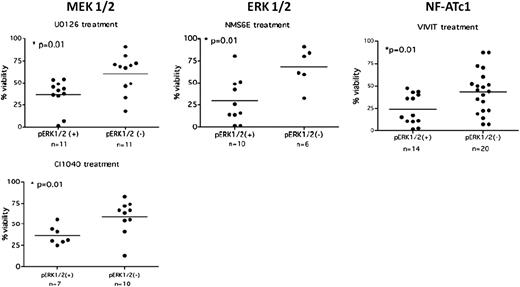
Inhibition of the anergic signaling pathway selectively reduces survival in anergic CLL cells. CLL cells displaying an anergic phenotype (pErk1/2+) or a nonanergic phenotype (pErk1/2−) were treated with agents targeting MEK 1/2, ERK 1/2, and NF-AT1c, molecules implicated in the maintenance of anergy. Cells displaying an anergic phenotype showed decreased viability in the presence of these agents compared with nonanergic cells. Adapted from Figure 5 in the article by Apollonio et al that begins on page 3789.
Inhibition of the anergic signaling pathway selectively reduces survival in anergic CLL cells. CLL cells displaying an anergic phenotype (pErk1/2+) or a nonanergic phenotype (pErk1/2−) were treated with agents targeting MEK 1/2, ERK 1/2, and NF-AT1c, molecules implicated in the maintenance of anergy. Cells displaying an anergic phenotype showed decreased viability in the presence of these agents compared with nonanergic cells. Adapted from Figure 5 in the article by Apollonio et al that begins on page 3789.
CLL is a biologically heterogeneous disease with some patients exhibiting indolent disease for many years and others progressing rapidly and requiring early treatment. The distinction between and even within these groups has become more clear with progressively more sophisticated molecular profiling of this disease. Although common cancer pathways such as p53 play a significant role, more recently B-cell−specific abnormalities have been identified and characterized. The most well-defined B-cell−specific markers, immunoglobulin heavy chain (IgVH) mutational status,2,3 Zap70 expression,4 and methylation,5 identify a subgroup of patients with increased responsiveness of the B-cell receptor (BCR) signaling pathway to external stimulation, constitutive activity of this pathway, and more aggressive disease.
In contrast, this article focuses on the subset of patients whose CLL cells display a phenotype associated with molecular anergy, where immunoglobulin M ligation of the BCR does not lead to phosphotyrosine induction or calcium flux. Previously, this group has shown that CLL cells from this subset of patients display constitutive activation of phosphorylated Erk1/2 and activated nuclear factor of activated T cells c1 (NF-ATc1) without Akt phosphorylation.6 In the current article, this group extends these observations by further characterizing these cells. Similar to previous observations, constitutive activation of phosphorylated extracellular signal-regulated kinase (pErk) was found in 23 of 52 patients. The expression of pErk was correlated with NF-ATc1 nuclear translocation and prolonged survival in culture, as well as markers associated with indolent disease biology, including absence of CD38 expression and low Zap70 expression. Importantly, supporting the idea that these cells are anergic as the result of chronic antigen stimulation, in vitro culture reversed this phenotype and restored sensitivity to BCR stimulation. These finding therefore show that CLL cells are anergic in a subset of patients exhibiting indolent disease, that these cells are likely exposed to chronic antigen stimulation in vivo, and that these cells show prolonged in vitro survival, suggesting that anergy can be a mechanism of cell survival.
To prove that anergy does indeed lead to cell survival and can potentially be a therapeutic target, the authors treated anergic (pErk1/2+) and nonanergic (pErk1/2−) CLL cells in culture with inhibitors of mitogen-activated protein kinase kinase 1 and 2 (MEK1/2), extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), or NF-AT.1 Inhibitors of MEK1/2 and NF-AT were both able to reduce phosphorylation of Erk1/2 and nuclear translocation of NF-AT and restore sensitivity to BCR engagement with short periods of exposure. As well, At 48 hours, anergic cells showed significantly reduced survival compared with nonanergic cells treated with any of these inhibitors (see figure). In addition, an in vivo xenograft experiment in which the authors used the phenotypically anergic Mec-1 cell line showed improved survival in mice treated with an NF-AT inhibitor.
One question raised by these data is whether maintenance of anergy in CLL might be beneficial in some cases, as it is associated with favorable CLL biomarkers as well as potentially an indolent disease course.1 Although targeting anergy in vitro and in a xenograft model promotes apoptosis, this does not always translate well to the clinic. It will be interesting to discover whether anergy is maintained even in the presence of disease progression and whether the anergic phenotype is maintained after therapeutic intervention. These answers may help determine whether anergy reversal is a viable therapeutic target in a subset of patients.
These data also raise the question of how best to target anergy in CLL. One intriguing possibility is combination with other targeted CLL therapies. Currently, much attention is focused on the treatment of CLL with inhibitors of the BCR signaling pathway, especially the phosphatidylinositol3-kinase inhibitor GS-1101 and the Bruton tyrosine kinase inhibitor ibrutinib. Preliminary data with ibrutinib suggest that patients with IgVH-unmutated disease, associated with more active BCR signaling, respond earlier than patients with IgVH-mutated disease.7 Apollonio et al show that inhibitors of markers of anergy restore BCR sensitivity—perhaps inhibition of anergy before BCR pathway inhibition might improve response. Alternatively, the concept of anergy maintenance through chronic antigen stimulation opens another avenue to target these cells. Biased immunoglobulin gene usage in CLL suggests that in at least a subset of patients’ CLL pathogenesis is antigen driven.8 If continued antigen stimulation is required for maintenance of anergy, as suggested by these data, inhibition of antigen binding may be another therapeutic strategy to promote apoptosis.
As we move toward more distinct molecular classification of CLL, Apollonio et al identify a subset of CLL patients with a phenotype that could potentially be targeted therapeutically. These data, building on previous work from this group, enhance our knowledge of the biology of this heterogeneous disease and continue to move us forward toward the goal of individualizing targeted therapies for our patients.
Conflict-of-interest disclosure: The author declares no competing financial interests.