcutaneously in the left flank of MEC1 cells (10 × 106 cells). Ten days later, mice bearing MEC1 tumors were randomly assigned to one of the following daily intraperitoneal treatments: saline solution (PBS, n = 7) or 10 mg/kg VIVIT (n = 8). We evaluated tumor size by measuring perpendicular diameters with a caliper. Animals were sacrificed when the tumor volume reached 1000 mm3 or when animals showed clinical signs and symptoms. Measurements were stopped when 75% of originally treated mice were still surviving. Statistical analysis was performed with the Student t test (day 33, day 38 and day 40 PBS vs VIVIT, P < .05). (B) In vivo VIVIT administration improves mice survival. Kaplan-Meier survival plot for Rag2−/−γc−/− mice challenged subcutaneously in the left flank with MEC1 cells (10 × 106 cells). Ten days later, mice bearing MEC1 tumor were randomly assigned to one of the following daily intraperitoneal treatments: saline solution (PBS, n = 15) or 10 mg/kg VIVIT (n = 14). Animals were sacrificed when the tumor volume reached 1000 mm3 or when animals showed clinical signs and symptoms (median survival of untreated group 46 days, median survival of VIVIT treated group 52.5 days, P = .006). Data are from three independent experiments. (C) In vivo anergy reversal: pERK1/2. pERK1/2 expression on MEC1 cells present in the spleen of animals injected with the cell line. At time of sacrifice, spleens were collected, processed, and stained for CD19 and phoshorylated ERK1/2, as described in the “Materials and methods” section. Statistical analysis was performed by use of the Student t test.
11R-VIVIT slows leukemia progression in CLL xenograft model. MEC1 CLL cell line was injected subcutaneously into Rag2−/−γc−/− mice. Mice were challenged with 11R-VIVIT (daily intraperitoneal administration of 10 mg/kg) or with vehicle alone starting from day 10 after tumor injection. (A) In vivo VIVIT administration reduces tumor growth. Tumor growth curves obtained in Rag2−/−γc−/− mice that received a transplant subcutaneously in the left flank of MEC1 cells (10 × 106 cells). Ten days later, mice bearing MEC1 tumors were randomly assigned to one of the following daily intraperitoneal treatments: saline solution (PBS, n = 7) or 10 mg/kg VIVIT (n = 8). We evaluated tumor size by measuring perpendicular diameters with a caliper. Animals were sacrificed when the tumor volume reached 1000 mm3 or when animals showed clinical signs and symptoms. Measurements were stopped when 75% of originally treated mice were still surviving. Statistical analysis was performed with the Student t test (day 33, day 38 and day 40 PBS vs VIVIT, P < .05). (B) In vivo VIVIT administration improves mice survival. Kaplan-Meier survival plot for Rag2−/−γc−/− mice challenged subcutaneously in the left flank with MEC1 cells (10 × 106 cells). Ten days later, mice bearing MEC1 tumor were randomly assigned to one of the following daily intraperitoneal treatments: saline solution (PBS, n = 15) or 10 mg/kg VIVIT (n = 14). Animals were sacrificed when the tumor volume reached 1000 mm3 or when animals showed clinical signs and symptoms (median survival of untreated group 46 days, median survival of VIVIT treated group 52.5 days, P = .006). Data are from three independent experiments. (C) In vivo anergy reversal: pERK1/2. pERK1/2 expression on MEC1 cells present in the spleen of animals injected with the cell line. At time of sacrifice, spleens were collected, processed, and stained for CD19 and phoshorylated ERK1/2, as described in the “Materials and methods” section. Statistical analysis was performed by use of the Student t test.
At the time of sacrifice, organs and tissues (spleen, bone marrow, peritoneal exudate, lymph nodes when enlarged and peripheral blood) were collected, processed, and stained for human CD19 to identify human leukemic cells.
To further explore in vivo anergy reversal, cells processed from spleen were also stained for pERK1/2 (Figure 6C). VIVIT treatment significantly decreased ERK1/2 phosphorylation in CD19+ cells, indicating that the biochemical anergic program can be reverted also in vivo, in a mouse model of CLL, and anergy reversal is associated with a survival benefit for the animals.
Discussion
For many years CLL has been considered a leukemia of functionally inactive B lymphocytes. In apparent contrast, more recently it has been shown that a portion of cases is actually responsive to external stimuli, including the activation though the BCR. That notwithstanding, a sizable fraction of patients with CLL are indeed characterized by the expansion of clonal B cells that have anergic features.5,9,10 Anergy is one of the strategies adopted by the immune system to silence autoreactive B cells; the others are clonal deletion and tolerance. Studies in transgenic mouse models26 have demonstrated that anergy is the result of the continuous binding of low-affinity self-Ag to surface Ig that induces the activation of a biochemical program resulting in cell unresponsiveness when B lymphocytes are further triggered via the BCR. Phosphorylation of ERK and the nuclear translocation of NF-AT in the absence of AKT activation are the hallmarks of mouse B-cell anergy. We have previously shown that such features are present in a subset of patients with CLL who have an indolent clinical presentation.9
In this work, we demonstrate that in CLL cells this biochemical signature associates with cellular and functional features of anergy, including in vitro inability to mobilize intracellular calcium upon anti-IgM stimulation and reduced expression of surface IgM. The latter features are reminiscent of B cells facing chronic Ag-stimulation that favors continuous recycling of BCR.15
A recent work27 demonstrated that NF-ATc1 is constitutively activated in all CLL samples tested. We here show that this activation occurs at variable levels in the different patients. Interestingly, constitutive phosphorylation of ERK1/2 kinases correlates with greater NF-ATc1 activation, suggesting that the different basal levels of NF-ATc1 activation, being greater in the pERK(+) subset (Figure 1A), might induce a different downstream transcriptional program that culminates in the induction of B-cell anergy as the result of a “dosage-dependent” activation of the transcription factor and/or to different NF-AT–interacting partners.28 All these issues could be investigated in the future by the direct analysis of the NF-ATc1 interacting proteins.
In vivo, the activation of the biochemical program that culminates in the induction of B-cell anergy is known to be transient because it requires chronic BCR triggering. Accordingly we show here that Ag removal, obtained by culturing CLL cells in vitro, associates with the loss of the biochemical anergic signature (reduction of pERK and nuclear NF-ATc1 levels) along with the re-expression of surface IgM and restores the ability of CLL cells to fully respond to BCR triggering, as previously shown in a mouse model of B-cell anergy14 as well as in CLL.10 This transient capacity to be anergized after a chronic stimulation through the BCR is independent of and likely successive to the supposedly autonomous signaling occurring in all cases of CLL as recently suggested.6 The actual relationship between these 2 signaling activities deserves further investigation.
We also show that by inhibiting the signaling molecules involved in anergy we can revert anergy both in vitro and in vivo, as revealed by the restoration of BCR responsiveness after the loss of ERK phosphorylation and NF-AT nuclear translocation. Reversal of anergy could also be obtained by simply culturing cells for 48 hours after blood withdrawal, and this paralleled the delayed onset of the apoptosis among anergic leukemic lymphocytes in vitro. This finding is in line with the hypothesis that chronic antigen engagement may be responsible also for a prolonged cell survival in vivo. It came then with no surprise that by using of a number of small chemical inhibitors that specifically target, at different levels, the anergy-related pathways, we were able to induce apoptosis of the anergic CLL subset, opening a new possibility of therapeutic intervention. This result occurred both by blocking MAPK with 2 different classes of molecules that target both MEK1/2 and ERK1/2 and by inhibiting NF-ATc1 nuclear translocation with a cell permeable version of VIVIT peptide. Apoptosis occurred at later time points and the use of NF-AT and ERK1/2 inhibitors induced an initial phase of anergy reversal, as revealed by the restoration of BCR responsiveness that mirrored the loss of ERK phosphorylation and NF-AT nuclear translocation at biochemical level.
We also tested the effect of NF-AT inhibition by administering VIVIT in xenograft model of CLL with Rag2−/−γc−/− mice subcutaneously injected with MEC1 cell line.22 MEC1 cells have anergic features as they display constitutive ERK1/2 and NF-ATc1 activation, high basal level of calcium, and general unresponsiveness to BCR triggering together with low levels of surface IgM. The reason why features typical of a reversible biochemical program (anergy) are maintained in a cell line without any apparent ongoing antigen stimulation is unclear. A plausible explanation is that yet-unknown gene abnormalities acquired during the immortalization process may maintain the anergic program in an antigen-independent fashion. Alternatively it might be speculated that self antigens able to stimulate the MEC1 BCR are released by apoptotic cells in culture.
Mice treated with VIVIT had a delay in tumor growth and an increased survival compared with the untreated group. Of interest, leukemic cells from treated mice showed a decreased expression of ERK1/2 phosphorylation, thereby paralleling and confirming our in vitro findings. These data further support the idea that blocking anergic pathways may be highly effective not only in vitro but also in vivo with an obvious potential clinical implications at least for the subset of patients whose cells are characterized by anergic features.
The use of small molecules inhibitors is now becoming extremely promising (see Bruton tyrosine kinase and phosphatidylinositol-3-kinase inhibitors) because of the specific targeting and the limited side effects. For this reason, anergy reversal could be envisaged of potential use in the sizable fraction of patients who have a rather indolent still progressive disease. It may be asked whether the risk exists that the reversal of B-cell anergy in vivo might lead to a more aggressive disease, considering that absence of ERK1/2 phosphorylation is associated with a worse prognosis. However, according to our findings, it is reasonable to believe that blocking B-cell anergy in vivo would trigger an even greater apoptosis rate than that observed in vitro. This effect could be attributable to the combination of 2 different mechanisms: on one side the inhibition of a survival program (anergy) and on the other side the restoration of the signaling capacity, the latter being involved in the induction of apoptosis in CLL, as previously reported.29,-31
In conclusion, our results demonstrate that a subset of patients with CLL, identified by the constitutive ERK1/2 phosphorylation, is characterized by the abnormal expansion of B cells with anergic properties. In these cells the constitutive activation of ERK and NF-ATc1, leading to functional unresponsiveness, can be efficiently targeted and cells regain their ability to respond to BCR stimulation. More importantly, B-cell anergy reversal is followed by apoptosis induction possibly opening new perspectives in the clinical management of these patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Acknowledgments
The authors thank Eleonora Maria Fonte, Sergey Krysov, and Federica Barbaglio for helpful suggestions and technical support.
This project was supported by the Associazione Italiana per la Ricerca sul Cancro AIRC (Investigator Grant and Special Program Molecular Clinical Oncology – 5 per mille #9965), “CLLGRF – U.S./European Alliance for the Therapy of CLL,” FIRB, and PRIN – Ministero Istruzione, Università e Ricerca (MIUR), Roma, Progetti Integrati Oncologia (PIO) – Ministero della Salute, Roma. B.A. conducted this study as partial fulfillment of her PhD in Molecular and Cellular Biology, San Raffaele University, Milan, Italy. C.S. was supported by the EHA Fellowship Program (2009/18). The compound NMS6E was a kind gift by Nerviano Medical Sciences. Rag2−/− γc−/− on BALB/c background were kindly provided by CIEA (Central Institute for Experimental Animals, Kawasaki, Japan) and Taconic.
Authorship
Contribution: B.A. designed the study, performed in vitro and in vivo experiments, analyzed the data, and wrote the manuscript; C.S., E.t.H., and P.R. performed in vitro experiments; M.T.S.B. performed in vivo experiments; L.S. provided patients samples and clinical data; F.S. and G.P. assisted in writing the manuscript; and P.G., M.M., and F.C.-C. designed the study, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paolo Ghia, Università Vita-Salute San Raffaele, Via Olgettina 58, 20132, Milano, Italy; e-mail: ghia.paolo@hsr.it.
References
Author notes
M.M. and F.C.-C. contributed equally to this study.

![Figure 1. Molecular determinants of B-cell anergy. (A) ERK1/2 constitutive phosphorylation significantly correlated with NF-ATc1 nuclear translocation (P = .004). Constitutive ERK1/2 and NF-ATc1 activation (each normalized to specific positive controls) were evaluated in a cohort of 52 freshly purified samples. Samples were grouped on the basis of the ERK1/2 phosphorylation status [23 pERK(+), with relative % pERK >10%, and 29 pERK(−), with relative % p-ERK <10%], and the percentage of NF-ATc1 activation was reported on the y-axis. (B) ERK1/2 activation and calcium flux. Leukemic cells from 32 patients were labeled with the calcium-sensitive dye Fluo3-AM and analyzed by flow cytometry before and after addition of F(ab′)2 anti-IgM. Samples were grouped on the basis of the ERK1/2 activation status. Graph shows individual data point and means (horizontal line). Data were analyzed by using Mann-Whitney U test (P value is indicated). (C) ERK1/2 activation and IgM expression. Leukemic cells from 24 patients were analyzed for surface IgM expression (mean fluorescence intensity) and grouped on the basis of pERK1/2 status. The graph shows individual data point and means (horizontal line). Data were analyzed with the Mann-Whitney U test (P value is indicated). (D) Anergic cells displayed an increasing survival in vitro. Anergic signature correlated with increased in vitro survival of CLL cells. Freshly purified leukemic cells (10 pERK(+) and 9 pERK(−) samples) were cultured for 24 or 48 hours, and cell viability was analyzed with Annexin/PI staining. Samples were grouped on the basis of the ERK1/2 activation status, and data were analyzed with the Mann-Whitney U test (P value is indicated).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/19/10.1182_blood-2012-12-474718/3/m_3879f1.jpeg?Expires=1767716960&Signature=NnIJcMpRLNk64mr-w7CyMVHbrIJx-3kel1H~3Esb6gTeLJsUNaZcOkqCX~bfv8fLAgbi8BkI-m5hcPn7UvbVVFpFPk0yAwrVym3GkjJVGBpq6WgkLS4ds-UptqbXWGwQQkjmjn45aCZFGUqlMkxup~ddPGUYGvHJo-qxyudFkybQ~-lcTbyQjIxssv8JUUSVrwmKKN1RyL6ZdiKRBk1CrBOTowphdGwmheS67wJFaXkYjnY59k4DcrxyuIlQNDrcashenfAm7~HEvv-JMDZQ7ckJKqxHxwhfSEa2CtSNe~NtnF-B9LOlRgx-~iUn-Q~lC6yGU-3569ecHOTsLsp8Dg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal