Key Points
We describe a novel homozygous mutation in exon 2 of the VHL gene causing congenital polycythemia.
We demonstrate the VHLP138L effect on the augmentation of erythropoiesis, along with structural and functional studies of this mutation.
Germline von Hippel–Lindau (VHL) gene mutations underlie dominantly inherited familial VHL tumor syndrome comprising a predisposition for renal cell carcinoma, pheochromocytoma/paraganglioma, cerebral hemangioblastoma, and endolymphatic sac tumors. However, recessively inherited congenital polycythemia, exemplified by Chuvash polycythemia, has been associated with 2 separate 3′ VHL gene mutations in exon 3. It was proposed that different positions of loss-of-function VHL mutations are associated with VHL syndrome cancer predisposition and only C-terminal domain-encoding VHL mutations would cause polycythemia. However, now we describe a new homozygous VHL exon 2 mutation of the VHL gene:(c.413C>T):P138L, which is associated in the affected homozygote with congenital polycythemia but not in her, or her-heterozygous relatives, with cancer or other VHL syndrome tumors. We show that VHLP138L has perturbed interaction with hypoxia-inducible transcription factor (HIF)1α. Further, VHLP138L protein has decreased stability in vitro. Similarly to what was reported in Chuvash polycythemia and some other instances of HIFs upregulation, VHLP138L erythroid progenitors are hypersensitive to erythropoietin. Interestingly, the level of RUNX1/AML1 and NF-E2 transcripts that are specifically upregulated in acquired polycythemia vera were also upregulated in VHLP138L granulocytes.
Introduction
The von Hippel-Lindau (VHL) tumor suppressor gene encodes a multifunctional protein that interacts with diverse partners and promotes degradation of hypoxia-inducible transcription factors (HIFs) by facilitating their ubiquitinization and eventual proteasomal degradation. Germline dominantly inherited mutations in VHL predispose patients to highly vascularized malignant tumors, including renal cell carcinoma of the clear-cell type, hemangioblastoma, and pheochromocytoma/paraganglioma.1,2 Less frequently, VHL mutations have been associated with benign tumors, including those of the inner ear (endolymphatic sac tumor), pancreas (pancreatic cysts, serous cystadenoma, and pancreatic neuroendocrine tumors), and testes (epididymal cystadenomas).2 The incidence of VHL disease is thought to be about 1 in 36 000 births with an estimated de novo mutation rate of 4.4 × 10−6 gametes per generation.3 VHL tumors generally develop after age of 124 and have more than 90% penetrance in the highest age classes (96% at 51–60 years of age, 99% at 61–70 years of age).3
Polycythemia (also known as erythrocytosis) is characterized by an increased red cell blood mass. Polycythemias can be primary or secondary. Primary polycythemias are caused by somatic or germline mutations leading to changes within the erythroid progenitors causing an augmented response to erythropoietin (EPO). Secondary polycythemias are caused by either an appropriate or inappropriate increase in the red cell mass as a result of augmented levels of EPO. In Chuvash polycythemia, the first known congenital disorder of hypoxia-sensing,5 erythroid progenitors in in vitro cultures are hypersensitive to EPO; thus, Chuvash polycythemia shares features of both primary and secondary polycythemia.6
Because different positions of loss-of-function mutations of the VHL gene are associated with different type of cancers, it has been proposed that only C-terminal domain–encoding VHL mutations would cause polycythemia.7 There are 2 known homozygous VHL gene mutations causing polycythemia that are not associated with any VHL syndrome malignant or benign tumors; these are the VHLR200W mutation, constituting the disorder of augmented hypoxia-sensing in normoxia (ie, Chuvash polycythemia5 ) and the VHLH191D mutation found in a Croatian subject.8 Both are located in the distal portion of exon 3 of the VHL gene in its C-terminal domain. In addition, there are several reports of compound heterozygotes associated with congenital polycythemia, 3 in combination with VHLR200W mutation, and others employing different exon 3 VHL missense mutations (Table 1).9,,,-13 It has been suggested that the genomic configuration of the 3′ region of VHL exon 3 gene has a specific erythropoiesis-promoting effect independent of EPO by Janus Kinase-2 (JAK2) hyperactivation, because JAK2 is the crucial component of intracellular activation of EPO/EPO receptor signaling.14 Compatible with this report, to date no congenital polycythemia associated with VHL homozygous or compound heterozygous mutations outside of VHL exon 3 have been described. We now challenge this premise by reporting a family with congenital polycythemia with a new homozygous VHL mutation in exon 2 along with structural and functional studies of the mutation (Figure 1).
Homozygous and compound heterozygous VHL mutations in individuals with erythrocytosis/polycythemia reported in the literature
| VHL genotype . | Ethnicity . | Notes . | |
|---|---|---|---|
| Compound heterozygous VHL genotype | |||
| 235 C→T/586 C→G | R79C/L188V | Caucasian9 | |
| 376 G→A/548 C→T | D126N/S183L | British10 | |
| 598 C→T/574 C→T | R200W/P192A | American (white)11 | |
| 598 C→T/562 C→G | R200W/L188V | American (white)11 | |
| 598 C→T/388 G→C | R200W/V130L | American (white)8 | |
| Homozygous VHL genotype | |||
| 598 C→T/598 C→T | R200W/R200W | Chuvash,5 Italian,12 Danish,11 German,13 Turkish,13 American (white)11 | Frequent thrombotic complications |
| 571 C→G/571 C→G | H191D/H191D | Croatian11,27 | |
| 413 C→T/413 C→T | P138L/P138L | Punjabi | |
| VHL genotype . | Ethnicity . | Notes . | |
|---|---|---|---|
| Compound heterozygous VHL genotype | |||
| 235 C→T/586 C→G | R79C/L188V | Caucasian9 | |
| 376 G→A/548 C→T | D126N/S183L | British10 | |
| 598 C→T/574 C→T | R200W/P192A | American (white)11 | |
| 598 C→T/562 C→G | R200W/L188V | American (white)11 | |
| 598 C→T/388 G→C | R200W/V130L | American (white)8 | |
| Homozygous VHL genotype | |||
| 598 C→T/598 C→T | R200W/R200W | Chuvash,5 Italian,12 Danish,11 German,13 Turkish,13 American (white)11 | Frequent thrombotic complications |
| 571 C→G/571 C→G | H191D/H191D | Croatian11,27 | |
| 413 C→T/413 C→T | P138L/P138L | Punjabi | |
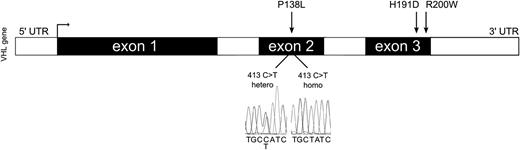
The schematic structure of the VHL gene. Sequencing of the second exon of the VHL gene revealed c.413C>T:P138L homozygous VHL mutation in the propositus, which was inherited from her parents, both VHLP138L heterozygous. UTR, untranslated region.
The schematic structure of the VHL gene. Sequencing of the second exon of the VHL gene revealed c.413C>T:P138L homozygous VHL mutation in the propositus, which was inherited from her parents, both VHLP138L heterozygous. UTR, untranslated region.
Materials and methods
Patient samples
The propositus is a 15-year-old girl of Asian Indian extraction (Punjabi ethnicity), who has been known to be polycythemic from infancy. Her parents are hematologically normal and are of the same ethnicity but not known to be related. Peripheral blood of the propositus and her parents was obtained by venipuncture after obtaining a signed Institutional Review Board informed consent in accordance with the Declaration of Helsinki. This study received approval from Institutional Review Board protocol #17665; molecular biology of polycythemia and thrombocytosis. Granulocyte and mononuclear cell fractions were isolated according to a previously published protocol.15
Mutation screening
Genomic DNA was isolated from granulocytes using Gentra-Puregene Kit (Qiagen, Germantown, MD), and VHL gene was amplified using Hot Star Master Mix (Qiagen) and the following primers: VHL_exon1_F 5′CGAAGACTACGGAGGTC GAC; VHL_exon1_R 5′GGCTTCAGACCGTGCTATCG; VHL_exon2_F 5′GTGTGGCTCTTTAACAACC; VHL_exon2_R 5′CTGTACTTACCACAACAACC; VHL_exon3_F 5′TCCTTGTACTGAGACCCTAG; and VHL_exon3_R 5′AGCTGAGATGAAACAGTCTA. Sequencing was performed using the same amplification primers and protocol established by the University of Utah, Core DNA Sequencing Facility.
In vitro assay of erythroid progenitors’ sensitivity to EPO
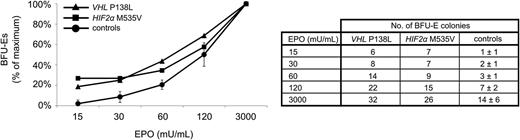
In vitro sensitivity of erythroid progenitors to EPO was performed on mononuclear cells isolated from the peripheral blood using Histopaque (Sigma-Aldrich, St. Louis, MO) density gradient centrifugation and plating (2.3 × 105/mL) to methylcellulose media (MethoCult H4531; StemCell Technologies, Vancouver, BC) with addition of various concentrations of EPO (StemCell Technologies), ranging from 0.015 to 3.0 U/mL. Cell cultures were maintained in humidified atmosphere of 5% CO2 at 37°C for 14 days. Erythroid burst-forming unit colonies (BFU-Es) were scored by standard morphologic criteria. The number of BFU-Es grown in individual concentrations of EPO was expressed as a percentage of maximum vs the concentration of EPO. Maximum growth (100%) represents the highest colony number grown in culture. The assay was carried out on erythroid progenitors from a VHLP138L-homozygous patient (n = 1), from patient with heterozygous gain-of-function HIF2αM535V mutation (n = 1), and 8 healthy controls (n = 8). Results for healthy controls are pooled and T bars designate standard deviations.
Quantitative analysis of HIF target genes expression
RNA was isolated from the patient’s granulocytes using TRI reagent and residual DNA was removed by DNA-free DNase Treatment & Removal Reagents (Ambion, Life Technologies, NY). Gene expression experiments were performed on a FastPCR 7500 instrument using TaqMan Gene Expression assays TFRC (Hs00951083), SLC2A1 (Hs00892681), HK1 (Hs00175976), RUNX1 (Hs00231079), NF-E2 (Hs00232351), and reference genes HPRT (4333768F) and GAPDH (4333764F). All samples were investigated in triplicate. The data represent the mean of 3 independent experiments; T bars designate SEM. The statistical significance of relative expression changes of target mRNA levels was analyzed using REST 2009 software.16
Cell culture, plasmids, and transfection
The 786-0 renal cell carcinoma cell line was purchased from ATCC (CRL-1932; Manassas, VA) and VHL human cDNA open reading frame clone was obtained from OriGene (RC216151; Rockville, MD). Site-directed mutagenesis was performed using the QuickChange II Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) and allele-specific oligonucleotides for VHLP138L and VHLH191D mutations. Transfection of mutated and empty plasmids was done using Lipofectamine 2000 reagent (Invitrogen, Life Technologies). Cells were selected 48 hours after transfection using 1 mg/mL G418 (Invitrogen, Life Technologies) and cultured for 21 days. Resistant clones were isolated using 96-well limiting dilution. Thirty single clones were picked up for each VHL construct and tested for expression of VHL(Myc-DDK) by TaqMan Gene Expression assay on demand (Applied Biosystems, Carlsbad, CA).
Determination of pVHL stability
To determine the half-life of protein VHL (pVHL), 786-0 stable transfected clones (VHLwt, VHLP138L, and VHLH191D) were treated with 200 μM cycloheximide (Sigma-Aldrich) and cells were harvested at different time points (0, 2, 4, 6, 8, and 10 hours). Western blot was performed with antibodies VHL (FL-181, Santa Cruz, 1:500) and actin (Sigma-Aldrich, 1:1000). Quantitation of pVHL signals was performed using densitometric analysis by ImageJ software according to the software manual.17 The data represent the mean of 3 independent experiments; T bars designate standard deviations.
Structural and energetic modeling
Computational analysis (molecular graphics, structural manipulations, energy minimization, molecular docking, and molecular dynamics simulations) were carried out using Internal Coordinate Mechanics molecular modeling program (Molsoft, La Jolla, CA). Energy grids representing the active site (van der Waals, hydrogen bonding, electrostatics, and hydrophobic interactions) were calculated with 0.5 Å grid spacing, and protein ligand docking experiments were performed using the defined site map–binding pocket with the application of our docking workflow.18
Immunoprecipitation assay
HIF1α ubiquitination and pVHL binding was determined through immunoprecipitation assay as previously described.19,20 Briefly, 786-0 cells were co-transfected with VHL plasmids (VHLwt, VHLP138L, VHLH191D, and VHLR200W) together with hemagglutinin (HA)-tagged HIF1α plasmid using Lipofectamine 2000 (Invitrogen; Life Technologies). Cells were lysed in either NP40 lysis buffer for pVHL binding or radio-immunoprecipitation assay lysis buffer supplemented with 1% sodium dodecyl sulfate for ubiquitination assay. Lysate was immunoprecipitated using Protein G Dynabeads co-immunoprecipitation kit (Invitrogen, Life Technologies) and anti-HA antibody (Covance, Gaithersburg, MD). Bound protein was eluted in loading buffer supplemented with 1% sodium dodecyl sulfate. Eluents were subsequently analyzed by western blot and quantitation of signals was performed using densitometric analysis by ImageJ software.
Results
VHLP138L mutation
We report a novel homozygous variant of the VHL gene located in the middle of exon 2: c.413C>T, VHLP138L. The propositus is a 15-year-old Punjabi female with congenital polycythemia (hemoglobin 19-20 g/dL), elevated EPO at 40 mIU/mL, no evidence of high-affinity hemoglobin mutations or 2,3-DPG deficiency as determined by a normal p50,21 and absence of JAK2V617F or exon 12 JAK2 mutations. Her parents were found to be VHLP138L heterozygous. No VHL syndrome malignant or benign tumors have been encountered in the propositus’ parents or in their extended family.
In vitro analysis of VHLP138L native erythroid progenitors
We then analyzed the VHLP138L propositus early erythroid progenitors in BFU-E assay and found them to be hypersensitive to EPO (a feature of primary polycythemias), as shown in Figure 2.
Response of BFU-E erythroid progenitors to EPO. EPO dose–response curves derived from the homozygous VHLP138L patient (▵), patient with heterozygous gain-of-function HIF2αM535V mutation (□), and healthy controls (●; n = 8, T bars = SD). VHLP138L-affected erythroid progenitors display hypersensitivity to low concentration of EPO (15–60 mU/mL). There was a relatively higher number of BFU-Es in comparison with healthy controls (number of colonies ± SD) in all analyzed EPO concentrations. The assays of VHLP138L and HIF2αM535V erythroid progenitors were not done concomitantly.
Response of BFU-E erythroid progenitors to EPO. EPO dose–response curves derived from the homozygous VHLP138L patient (▵), patient with heterozygous gain-of-function HIF2αM535V mutation (□), and healthy controls (●; n = 8, T bars = SD). VHLP138L-affected erythroid progenitors display hypersensitivity to low concentration of EPO (15–60 mU/mL). There was a relatively higher number of BFU-Es in comparison with healthy controls (number of colonies ± SD) in all analyzed EPO concentrations. The assays of VHLP138L and HIF2αM535V erythroid progenitors were not done concomitantly.
The effect of VHLP138L mutation on HIFs signaling
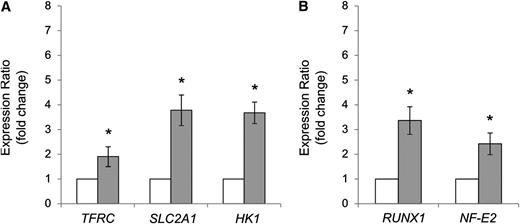
The accumulation of transcripts of HIF-regulated target genes, including those for glucose transporter-1 (SLC2A1), transferrin receptor (TFRC), and hexokinase-1 (HK1), was measured in the propositus’ granulocytes. As shown in Figure 3A, the transcripts of these HIF-regulated genes were significantly increased.
Relative expression of HIF target genes induced by hypoxia and AML1/RUNX1 and NF-E2 genes. (A) Expression of TFRC, SLC2A1, and HK1 genes and (B) AML1/RUNX1 and NF-E2 genes was evaluated by quantitative polymerase chain reaction in granulocytes isolated from homozygous patient for VHLP138L mutation (grey columns), and normal controls (n = 8, white columns). Data are normalized to HPRT and GAPDH reference genes. T bars = SEM. *P < .01.
Relative expression of HIF target genes induced by hypoxia and AML1/RUNX1 and NF-E2 genes. (A) Expression of TFRC, SLC2A1, and HK1 genes and (B) AML1/RUNX1 and NF-E2 genes was evaluated by quantitative polymerase chain reaction in granulocytes isolated from homozygous patient for VHLP138L mutation (grey columns), and normal controls (n = 8, white columns). Data are normalized to HPRT and GAPDH reference genes. T bars = SEM. *P < .01.
RUNX1/AML1 transcript levels in granulocytes
The upregulation of RUNX1/AML1 transcript levels was reported in erythroid progenitors and granulocytes and claimed to be specific for an acquired polycythemic disorder (ie, polycythemia vera [PV]) and to be responsible for PV EPO hypersensitivity.22,23 However, we considered the possibility that the increased transcripts of these genes may be a feature associated with other primary polycythemic states with increased EPO sensitivity.8 We thus analyzed RUNX1/AML1 and NF-E2 transcripts in the propositus and found them to be increased (Figure 3B).
In vitro analysis of VHLP138L effect in VHL-null 786-0 renal carcinoma cells
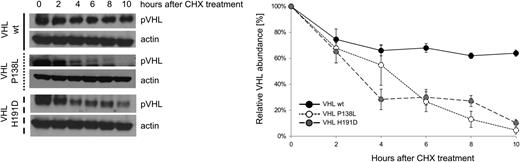
We analyzed the effect of homozygosity of VHLP138L on pVHL stability. Renal carcinoma cells 786-0, which do not express a detectable pVHL, were stably transfected with plasmids expressing VHLwt, VHLP138L, and VHLH191D proteins. Mutant and wild-type cell lines were treated with cycloheximide (CHX) for different lengths of time and pVHL levels were determined by western blot. We show a decreased stability of both mutated VHL peptides (VHLP138L and VHLH191D) in the transfected cells (Figure 4).
CHX assay measuring pVHL stability, showing mutant VHL proteins decreased half-life in vitro. The 786-0 cells were stably transfected with plasmids expressing VHLwt, VHLP138L, and VHLH191D mutants. Clones were treated with cycloheximide (CHX, 200 μM) for 0, 2, 4, 6, 8, and 10 hours, and lysates were subjected to western blot as indicated. Actin was used as the loading control. (Right) The relative quantitation of pVHL.
CHX assay measuring pVHL stability, showing mutant VHL proteins decreased half-life in vitro. The 786-0 cells were stably transfected with plasmids expressing VHLwt, VHLP138L, and VHLH191D mutants. Clones were treated with cycloheximide (CHX, 200 μM) for 0, 2, 4, 6, 8, and 10 hours, and lysates were subjected to western blot as indicated. Actin was used as the loading control. (Right) The relative quantitation of pVHL.
Molecular dynamics simulation study of VHLP138L mutation effect
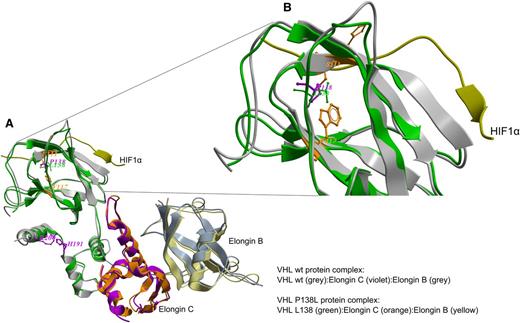
Molecular dynamics simulation showed that the VHLP138L mutation, which lies in the catalytic HIF1α peptide ligand–binding region, perturbs HIF1α/pVHL interaction because of the conformational effect on the Trp117 and Ser111 residues (Figure 5A-B), both within 2.8 to 4.3 Å distance from the mutated Leu138. The effect of this single mutation on overall structure was calculated as a shift of 1.9 Å root mean square deviation from the wild-type complex structure. Our model also predicted that HIF1α binding energy would change from −34.86 kcal/mol to −29.84 kcal/mol, mainly from the loss of 2 ligand hydrogen-bonding interactions within the residues Ser111 and Arg107.
Molecular dynamics simulations study of pVHL–ElonginC–ElonginB complex and interaction with HIF1α. (A) Superimposition of wild-type (wt; grey) and mutated pVHL (green) is shown. The wt P138 (violet) and mutated L138 (green) sites and the critical active site residues for the HIF1α peptide (PDB:1LM8)-binding region are depicted. The P138L mutation perturbs pHIF1α interactions with pVHL because of the conformational effect on the W117 and S111 residues (orange) at 2.8 to 4.3 Å distance from mutated L138. (B) Detail superimposition of wt and P138L pVHL in the interaction with HIF1α.
Molecular dynamics simulations study of pVHL–ElonginC–ElonginB complex and interaction with HIF1α. (A) Superimposition of wild-type (wt; grey) and mutated pVHL (green) is shown. The wt P138 (violet) and mutated L138 (green) sites and the critical active site residues for the HIF1α peptide (PDB:1LM8)-binding region are depicted. The P138L mutation perturbs pHIF1α interactions with pVHL because of the conformational effect on the W117 and S111 residues (orange) at 2.8 to 4.3 Å distance from mutated L138. (B) Detail superimposition of wt and P138L pVHL in the interaction with HIF1α.
Immunoprecipitation assay of HIF1α ubiquitination and pVHL binding
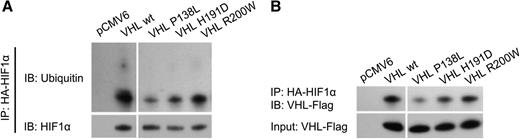
To determine the effect of the Pro138Leu substitution on pVHL function, we carried out an in vitro ubiquitination assay. The level of HIF1α ubiquitination was markedly decreased in cells expressing all 3 mutant proteins (P138L, H191D, and R200W), with the highest reduction associated with pVHLP138L (Figure 6A). To further investigate the impact of the VHLP138L mutation on HIF1α signaling, we analyzed the binding of mutated pVHL to the HIF1α peptide. Radioactively labeled VHLP138L protein showed decreased affinity to HIF1α peptide (Figure 6B), with only 26.6% of the VHL protein bound. By comparison, the wild-type VHL protein (100%) and also proteins with other homozygous polycythemic mutations (H191D, R200W) have much stronger affinity for the HIF1α peptides, with 49.0% and 62.2% of the bound VHL protein, respectively. These data indicate that the P138L mutation specifically reduced the affinity of pVHL for HIF1α, resulting in a reduced rate of ubiquitination under nonhypoxic conditions.
Immunoprecipitation assay of HIF1α ubiquitination and VHL binding. The 786-0 cells were cotransfected with plasmids expressing pVHL (pCMV6 denotes empty plasmid without VHL open reading frames) and HA-tagged HIF1α. Total protein was extracted from cells and HIF1α was precipitated with magnetic beads coupled with anti-HA antibody. pVHL binding and HIF1α ubiquitination was then tested by western blot. Samples were run on the same gel but were noncontiguous, as indicated by white spaces.
Immunoprecipitation assay of HIF1α ubiquitination and VHL binding. The 786-0 cells were cotransfected with plasmids expressing pVHL (pCMV6 denotes empty plasmid without VHL open reading frames) and HA-tagged HIF1α. Total protein was extracted from cells and HIF1α was precipitated with magnetic beads coupled with anti-HA antibody. pVHL binding and HIF1α ubiquitination was then tested by western blot. Samples were run on the same gel but were noncontiguous, as indicated by white spaces.
Discussion
We describe a new homozygous VHL exon 2 mutation, VHLP138L, which is associated in the affected homozygote with congenital polycythemia but not in her parents or her relatives, with cancer or other VHL syndrome tumors. This contrasts with reports of other heterozygous VHL mutations encoding the same amino acid residue (VHL P138R, P138T) that have been reported in VHL syndrome and renal cancer.24,-26 We also show that this mutation is not only associated with elevated EPO levels but also with a hallmark of primary polycythemia (ie, EPO hypersensitivity).
This novel, polycythemia-associated germline homozygosity for the hypomorphic VHLP138L allele, albeit present in a different domain of the VHL protein, has a similar erythropoiesis-stimulating effect to the Chuvash VHLR200W mutation in regard to EPO levels and BFU-Es EPO hypersensitivity (Table 2). It has been suggested that these features are due to a conformational change of SOCS1’s binding groove encoded by VHL gene’s exon 3. SOCS1 is a negative regulator of JAK2, and it has been suggested that the impaired interaction of VHLR200W and VHLH191D with SOCS1 is a cause of EPO hypersensitivity.9 However, this putative mechanism is contradicted by our data of EPO-hypersensitive VHLP138L erythroid progenitors (Figure 2) and also by the fact that VHLH191D native erythroid progenitors are not EPO hypersensitive,27 even though VHLH191D is located in the SOCS1’s binding groove.9 Further, our data reveal that some HIF2α gain-of-function mutation (c.1603A>G:M535V)28 also have EPO-hypersensitive erythroid progenitors. We conclude that the molecular mechanisms of EPO-hypersensitive stimulation of erythroid proliferation (a feature of primary polycythemia) remain unexplained and await further studies.
Characteristics of patients with VHL homozygous mutation (adjusted to age)
| VHL mutation . | Age, y . | Sex . | Hct, % . | MCV, (fL) . | EPO, (mIU/mL) . | In vitro assay . |
|---|---|---|---|---|---|---|
| VHLP138L | 15 | Female | 59.2 | 88.1 | 40 | Hypersensitive |
| VHLH191D | 5 | Female | 53.1 | 63.1 | 201.6 | Normal27 |
| VHLR200W | 16 | Male | 53.4 | 81.2 | 31 | Hypersensitive27 |
We show that the loss of function of the VHLP138L mutation (as well as VHLH191D) is at least in part from decreased stability of VHL protein (Figure 4). Similar protein instability was previously shown for the Chuvash VHLR200W mutation.29 Further, our computer modeling of the pVHLP138L structure predicted significant interference with binding of the α subunit of the HIF1 peptide, which was confirmed by HIF1α ubiquitination and a pVHL-binding assay (Figure 6). These functional abnormalities would be expected to prolong the half-life of HIF1 or HIF2, essential transcriptional factors regulating hypoxia sensing. Indeed, this assumption is directly confirmed by the enhanced expression of HIF-regulated genes SLC2A1, TFRC, and HK-1 (Figure 3A). These data suggest that the impaired stability, together with reduced affinity for the HIF α subunit of the polycythemic pVHL mutants, are common features resulting in the delayed ubiquitinization and degradation of HIFs.30
We also demonstrate that granulocytes from the VHLP138L subject have increased transcripts of RUNX1 and NF-E2 genes (Figure 3B). It was reported that increased transcription of NF-E2 is specific for the acquired primary polycythemic disorder, PV.22,23 Our results contradict this assumption. The nonspecificity of increased transcripts of these genes in PV is further supported by our unpublished data in the study of HIF2α germline gain-of-function mutation. These data suggest that the activation of RUNX1 and NF-E2 genes is likely secondary to augmented erythropoiesis in polycythemia and not a candidate “driver” of PV augmented erythropoiesis. It remains to be shown if the increased transcripts of these genes in PV may be due to augmentation of the hypoxia sensing pathway, also possibly present in PV.
This report provides further evidence for the heterogeneity of clinical phenotypes of VHL mutations resulting in cancers1 or in augmented erythropoiesis. The molecular basis of these differences remains unclear at this time. One can only speculate that the relatively small VHL peptide comprising 213 codons yet encoded by a large >11.2-kb VHL gene may have multiple functions, possibly from interactions with other modifying factors, which await future clarification. We submit that descriptions of families with congenital disorders and unique phenotypes resulting from VHL mutations provide an attractive opportunity for structure-function relationship analyses of VHL protein and lead to an enhanced understanding of polycythemic disorders and diseases of hypoxia sensing.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Czech Science Foundation (project P301/12/1503), the European Structural Funds (project CZ.1.07/2.3.00/20.0164) and by grant LF_2012_016 (L.L. and V.D.).
Authorship
Contribution: L.L. designed the research, performed the research, analyzed data, and wrote the paper; F.L. performed the research and reviewed the paper; C.Y. performed the immunoprecipitation assay and wrote the paper; H.V. performed molecular dynamics simulations study and wrote the paper; R.D. recruited study subjects and reviewed the manuscript; V.D. analyzed data and reviewed the manuscript; and J.T.P. conceived the project, designed the study, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Josef T. Prchal, Division of Hematology, 30N 1900E, 5C402 SOM, University of Utah, Salt Lake City, UT 84132; e-mail: josef.prchal@hsc.utah.edu.