Abstract
Mediastinal large B-cell lymphoma (MLBL) represents 2% of mature B-cell non-Hodgkin lymphoma in patients ≤ 18 years of age. We analyzed data from childhood and adolescent patients with stage III MLBL (n = 42) and non-MLBL DLBCL (n = 69) treated with Group B therapy in the French-American-British/Lymphome Malins de Burkitt (FAB/LMB) 96 study. MLBL patients had a male/female 26/16; median age, 15.7 years (range, 12.5-19.7); and LDH < 2 versus ≥ 2 × the upper limit of normal, 23:19. Six MLBL patients (14%) had < a 20% response to initial COP (cyclophosphamide, vincristine, and prednisone) therapy. Central pathology revealed approximately 50% with classical features of primary MLBL. Five-year event-free survival for the stage III MLBL and non-MLBL DLBCL groups was 66% (95% confidence interval [CI], 49%-78%) and 85% (95% CI, 71%-92%), respectively (P < .001; 14%). The 5-year overall survival in the 42 MLBL patients was 73% (95% CI, 56%-84%). We conclude that MLBL in adolescent patients is associated with significantly inferior event-free survival compared with stage III non-MLBL DLBCL and can be of multiple histologies. Alternate treatment strategies should be investigated in the future taking into account both adult MLBL approaches and more recent biologic findings in adult MLBL.
Key Points
There are multiple histologic types of MLBCL in adolescents.
Event-free survival of MLBCL in adolescents after FAB/LMB 96 therapy is less compared to DLBCL.
Introduction
Mediastinal large B-cell lymphoma (MLBL) is a rare malignancy thought to arise from mature thymic B cells. Although previously considered by the World Health Organization (WHO) to be a subtype of diffuse large B-cell lymphoma (DLBCL),1 primary mediastinal large B-cell lymphoma (PMBL) is now classified as a distinct mature B-cell neoplasm.2 According to the Murphy staging system for childhood non-Hodgkin lymphoma (NHL), MLBL, by virtue of its primary location in the mediastinum, must be at least stage III disease even when tumor is localized above the diaphragm.3 The Children's Cancer Group (CCG) and Berlin-Frankfurt-Münster (BFM) group each reported results in children with mediastinal disease enrolled in a series of completed multicenter mature B-NHL trials, and demonstrated 5-year event-free survival (EFS) of 75% ± 10% and 75% ± 8%, respectively.4,5 In contrast, a considerably higher 5-year EFS of 85% ± 2% was reported for BFM patients with stage III non-primary-mediastinal B-cell NHL who received the same therapy as those with primary mediastinal disease, suggesting that MLBL may be an inherently different disease that requires alternative therapy than other identically grouped pediatric mature B-cell NHLs.
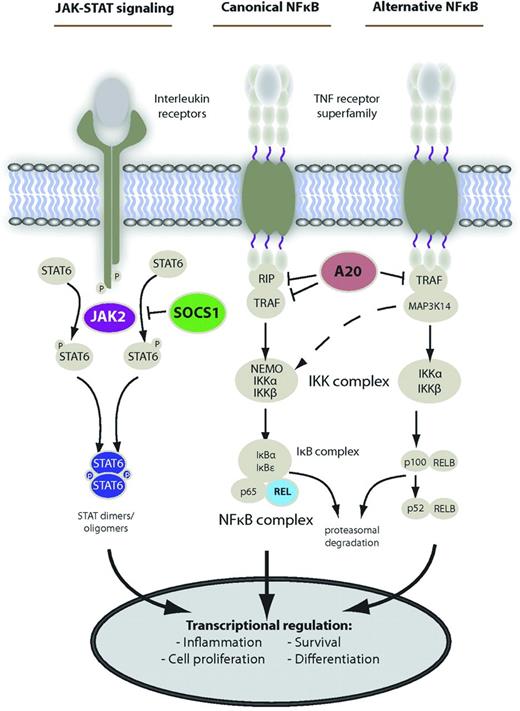
With the advent of gene-expression profiling, it has become apparent that adult PMBL differs biologically from other mature B-cell NHLs, including DLBCL subtypes1-3,6 and Burkitt lymphoma (BL),7 and may in fact be a separate disease entity altogether. DNA microarrays have demonstrated similarities between PMBL and Hodgkin lymphoma (HL), suggesting that PMBL falls somewhere along the biologic spectrum between DLBCL and HL.6,8 Recent studies have suggested that molecular hallmarks of PMBL include overexpression of genes in both the NF-κB and JAK/STAT pathways (Figure 1).9
Main deregulated signaling pathways in PMBCL. Shown are the main activation cascades of JAK-STAT and NF-κB signaling. Alternative pathway activation exists. Known gene alterations leading to constitutive pathway activity in PMBCL are shown in color. Reprinted from Steidl and Gascoyne9 by permission of the American Society of Hematology.
Main deregulated signaling pathways in PMBCL. Shown are the main activation cascades of JAK-STAT and NF-κB signaling. Alternative pathway activation exists. Known gene alterations leading to constitutive pathway activity in PMBCL are shown in color. Reprinted from Steidl and Gascoyne9 by permission of the American Society of Hematology.
In the present study, we report on the largest experience of pediatric patients with MLBL uniformly treated as part of the single international cooperative group study French-American-British/Lymphome Malins de Burkitt (FAB/LMB) 96, which enrolled pediatric patients with mature B-cell NHL from May 1996 to June 2001. Results for the low-, intermediate-, and high-risk cohorts have been described previously.10-12 However, this is the first report to focus specifically on the subset of 42 patients with stage III MLBL enrolled in the FAB/LMB 96 study to describe their unique clinical and pathologic characteristics, outcomes, and risk factors for EFS and compare them with patients with stage III non-MLBL DLBCL treated with identical therapy.
Methods
Study design
The FAB/LMB 96 study was an open-label, randomized cooperative international study involving 161 pediatric cancer centers in 3 national groups: the Societé Française d'Oncologie Pediatrique (SFOP: France, Belgium, and The Netherlands), Children's Oncology Group (COG; United States, Canada, and Australia), and the United Kingdom Children's Cancer Study Group (UKCCSG). It was a planned 5-year study that enrolled patients from May 1996 until June 2001. The protocol was approved by each participating center's institutional review board. Each national group was responsible for scientific, ethical, and administrative approvals, randomization, and data collection in a national database. Data were transferred every 6 months to the international database at Institut Gustave Roussy in France. Parents or patients over 18 years of age signed informed consent before randomization in accordance with the Declaration of Helsinki.
Eligibility
Patients under 18 (SFOP and UKCCSG) or 21 (COG) years of age with newly diagnosed de novo mature B-cell lymphoma classified as BL, Burkitt-like lymphoma, or DLBCL were eligible for the study. Ineligibility criteria included immunodeficiency, HIV positivity, and previous solid organ transplantation, malignancy, and/or chemotherapy.
Diagnosis
Staging was performed according to the Murphy classification system.3 Disease was categorized as low-risk (Group A) for resected stage I and completely resected abdominal stage II; high-risk (Group C) for BM disease (≥ 25% L3 blasts) and/or CNS disease defined by 1 or more of the following: any L3 cerebrospinal fluid blast, cranial nerve palsy, clinical spinal cord compression, isolated intracerebral mass, and/or cranial or spinal parameningeal extension; and intermediate-risk (Group B) for all others including MLBL.
The morphology and immunophenotype from the initial diagnostic material from each case was independently evaluated by each of the 6 hematopathologists from the 3 national cooperative groups (for SFOP, M. Raphael and M. J. Terrier-Lacombe; for COG, M. A. Lones and S. L. P.; and for UKCCSG, K.M., and A. Wotherspoon) to establish a diagnosis of PMBL. All cases were classified according to the criteria described in the revised European-American Lymphoma and WHO classifications.1,13 An international consensus diagnosis was established for each case based on independent agreement by the group of hematopathologists or following review by the national groups on a multiheaded microscope.14
A protocol-specific standard immunophenotyping panel was performed on each case and included CD20, CD79a, CD3, CD45RO, TDT, CD30, and p80, as described previously.14 In addition, a subset of eleven cases had additional immunophenotypic analysis for germinal center differentiation markers CD10, BCL-6, and IRF4/MUM-1 as described previously.15
Retrospective pathologic review of all cases was performed to confirm a diagnosis of NHL and to attempt to subclassify the lymphomas included in this analysis. The review was limited to morphology and the initial diagnostic immunophenotypic panels performed on the cases because no additional case materials were available for further workup. In particular, cases were evaluated for characteristic morphologic features of PMBL including compartmentalizing fibrosis, medium to large cells with abundant pale cytoplasm, and round to oval nuclei lacking prominent macronucleoli and demonstrating strong, uniform expression of CD20 and CD79a with variable, weak expression of CD30. Only 10 cases had sufficient materials to allow for staging with IRF4/MUM1. No additional staining for characteristic markers such as CD23, I MAL, TRAF1, or REL were performed because of a lack of additional unstained slides or blocks. Some cases were considered indeterminant, including “gray zone” lymphoma that is termed B-cell lymphoma, unclassifiable with features intermediate between DLBCL and classic HL in the most recent WHO classification.2
Workup included clinical examination; chest X-ray, abdominal ultrasound, or computed tomography; BM aspiration; cerebrospinal fluid cytology; and initial lactate dehydrogenase (LDH) level. Computed tomography scans and bone scintigraphy were performed as clinically indicated.
Treatment and randomization
Patients with stage III MLBL and stage III non-MLBL DLBCL were initially assigned to the Group B treatment arm as we described previously.10 Patients received a pre-phase consisting of low-dose cyclophosphamide, vincristine, and prednisone (COP). Patients with a ≥ 20% response at day 7 received a first induction course, COPADM1 (cyclophosphamide 1.5 g/m2, vincristine, prednisone, doxorubicin, high-dose methotrexate [HDMTX 3 g/m2 as a 3-hour infusion with intrathecal MTX]). On recovery, patients received a second COPADM induction course (randomized to receive either cyclophosphamide 1.5 or 3 g/m2), 2 consolidation courses with CYM (cytarabine and HDMTX), and, depending on randomization, either a single maintenance course of M1 (cyclophosphamide 500 mg/m2, vincristine, prednisone, doxorubicin, and HDMTX) or no maintenance. Response to treatment was measured at 3 time points. The first evaluation was performed after COP at day 7. Patients with > a 20% response to COP were considered responders to COP. Patients with < a 20% response to COP were switched to a more intensive, high-risk (Group C) regimen10 including MTX (8 g/m2), high-dose cytarabine, and etoposide. The second evaluation was performed after the first COPADM course and patients were randomized if there was no disease progression. The third evaluation was performed after the first consolidation CYM course (CYM1). Residual mass was recommended to be removed or biopsied. Patients with residual lymphoma after CYM1 were switched to the more intensive Group C regimen at CYVE1 (consisting of high-dose cytarabine 3 g/m2 and continuous cytarabine 50 mg/m2 plus etoposide), and subsequently received a second course of CYVE and 4 maintenance cycles: M1 (COPADM3 [same as M1 for Group B]), M2 (cytarabine plus etoposide), M3 (cyclophosphamide, vincristine, prednisone, and doxorubicin), and M4 (cytarabine plus etoposide) as we described previously.10,12 Response criteria differed by time point. After the COP reduction phase, less than a 20% reduction in the product of the 2 largest diameters of measured lesions was considered a poor response and these patients were switched to Group C therapy. An intermediate response was defined as a 20%-99% reduction in the product of the 2 largest diameters of measurable lesions. Patients with a 100% reduction were considered to have a complete response (CR). At the third evaluation, CR included the presence of residual masses if an adequate biopsy demonstrated no viable tumor. Persistent disease was defined as histologically proven residual mass and disease progression required a 25% or greater increase in the product of the 2 largest diameters of measurable lesions. Radiation therapy was not part of the primary treatment and was considered an off-therapy event if administered.
Statistical methods
The primary end point was EFS, defined as the time from randomization to disease progression, relapse, second malignancy, or death from any cause. Overall survival (OS) was a secondary end point and was defined as the time from randomization to death from any cause. For determination of EFS and OS, patients who did not experience an event were censored at the date of last follow-up contact. Probability of EFS and OS were determined using the Kaplan-Meier method. Comparisons of EFS and OS in patients with stage III MLBL and stage III non-MLBL DLBCL were performed using a log-rank test. Prognostic analyses of EFS according to gender (male vs female), age (10-14 vs 15-21 years), COP response (response [incomplete or CR] vs no response [NR]), cooperative group, histology (stage III MLBL vs stage III non-MLBL DLBCL), size (< 10 cm vs ≥ 10 cm), and LDH level (< 2 × the upper limit of institutional normal [ULN] vs ≥ 2 × ULN) were performed using a log-rank test.
Results
Demographics
A total of 42 patients with primary MLBL arising from the mediastinum were enrolled, constituting approximately 5% of the total number of patients registered in Group B therapy (n = 762). Demographic information for the MLBL subset is summarized in Table 1. All patients were adolescents (median age, 15.7 years) and the majority were female (ratio of female to male, 3:2). Patients were enrolled from all 3 cooperative groups, with the majority receiving treatment at COG sites.
Demographic data for 42 MLBL patients enrolled in the FAB/LMB 96 study
| Sex | |
| Female | 26 (62%) |
| Male | 16 (38%) |
| Age, y | |
| Median | 15.7 |
| Range | 12.5-19.7 |
| LDH, × ULN | |
| < 2 | 23 (55%) |
| ≥ 2 | 19 (45%) |
| Stage at diagnosis | III (100%) |
| Extranodal sites | 0 (0%) |
| Cooperative group | |
| COG | 29 (69%) |
| SFOP | 10 (24%) |
| UKCCSG | 3 (7%) |
| Sex | |
| Female | 26 (62%) |
| Male | 16 (38%) |
| Age, y | |
| Median | 15.7 |
| Range | 12.5-19.7 |
| LDH, × ULN | |
| < 2 | 23 (55%) |
| ≥ 2 | 19 (45%) |
| Stage at diagnosis | III (100%) |
| Extranodal sites | 0 (0%) |
| Cooperative group | |
| COG | 29 (69%) |
| SFOP | 10 (24%) |
| UKCCSG | 3 (7%) |
Pathology
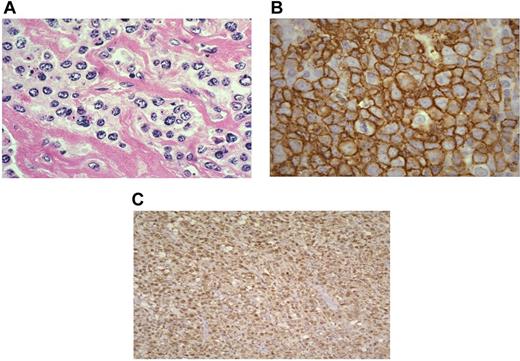
All MLBL cases showed compartmentalizing sclerosis surrounding medium- to large-sized tumor cells (Figure 2A). The tumor cells had moderate amounts of clear cytoplasm with round to oval vesicular nuclei. Nucleoli were not prominent. Immunophenotypic analysis showed expression of the B-cell markers CD20 and CD79a in all cases. The tumor cells were negative for CD3, CD45RO, TdT, and p80. All cases showed CD30 staining that was variable in intensity but usually weak and patchy in distribution in a predominantly cytoplasmic distribution. In the small subset of 11 cases stained for germinal center markers,15 it was noted that 2 expressed CD10 and BCL-6, 7 expressed BCL6 without CD10 (Figure 2B), and 2 showed only MUM-1 without CD10 or BCL-6 expression. Interestingly, all cases (11 of 11), irrespective of germinal center marker expression, showed IRF4/MUM-1 staining (Figure 2C).
Pathologic features of mediastinal large-cell lymphoma. (A) H&E histologic section of a MLBL tumor showing the characteristic large-cell infiltrate with intervening bands of compartmentalizing fibrosis. The tumor cells form individual cells and small groups (400× magnification). (B) CD20 immunoperoxidase staining demonstrating the mature MLBL infiltrate (1000× magnification). (C) IRF4/MUM 1 staining of a MLBL tumor showing characteristic nuclear staining (100× magnification; Olympus BX40 microscope).
Pathologic features of mediastinal large-cell lymphoma. (A) H&E histologic section of a MLBL tumor showing the characteristic large-cell infiltrate with intervening bands of compartmentalizing fibrosis. The tumor cells form individual cells and small groups (400× magnification). (B) CD20 immunoperoxidase staining demonstrating the mature MLBL infiltrate (1000× magnification). (C) IRF4/MUM 1 staining of a MLBL tumor showing characteristic nuclear staining (100× magnification; Olympus BX40 microscope).
Limited retrospective pathologic review of the MLBL cases based on morphologic features and available immunohistochemical stains that were able to be performed on 32 of 36 cases. Four cases had no histologic or immunophenotypic slides available. Eighteen of 36 cases had morphologic and immunophenotypic features that were characteristic of PMBL, whereas 5 cases had features that were more consistent with DLBCL not otherwise specified (DLBCL-NOS). The remaining cases (9 of 36) had features that were indeterminant. Of the indeterminant cases, 5 cases had features that were indeterminant between classic HL and PMBL, including thick bands of sclerosis, prominent macronucleoli, multinucleation of a subset of tumor cells, and aberrant immunophenotypes with strong, uniform CD30 and variable expression of CD79a and CD30. These indeterminate cases were considered to be suspicious for gray zone lymphoma, although this diagnosis could not be confirmed without additional immunophenotypic studies. Review of submitted pathology reports on these cases indicated that all tumor cells were strongly positive for CD45 and lacked definitive CD15 expression, although these findings could not be confirmed retrospectively. The remaining 4 cases had features that were indeterminate between DLBCL-NOS and PMBL, including large-cell morphology with no discernible fibrosis and variable, weak CD30 expression.
Response to treatment
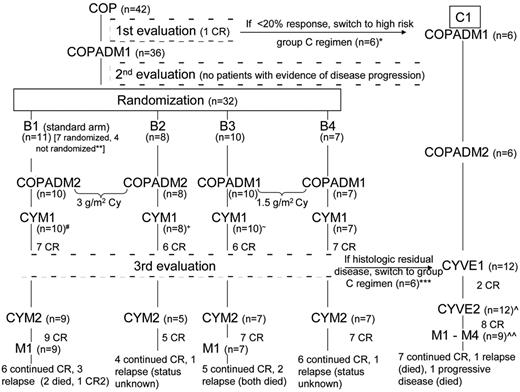
The treatment course and response for each of the 42 MLBL patients is summarized in Figure 3. There were no toxic deaths during the study. Of the 42 MLBL patients who were received COP therapy, 36 (84%) had at least a 20% response. No patient had evidence of disease progression at the second evaluation (after COPADM1). Six patients were transferred to Group C after COP therapy. Of the remaining 36 patients, 32 were randomized according to Group B therapy randomization, as described previously12 ; 4 patients were not randomized and were treated on the B1 arm by physician choice. Eleven patients (7 randomized and 4 nonrandomized) were treated on the standard B1 arm; 8 were randomized and treated on the B2 arm, 10 on the B3 arm, and 7 on the B4 arm. Twenty-nine subjects on the B arms achieved a CR during the course of Group B therapy, although 1 of these patients relapsed before the third evaluation and was therefore no longer included among the CRs at CYM2 (Figure 3). At the end of CYM1, 29 of 36 patients had a residual mass on imaging. Only 23 of 29 patients who underwent a biopsy or excision and only 4 of 23 (17%) had residual viable tumor. Two additional patients treated on the B arms stopped therapy before achieving CR. One Group B patient who came off study because of disease progression after CYM1 achieved a CR after BEAM (carmustine, etoposide, cytarabine, and melphalan) therapy, but subsequently died of relapse.
Treatment course for each MLBL patient treated in the FAB/LMB 96 study. All MLBL patients received Group B therapy initially. After the COP reduction phase, patients with a CR or intermediate response (defined as a 20%-99% reduction in the product of the 2 largest diameters of evaluable lesions) continued on Group B therapy. At the third evaluation, a CR included residual masses as long as adequate biopsy demonstrated no viable tumor. Patients with residual viable tumors (persistent disease) were switched to Group C therapy beginning with CYVE1 consolidation. Nine patients in CR continued onto Group C M1-M4 therapy, 1 patient from Group B and 8 patients from Group C. Patients with disease progression (> 25% increase in the product of 2 largest diameters) were taken off study. Disease status at last follow-up for all patients completing therapy is included.
Treatment course for each MLBL patient treated in the FAB/LMB 96 study. All MLBL patients received Group B therapy initially. After the COP reduction phase, patients with a CR or intermediate response (defined as a 20%-99% reduction in the product of the 2 largest diameters of evaluable lesions) continued on Group B therapy. At the third evaluation, a CR included residual masses as long as adequate biopsy demonstrated no viable tumor. Patients with residual viable tumors (persistent disease) were switched to Group C therapy beginning with CYVE1 consolidation. Nine patients in CR continued onto Group C M1-M4 therapy, 1 patient from Group B and 8 patients from Group C. Patients with disease progression (> 25% increase in the product of 2 largest diameters) were taken off study. Disease status at last follow-up for all patients completing therapy is included.
Five patients with evidence of residual disease (4 with histologic disease and 1 positron emission tomography positive) were switched to Group C therapy after the third evaluation (after CYM1). One additional patient was transferred to Group C despite having achieved CR.by physician choice (Figure 3). Excluding the 1 Group C subject already in CR at the time of transfer, 8 of 11 patients in Group C attained CR. In Group C, 3 patients failed to achieve a CR after CYVE2 (induction failure); all 3 subsequently died from progressive disease. Lastly, among the different histologies, within the group of 18 patients with PMBL histology, 13 remain in CR; within the group of 5 with DLBCL histology, 3 remain in CR; and within the group of 9 with indeterminate histology, 7 remain in CR.
EFS and OS
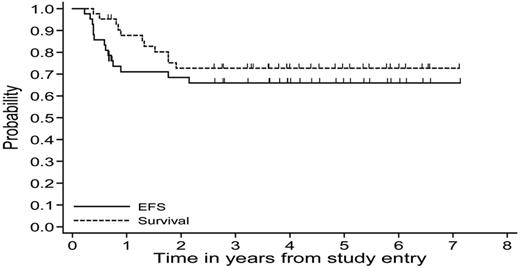
In the 42 MLBL patients, the probabilities of 5-year EFS and OS were 66% (95% confidence interval [CI], 50%-78%) and 73% (95% CI, 56%-84%), respectively (Figure 4). At the time of final data analysis, a total of 28 patients remained in CR1. Among those who completed treatment in the 4 intermediate-risk Group B treatment arms (n = 28), 21 remain in CR1 at the time of this analysis and 7 have relapsed. Among the 6 patients who responded poorly to COP and were switched to Group C therapy prior at COPADM1 according to the protocol, 3 remained in CR1 after completion of therapy and 3 progressed. Among the 6 patients who were switched to Group C therapy after CYM1 according to protocol, 4 remained in CR1 at the time of final analysis and 2 died of disease progression (Figure 3). One patient who withdrew from therapy to move to a foreign country died of intracranial hemorrhage unrelated to therapy. The majority of relapses and progression occurred within the first year of diagnosis, with only a few events occurring in the second year of diagnosis.
Probability of 5-year EFS and OS calculated using the Kaplan-Meier method for MLBL patients treated on Group B therapy in the FAB/LMB 96 study.
Probability of 5-year EFS and OS calculated using the Kaplan-Meier method for MLBL patients treated on Group B therapy in the FAB/LMB 96 study.
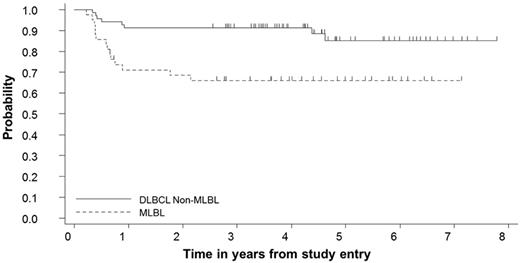
The probability of 5-year EFS in the MLBL group (n = 42) was compared with the probability of 5-year EFS in patients with stage III non-MLBL DLBCL (n = 69).12 The MLBL group had a significantly lower 5-year EFS compared with the non-MLBL DLBCL stage III subgroup: 66% (95% CI, 49%-78%) vs 85% (95% CI, 71%-92%), respectively (P < .001; Figure 5).
Probability of 5-year EFS calculated using the Kaplan-Meier method for patients with MLBL compared with stage III non-MLBL DLBCL patients treated on Group B therapy in the FAB/LMB 96 study. The 5-year EFS for MLBL patients was 66% (95% CI, 49%-78%) and for stage III non-MLBL DLBCL patients, it was 85% (95% CI, 71%-92%; P < .001).
Probability of 5-year EFS calculated using the Kaplan-Meier method for patients with MLBL compared with stage III non-MLBL DLBCL patients treated on Group B therapy in the FAB/LMB 96 study. The 5-year EFS for MLBL patients was 66% (95% CI, 49%-78%) and for stage III non-MLBL DLBCL patients, it was 85% (95% CI, 71%-92%; P < .001).
Risk factors associated with EFS
To determine the effect of various risk factors on the probability of 5-year EFS, log-rank testing with stratification for sex, age, COP response, LDH level, and size of mass (> 10 vs ≤ 10 cm) was performed. There was no difference in probability of 5-year EFS among each of the risk factors (CR to COP vs NR, 67.7 vs 42.9%, P = .14; LDH ≥ 2 × ULN vs < 2 × ULN, 53.8 vs 73.2%, P = .2; and tumor size > 10 vs ≤ 10 cm, 53.8% vs 61.3%, P = .07). However, the numbers in each group were far too low to have a high level of confidence. Larger numbers would be required to determine the independent effect of each of these variables.
Discussion
MLBL is a rare subtype of B-cell NHL, accounting for approximately 2% of childhood and adolescent lymphomas.16 Despite its rarity, treatment of adolescent MLBL is a topic of considerable interest because pediatric and adolescent patients with MLBL tend to have worse outcomes than those with other stage III mature B-cell NHLs.5 The BFM group was the first to report a decreased EFS in pediatric MLBL patients (75% vs 85% 5-year EFS), but the result was not statistically significant (P = .19) and included 30 patients treated during the course of 3 trials and an interim treatment period. More recently, the BFM has reported a 34% relapse rate among 44 PMBL patients treated over a 23-year period in 4 different protocols (NHL-BFM 86, NHL-BFM 90, NHL-BFM 95, and B-NHL BFM 04).17 The results from the current FAB/LMB 96 study confirm the findings reported by the BFM group and provide important additional data to support the need for improved therapies for adolescent patients with MLBL. Because our results come from the largest single uniform treatment study of adolescent MLBL and show for the first time a highly significant decrease in 5-year EFS compared with other stage III non-MLBL pediatric DLBCL patients, it is increasingly clear that the currently used treatment regimens, consisting of dose-intense courses of steroids, vincristine, methotrexate, cyclophosphamide (or ifosfamide), doxorubicin, cytarabine, etoposide and intrathecal chemotherapy are not as effective for MLBL compared with other pediatric mature B-cell NHLs (BL and DLBCL) treated on similar treatment regimens.
To further support the importance of studying new approaches for the treatment of adolescent MLBL, we refer to recent improvements in the treatment of adult PBML. Various chemotherapy regimens, including MACOP-B (methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin) and VACOP-B (etoposide instead of methotrexate) have been studied in adult patients with PMBL and have been shown to offer a survival benefit over conventional CHOP (Cytoxan, doxorubicin, vincristine, and prednisone) therapy.18-20 In addition, in a small series of adult patients with newly diagnosed PMBL, VACOP-B followed by autologous stem cell transplantation led to a disease-free survival rate of 93% after 35 months median follow-up.21
Rituximab, the CD20 mAb known to inhibit the antiapoptotic NF-κB pathway, has also been extensively studied in adults with DLBCL. Rituximab is of interest in the treatment of B-cell NHL because up-regulation of subsets of NF-κB signaling genes in PMBL and activated B-cell-like DLBCL has been demonstrated by gene-expression profiling.6,22 The addition of rituximab to CHOP therapy (CHOP-R) has been shown to provide a benefit over CHOP alone in adults with DLBCL.23-25 Similarly, the addition of rituximab to DA-EPOCH (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) has been shown to benefit adults with DLBCL whose tumors overexpress Bcl-2.26 Although this latter study did not include patients with PMBL, a separate study of 32 adult patients with PMBL (Bcl-2 status not reported) treated with DA-EPOCH and rituximab demonstrated that the regimen was highly effective, with 95% EFS.27 Among adult patients with PMBL who also received an intensified CHOP regimen and rituximab, this strategy has also shown very encouraging results.28,29
The pathology review, although limited by the materials available, showed the need for careful clinical, morphologic, and immunophenotypic examination to ensure that the mediastinal lymphoma was appropriately diagnosed. Although most cases of PMBL were relatively easily diagnosed, awareness of the gray zone lymphomas or those lymphomas with features that were indeterminant between classic HL and PMBL was necessary because these tend to be seen (albeit in low incidence) in adolescents and young adults, as was demonstrated recently in a retrospective study.17 We noted 5 cases that would need additional immunophenotypic work-up to distinguish between PMBL and gray zone lymphoma, but there were not sufficient materials available in this retrospective study to allow for this immunophenotyping to be performed. However, this incidence of cases is similar to that seen in a previous study of mature B-cell lymphoma in children and adolescents.17 It is also important to recognize that all DLBCL cases that occur in the chest or mediastinum are not PMBL or gray zone lymphomas; there are some cases of DLBCL-NOS that may be present initially in this anatomic site, but they often have evidence of disease in other sites. Both DLBCL-NOS and gray zone lymphomas may require different approaches to therapy than PMBL and have different prognostic implications and clinical behavior. Because of the diversity of lymphoid neoplasms that may present in the mediastinum, it is essential that a sufficient amount and quality of diagnostic materials be collected to allow for the appropriate diagnostically important ancillary studies. In addition, some cases may require review by an experienced hematopathologist with appropriate immunophenotyping to meet the defined diagnostic criteria set forth in the 2008 WHO classification for accurate diagnosis and differentiation between PMBL, DLBCL-NOS, and gray zone lymphoma.2,17
Aside from rituximab, other inhibitors of the NF-κB pathway could be of benefit to patients with PMBL. NF-κB–blocking agents, such as proteasome inhibitors and small-molecule inhibitors of the IKK protein, may work synergistically with chemotherapy and rituximab and warrant further study. By blocking the pathway at various points in the NF-κB signal transduction pathway, it may be possible to overcome any compensatory up-regulation that may occur. Preliminary activity of proteasome inhibitors and small-molecule IKK inhibitors has already been demonstrated in vitro in a PMBL cell line, with increases in apoptosis reported after incubation with a proteasome inhibitor, a small-molecule IKK inhibitor, or the combination of both. In addition, these increases in apoptosis were shown to be associated with inhibition of multiple apoptosis-associated proteins, including members of the NF-κB family of transcription factors.30
The NF-κB signaling pathway is not the only pathway that is overexpressed in PMBL. Constitutive activation of the JAK-STAT signaling pathway due to recurrent gene mutations affecting the STAT6 DNA-binding domain has been demonstrated in PMBL tumor samples, but not in DLBCL tumor samples.9,31 Targeted agents that block the JAK-STAT pathway may therefore also prove useful in the treatment of PMBL (Figure 1).9,31 Recent studies have demonstrated a consistent 9p24.1 amplification and enhanced expression of the PD-1 ligand in adult PMBL.32 Lastly, reduced expression of MHC class II genes is an additional defining feature of adult PMBL with a strong correlation with expression of the master transcriptional regulator of class II expression.33-35 Immunologic approaches attempting to block PD-1 and/or increasing T-cell effector cell infiltration into the microenvironment may be additional alternative treatment strategies to consider in the future.
It is important to consider alternative chemotherapy regimens and the addition of targeted agents early in the treatment of all patients with adolescent MLBL because patients who do not achieve a CR with first-line therapy are difficult to retrieve with subsequent therapies. This is evidenced by the finding that all 3 of the patients switched to Group C therapy who were not in CR after the second CYVE2 intensification cycle went on to die of their disease. Although more intensive and targeted therapies may be important for all adolescent MLBL patients, they are most urgently needed for the treatment of patients without an early response to chemotherapy. Although COP nonresponders were switched to the more intensive Group C therapy, 2 of these patients still failed to achieve a CR and subsequently died from disease progression. Furthermore, the single patient who did not respond to COP but remained on Group B therapy also went on to relapse after the completion of treatment and subsequently died.
Given the poorer outcome for adolescent patients with MLBL compared with pediatric patients with non-MLBL stage III DLBCLs, and taking into account the recent encouraging results in adults with MLBLs treated with rituximab and CHOP or DA-EPOCH like regimens, alternative therapeutic strategies should be explored in the future in this small subgroup of adolescent patients.36 Taking into account the difference in biology in adult PMBLs versus DLBCLs, targeted approaches, especially inhibitors of NF-κB and/or JAK/STAT pathways, should be investigated in the relapsed setting.37-39 In addition, this is a prototype disease that should be studied by both pediatric and adult cooperative groups together in adolescent and young adult patient initiatives.40 Based on the results of the present study, an international pediatric trial of dose-adjusted EPOCH with rituximab in adolescents with MLBL has been initiated (Catherine Patte, personal communication).
Presented in part at the American Society of Clinical Oncology (ASCO) annual meeting, Orlando, Florida, June 1, 2009.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the Data and Safety Monitoring Committee, Professors Michael Link, Alfred Reiter, David Harrington, and Robert Souhami, for continued and diligent oversight of this study; Linda Rahl and Virginia Davenport for assistance in the administration of this trial; Amber Quinlan and Erin Morris, RN, for editorial assistance in the development of this manuscript; the other members of the international pathology review panel group (Drs M. J. Terrier-Lacombe, C. Bayle, P. Felman, M. Lones, and A. Wotherspoon) and the international cytogenetics review panel (Drs H. Poirel, W. Sanger, N. Heerema, J. Swansbury, P. Talley, and A. Bernheim); and all of the members and investigators of the COG, SFOP, and UKCSSG.
This work was supported by grants from the Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, Department of Health and Human Services (COG); the Cancer Research Campaign (UKCSSG); and the Association pour la Recherche contre le Cancer, La Ligue Nationale Contre le Cancer, Institut Gustave Roussy (SFOP).
National Institutes of Health
Authorship
Contribution: M.G. and M.S.C. designed and performed the research, analyzed the data, and wrote the manuscript; I.M.W. and S.G. analyzed the data and wrote the manuscript; R.S. designed the research and analyzed the data; A.A. collected the data; A.A. and C.P. designed and performed the research, analyzed the data, and revised the manuscript; S.L.P., K.M., and M.R. performed the research and analyzed the data; L.H. collected and analyzed the data; and R.P. designed and performed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The FAB/LMB 96 International Study Committee includes the Children's Oncology Group (COG; Arcadia, CA), the Societé Française d'Oncologie Pediatrique (SFOP; Paris, France), and the United Kingdom Children's Cancer Study Group (UKCCSG; Leicester, United Kingdom). For a complete list of FAB/LMB 96 International Study Committee members, please see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Mitchell S. Cairo, MD, Chief, Division of Pediatric Oncology, Hematology and Stem Cell Transplantation, Professor of Pediatrics, Medicine, Pathology, Microbiology and Immunology, and Cell Biology and Anatomy, Associate Chairman, Department of Pediatrics, New York Medical College, Munger Pavilion, Rm 110, Valhalla, NY 10595; e-mail: mitchell_cairo@nymc.edu.
References
Author notes
M.G. and I.M.W. contributed equally to this work.
C.P. and M.S.C. are co–senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal