Abstract
von Willebrand factor (VWF) is amongst others synthesized by endothelial cells and stored as ultra-large (UL) VWF multimers in Weibel-Palade bodies. Although UL-VWF is proteolysed by ADAMTS13 (a disintegrin-like and metalloprotease domain with thrombospondin type-1 motif, number 13) on secretion from endothelial cells, in vitro experiments in the absence of ADAMTS13 have demonstrated that a proportion of these UL-VWF multimers remain anchored to the activated endothelium. These multimers unravel, bind platelets, and wave in the direction of the flow. These so-called VWF “strings” have also been visualized in vivo, lining the lumen of activated mesenteric veins of Adamts13−/− mice. Various studies have demonstrated the extraordinary length of these VWF strings, the availability of their platelet binding and ADAMTS13 cleavage sites, and the possible nature of their endothelial attachment. VWF strings are also capable of tethering leukocytes and parasite-infected red blood cells. However, the majority of studies have been performed in the absence of ADAMTS13, a condition only experienced in thrombotic thrombocytopenic purpura. A normal functional role of VWF strings in healthy persons or in other disease pathologies remains unclear. In this review, we discuss some of the puzzling characteristics of VWF strings, and we debate whether the properties of VWF strings in the absence of ADAMTS13 might be relevant for understanding (patho)physiologic mechanisms.
Introduction
von Willebrand factor (VWF) strings correspond to ultra-large (UL) VWF multimers that, after secretion from the endothelium, remain anchored to the cell surface. As a result of this tethering and the rheologic forces of flow, these VWF multimers unravel, can bind platelets, and wave in the direction of the blood flow1 (Figure 1). Since their discovery, platelet-decorated VWF strings have proved fascinating, and yet puzzling entities, with interesting and rather unique characteristics. VWF strings can be extraordinarily long, can be anchored to endothelial cells through a discrete number of attachment sites, contain active platelet binding sites, and are susceptible to specific proteolysis by its regulating protease, ADAMTS13 (a disintegrin-like and metalloprotease domain with thrombospondin type-1 motif, number 13). VWF strings are considered prothrombotic and might therefore contribute to the pathophysiology of thrombotic disorders, such as thrombotic thrombocytopenic purpura (TTP) and arterial thrombosis. In addition, platelet-decorated VWF strings can also provide a reactive surface for leukocytes and parasite-infected red blood cells, which has suggested possible roles in both inflammation and malaria.
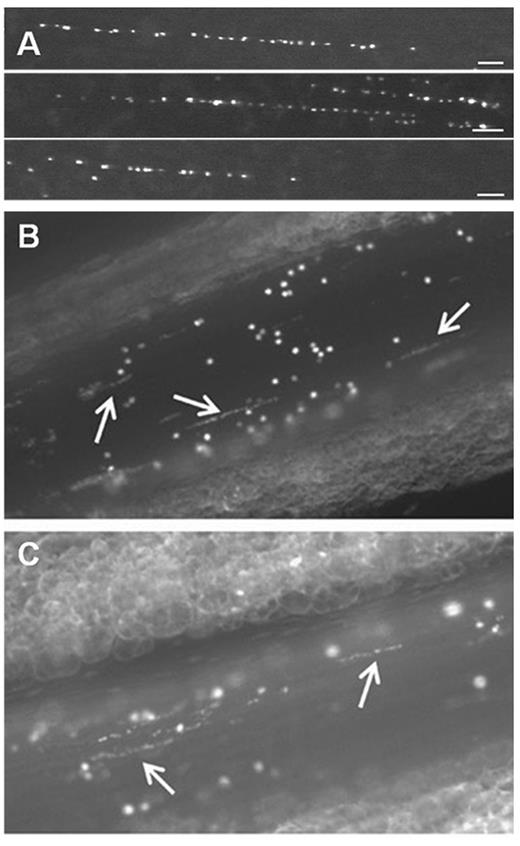
Platelet-decorated VWF strings rapidly appear on the surface of activated endothelial cells. (A) In vitro, platelet-decorated VWF strings are visualized by fluorescence microscopy on the surface of blood outgrowth endothelial cells perfused with washed DIOC6-labeled platelets at a shear rate of 250 seconds−1.43 Scale bar represents 50 μm. (B-C) Platelet-decorated VWF strings (white arrows) appear on the surface of mesenteric veins of Adamts13−/− mice after activation with 10% FeCl3 solution (B) or calcium ionophore A23187 (C). Endogenous platelets were labeled by intravenous administration of rhodamine 6G.42 Microscopy was performed using a Nikon Eclipse TE200 inverted fluorescence microscope equipped with a 20× PLAN objective (numeric aperture 0.4) coupled to an ORCA-R2 Hamamatsu CCD camera. Images were recorded using Hokawo software (Hamamatsu Photonics).
Platelet-decorated VWF strings rapidly appear on the surface of activated endothelial cells. (A) In vitro, platelet-decorated VWF strings are visualized by fluorescence microscopy on the surface of blood outgrowth endothelial cells perfused with washed DIOC6-labeled platelets at a shear rate of 250 seconds−1.43 Scale bar represents 50 μm. (B-C) Platelet-decorated VWF strings (white arrows) appear on the surface of mesenteric veins of Adamts13−/− mice after activation with 10% FeCl3 solution (B) or calcium ionophore A23187 (C). Endogenous platelets were labeled by intravenous administration of rhodamine 6G.42 Microscopy was performed using a Nikon Eclipse TE200 inverted fluorescence microscope equipped with a 20× PLAN objective (numeric aperture 0.4) coupled to an ORCA-R2 Hamamatsu CCD camera. Images were recorded using Hokawo software (Hamamatsu Photonics).
In this review, we discuss the typical features of these intriguing VWF strings and highlight questions that remain to be resolved and that would provide a better understanding of VWF string biochemistry. VWF strings were first discovered and studied in the absence of ADAMTS13, a situation that does not mimic normal physiologic conditions. In the majority of pathophysiologic conditions, plasma ADAMTS13 activity levels are at most decreased, but very rarely absent. To this end, a discussion on the possible involvement of VWF strings in disease pathology might serve to put VWF string research into perspective.
VWF, UL-VWF multimers, and ADAMTS13
VWF is the largest biopolymer present in plasma, and consists of multimers ranging in size from 500 to 10 000 kDa, with the largest multimers being the most hemostatically active.2,3 The building block of these multimers is a mature VWF subunit consisting of D, A, B, C, and CK domains,4,5 although the domain organization of VWF has recently been reannotated.6 In circulation, VWF multimers adopt a folded, globular conformation that shields the platelet glycoprotein Ib (GPIb) binding sites in the VWF A1 domain.7,8 This folding serves to prevent spontaneous binding of platelets to VWF in circulation. However, immobilization of VWF at sites of vascular injury (via the VWF A3-collagen interaction) leads to VWF unfolding in response to the shear forces exerted by the flowing blood. This exposes VWF A1 domains that, in turn, reveal the binding sites for the platelet GPIb receptor. The VWF-GPIb interaction is characterized by a fast association/dissociation rate, which enables platelets to “roll” over the VWF surface and provide the opportunity to establish firm platelet adhesion through the collagen receptors.9-12 This adhesion step, which is particularly relevant under high shear conditions, initiates platelet activation and aggregation, resulting in the formation of a platelet plug that seals the damaged vessel wall. VWF also plays a role in secondary hemostasis as a carrier protein for coagulation factor VIII through interaction with the VWF D′D3 domain, which reduces both the clearance and degradation of this procofactor.13,14
Megakaryocytes and endothelial cells are the only cells that express VWF, although all circulating VWF is derived from the endothelium. Both endothelial cells and platelets store UL-VWF (> 10 000 kDa) in Weibel-Palade bodies (WPBs)15 and α-granules,16 respectively, for regulated release.3 Given the unusually large molecular size of these stored UL-VWF multimers, specialized molecular mechanisms exist that enable both compact storage of VWF and its ordered unfurling during secretion.17,18 In WPB, UL-VWF assembles into well-defined helical tubules,19 which align in parallel and often extend the entire length of the organelle. This organization not only allows packing of UL-VWF multimers into WPB that are 1-5 μm in length but also permits organized release of UL-VWF molecules.19,20 VWF exocytosis can occur from single WPB21 or from secretory pods that are formed through the coalescence of multiple WPB.17,22 UL-VWF released from WPB is hyperreactive compared with normal circulating plasma VWF because they can induce spontaneous micro-aggregate formation in blood. Although the exact mechanism underlying UL-VWF hyperreactivity is not fully understood, it is probable that this is a combination of its extraordinary length, the higher number of platelet and collagen binding sites,23 and its propensity to adopt an open, platelet-binding conformation.24 This latter point is exemplified by the observation that spontaneous high-strength bonds can be formed between UL-VWF and GPIb, whereas this does not occur using normal circulating plasma VWF multimers.24 Similarly, the nanobody, AU/VWF-a12, which specifically recognizes “active” or “open” A1 domains, only binds UL-VWF in solution and not normal plasma VWF.25 Given this hyperreactivity, UL-VWF is more potent in facilitating shear-induced platelet aggregation than plasma VWF.26 For this reason, UL-VWF multimers are potentially harmful in the normal circulation and must be processed into smaller less reactive molecules that will not precipitate unwanted platelet aggregation. The major enzyme that processes UL-VWF is ADAMTS13.27,28
ADAMTS13 is constitutively active in plasma, as it is expressed and secreted as an active enzyme. Unusually, therefore, VWF proteolysis by ADAMTS13 is regulated by conformational changes in the VWF substrate (rather than by activation/inhibition of the enzyme).29,30 The ADAMT13 cleavage site in VWF (Met1605-Tyr1606)31 is normally hidden within the middle of the folded A2 domain, whereas VWF is in its circulating globular conformation, making globular VWF essentially resistant to proteolysis by ADAMTS13.32,33 However, shear-induced unfolding of this domain not only exposes the scissile bond but also reveals multiple exosites that interact with different ADAMTS13 domains that dictate substrate specificity.34 After secretion from the endothelium, UL-VWF multimers spontaneously unfold in response to the high shear forces exerted by the flowing blood, enabling ADAMTS13 to both access and cleave the exposed A2 domains.29,35 As the VWF multimers that arise after proteolysis are smaller, they can adopt a folded conformation that does not spontaneously unravel in response to the normal circulating shear forces, which serves to conceal both ADAMTS13 cleavage and platelet binding sites.
When ADAMTS13 is deficient, UL-VWF multimers have the potential to accumulate in plasma, which can cause spontaneous platelet binding and agglutination. This can, in turn, precipitate TTP, a serious microangiopathic disorder in which platelet-rich microthrombi block arterioles and capillaries, leading to organ failure and death, if left untreated.36,37 Hereditary TTP is caused by homozygous or compound heterozygous mutations in the ADAMTS13 gene,27 although ADAMTS13 deficiency more commonly arises through acquired TTP involving the formation of inhibitory anti-ADAMTS13 autoantibodies.38
VWF strings
Interestingly, a proportion of UL-VWF multimers that are released from WPB (particularly in the absence of ADAMTS13 activity) remain attached to the surface of activated endothelial cells and can bind platelets.1,39 Dong et al showed that perfusion of stimulated HUVECs in vitro with washed platelets or with CHO cells expressing GPIb-IX-V resulted in the appearance of “beads-on-a string-like” structures that wave in the direction of flow1 (Figure 1A). These platelet-decorated strings were shown to be composed of VWF, as antibodies inhibiting the GPIb-VWF interaction specifically prevented visualization of any platelet binding and endothelial cells defective in VWF synthesis failed to form strings. Rather surprisingly, platelets attached firmly to these VWF strings,1 which is in contrast to the rolling behavior of platelets on VWF bound to collagen.9 VWF strings could be formed by various different endothelial cell lines, at both low and high shear stresses, and could reach exceptional lengths of up to 1 mm. Finally, these VWF strings could be rapidly cleaved after perfusion with ADAMTS13 in a process that could be inhibited by autoantibodies from acquired TTP patients.1 More recently, platelet-decorated VWF strings have been observed over the surface of VWF-expressing human embryonic kidney cells, indicating that the ability to form VWF strings is not endothelial cell specific.40
In vivo, platelet-decorated VWF strings can be observed transiently on the surface of the activated endothelium, but these are rapidly proteolytically removed by endogenous ADAMTS13 in WT mice.39,41 However, in mice with ADAMTS13 deficiency, the lifetime of these VWF strings is significantly prolonged (Figure 1B-C).41,42
How long are VWF strings?
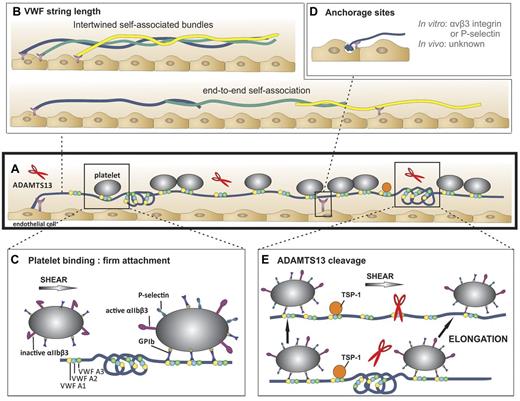
The length of VWF strings is remarkable. They are typically several 100 μm long but can reach extraordinary lengths of up to 1 mm.1 A detailed analysis of VWF string length in vitro revealed a median length of ∼ 100 μm, with 500 μm being the length of the longest strings observed.43 Hypothetically, a VWF tubule that extends the entire length of a 5 μm WPB would be predicted to be composed of ∼ 3700 VWF monomers (assuming a 2.7-nm length per VWF dimer in a helix).19 As each monomer spans ∼ 60 nm while in its extended conformation,44 a completely extended VWF multimer originating from a single 5-μm tubule should measure 220 μm in length. However, as VWF strings are still able to elongate further under flow conditions,43 this suggests that VWF strings are not necessarily fully extended on the endothelial surface. Therefore, based on these observations, strings with lengths > ∼ 200 μm must presumably be composed of different self-associated VWF multimers, rather than single VWF molecules (Figure 2A-B). Such VWF strings composed of different VWF multimers that have formed intertwined bundles through self-association have indeed been visualized by electron microscopy.45 Thus, extraordinarily long VWF strings as long as 1 mm could arise through end-to-end self-association of VWF multimers (Figure 2B bottom). Of particular note, as long strings form in the absence of VWF in the perfusate, it is probable that self-association occurs between VWF multimers secreted from WPB. However, when VWF is present in the perfusate, network-like VWF bundles can form that are thicker and longer than single VWF multimers.46-48 This process appears to be regulated by exposed thiol/disulfide exchange in VWF.46,47 In vivo, it is debatable whether extraordinarily long VWF strings actually form either through self-association, or by association of plasma multimers with VWF strings, as the length of the strings visualized in vivo range from 20 to 100 μm and VWF networks are not generally observed.42,49
VWF string characteristics. (A) A representative VWF string with VWF A1 (yellow circles), VWF A2 (blue circles), and with VWF A3 domains (green circles) in folded and unfolded parts of the VWF multimer (blue line). Attachment sites are represented by purple Y-shaped symbols. Platelets (gray circles) bind to open and accessible A1 domains. ADAMTS13 (red scissors) can digest unfolded VWF A2-domains. (B) VWF string length in vitro. Top: VWF multimers may consist of multiple self-associated multimers. Bottom: Occasionally, extraordinary long VWF strings (> 1 mm) are observed. Hence, these strings are probably formed by end-to-end self-association of different VWF multimers. (C) Platelet binding to VWF strings. Platelets bind to open VWF A1 domains (yellow circles) via their GPIb receptors. This binding does not result in a rolling movement of the platelets but in a firm adhesion to VWF strings. Bound platelets become activated as evidenced by the presence of P-selectin and activated αIIbβ3 on their surface.51 (D) Anchorage of VWF strings to endothelial cells. VWF strings are expelled from the WPB and remain anchored to the endothelial cells. P-selectin and αvβ3 have been identified as possible receptors involved in VWF string attachment in vitro, whereas these receptors do not seem to play a role in vivo. (E) ADAMTS13-mediated proteolysis of VWF strings. Top: ADAMTS13 (red scissors) mediated proteolysis of VWF strings is regulated by conformational changes in VWF and unfolding of its A2 domain (blue circle) induced by shear stress. Proteolysis occurs preferentially at sites of local elongations of VWF strings, which can be monitored by an increase in interplatelet distances (arrows).43 Bottom: ADAMTS13 cannot access the cleavage site in VWF A2 domains (blue circle) that are folded or cannot bind when TSP-1 (orange circle) interacts with the VWF A2-A3 domains.
VWF string characteristics. (A) A representative VWF string with VWF A1 (yellow circles), VWF A2 (blue circles), and with VWF A3 domains (green circles) in folded and unfolded parts of the VWF multimer (blue line). Attachment sites are represented by purple Y-shaped symbols. Platelets (gray circles) bind to open and accessible A1 domains. ADAMTS13 (red scissors) can digest unfolded VWF A2-domains. (B) VWF string length in vitro. Top: VWF multimers may consist of multiple self-associated multimers. Bottom: Occasionally, extraordinary long VWF strings (> 1 mm) are observed. Hence, these strings are probably formed by end-to-end self-association of different VWF multimers. (C) Platelet binding to VWF strings. Platelets bind to open VWF A1 domains (yellow circles) via their GPIb receptors. This binding does not result in a rolling movement of the platelets but in a firm adhesion to VWF strings. Bound platelets become activated as evidenced by the presence of P-selectin and activated αIIbβ3 on their surface.51 (D) Anchorage of VWF strings to endothelial cells. VWF strings are expelled from the WPB and remain anchored to the endothelial cells. P-selectin and αvβ3 have been identified as possible receptors involved in VWF string attachment in vitro, whereas these receptors do not seem to play a role in vivo. (E) ADAMTS13-mediated proteolysis of VWF strings. Top: ADAMTS13 (red scissors) mediated proteolysis of VWF strings is regulated by conformational changes in VWF and unfolding of its A2 domain (blue circle) induced by shear stress. Proteolysis occurs preferentially at sites of local elongations of VWF strings, which can be monitored by an increase in interplatelet distances (arrows).43 Bottom: ADAMTS13 cannot access the cleavage site in VWF A2 domains (blue circle) that are folded or cannot bind when TSP-1 (orange circle) interacts with the VWF A2-A3 domains.
Which factors regulate platelet binding to VWF strings?
A remarkable feature of VWF multimers anchored to endothelial cells is that they can rapidly recruit platelets from the circulation, which is in contrast to plasma VWF in which the platelet-binding sites are hidden by its folded globular conformation (Figure 1). Because VWF strings are derived from WPB-released UL-VWF, a logical explanation for the rapid VWF-platelet interactions that ensue is because of the presence of active A1 domains that, as mentioned previously, appear typical for UL-VWF. Further platelet binding sites could also become exposed in VWF through the effects of the shear stress, which is known to induce conformational changes in VWF and expose cryptic A1 domains.48,50
Once bound, platelets do not roll over VWF strings but, rather, remain firmly attached. Why the typical rolling behavior of platelets that is classically seen over VWF bound to collagen is not seen on VWF strings remains unclear. It is possible that the active A1 domains in UL-VWF strings are able to interact strongly with GPIb, allowing immediate arrest. Rolling of platelets over VWF surfaces is normally characterized by high association and dissociation rates and by an increase in bond life with increasing shear rates (catch bond).11 Therefore, perhaps in a manner analogous to type 2B VWF,11 the open conformation of A1 domains in VWF strings might result in longer bond lifetimes with GPIb, allowing engagement of other platelet receptors, such as activated αIIbβ3. Consistent with this hypothesis is the finding that platelets stably attached to VWF strings are activated, as evidenced by the exposure of both P-selectin and activated αIIbβ3 on their surface51 (Figure 2C right).
It appears that not all A1 domains present in VWF strings interact with platelets as the periodicity of the platelet-binding pattern on VWF strings is not consistent (Figure 1). Why platelets only bind to particular sites is not totally clear. It could be that not all A1 domains exist in the open conformation necessary for platelet binding or that some of the A1 domains remain hidden in certain sections of the VWF string (Figure 2C left). An alternative and interesting hypothesis is that a subset of A1 domains may be shielded by proteins that colocalize with VWF in the WPBs or that bind VWF in plasma. Osteoprotegerin,52,53 angiopoeitin,54 and galectins-1 and -355 all colocalize with VWF in WPBs and are known to bind and also remain associated with the VWF A1 domain or VWF strings after secretion from endothelial cells. However, to date, none of these proteins has been shown to interfere with platelet binding to VWF strings, as inhibition experiments were either negative or are yet to be performed. Conversely, the plasma protein β2-glycoprotein I both binds to open VWF A1 domains and also inhibits GPIb binding, which could theoretically modulate availability of platelet binding sites in VWF strings.56 However, it is yet to be demonstrated that β2-glycoprotein I can actually interact with VWF strings.
Accessibility or reactivity of A1 domains in VWF strings varies greatly within a single string as well as between different strings. Curiously, not all VWF strings released from endothelial cells bind platelets, suggesting that some strings have no accessible and/or open A1 domains.45 Why platelet-free and platelet-decorated VWF strings exist remains to be determined.
How are VWF strings anchored to endothelial cells?
Anchorage of VWF strings to endothelial cells appears to be mediated by just a few adhesion sites per string. It is this that allows (parts of) the string to wave in the direction of flow (Figure 2A,D).1,45 Specific contact points between VWF strings and the endothelial cell membrane have been visualized directly by electron microscopy predominantly at the upstream terminus of the string but also at sites along the length of the string.45 Unambiguous identification of the endothelial receptor or proteins involved in anchorage of VWF strings has proven difficult (Figure 2D) and appears to depend on experimental conditions and the species examined. In vitro, either P-selectin or αvβ3 was identified as receptors controlling VWF string attachment to endothelial cells depending on whether Ca2+ and Mg2+ are absent or present in the perfusate, respectively (Figure 2A,D).45,57 The mechanism(s) of string attachment to endothelial cells in vivo seems to be different. Neither P-selectin or αvβ3 are involved in string anchoring to stimulated veins in mice as the numbers and lengths of VWF strings in P-selectin or αvβ3 deficient mice do not differ from WT mice.58 In addition, although VWF strings can be generated in vitro over human endothelial cells under both venous and arterial flow conditions,1,45 they are only observed in the venous vasculature in mice.41,42,59 Whether this might be explained by the involvement of different attachment molecules in vitro and in vivo remains unclear.45 It must also be considered that differences in VWF expression and WPB biology in arterial and venous endothelial cells could also play an important role.
How is proteolysis of VWF strings by ADAMTS13 regulated?
VWF strings are rapidly removed in the presence of ADAMTS13. Similar to the GPIb binding sites in the A1 domain, ADAMTS13 cleavage site in the VWF A2 domains is also cryptic in normal circulating plasma VWF. As VWF strings are rapidly cleaved by ADAMTS13 both in vitro and in vivo, the scissile bond in the VWF A2 domain must therefore rapidly become exposed on VWF secretion (Figure 2A,E top). Unfolding of the A2 domain and exposure of the cleavage site are probably induced by shear forces exerted on the VWF string (Figure 2E top).60,61 Indeed, we recently demonstrated that platelet-decorated VWF strings are preferentially truncated under shear stress by ADAMTS13 specifically at sites of local elongation (Figure 2E). Such elongation reveals both ADAMTS13 binding exosites as well as the scissile bond in the VWF A2 domains, which permits ADAMTS13-mediated cleavage.43 When studied in vitro and in the absence of shear, expulsion of VWF from endothelial WPB alone is sufficient to expose ADAMTS13 cleavage sites, implying that VWF exocytosis can cause conformational changes in VWF A2 domains that permit proteolysis even in the absence of flow.62 Platelet-decorated and platelet-free VWF strings are cleaved by ADAMTS13 at similar rates, suggesting that platelet binding has little influence on proteolysis.45 This is in contrast to platelet binding to plasma VWF, which appreciably enhances ADAMTS13-mediated proteolysis under shear.61
Thrombospondin-1 (TSP-1; Figure 2E), a multidomain protein that interacts with a wide variety of receptors, integrins, and extracellular matrix proteins also seems to regulate cleavage of strings by ADAMTS13.63 In TSP-1–deficient (Thbs1−/−) mice, fewer VWF strings were observed compared with WT mice. Moreover, string formation could be increased by infusion of TSP-1 in Thbs1−/− mice. The inhibitory effect of TSP-1 may be explained by competition between TSP-1 and ADAMTS13 for the same binding sites in the VWF A2 and A3 domains.63,64
Does VWF string proteolysis generate the smaller VWF multimers found in plasma?
Platelet-decorated VWF strings anchored to endothelial cells are proteolytically released by ADAMTS13 in a limited number of steps (up to 8, but with a median of 3 cleavage events), and at sites randomly distributed through the VWF string.43 Based on these findings, the cleaved VWF fragments are still considered UL when released into the circulation,43,65 as their extended length ranges from 5 to > 100 μm.43 The fate of these circulating platelet-UL-VWF complexes is unknown. They could be further digested by ADAMTS13, as ADAMTS13 cleavage sites are likely to be rapidly exposed in these circulating platelet-UL-VWF multimers.35,61 Alternatively, because of their prothrombotic nature, they might be preferentially cleared from circulation via an as yet unidentified mechanism.
Do other proteases digest VWF strings?
In vitro, plasma VWF can be digested by proteases other than ADAMTS13, including leukocyte proteases proteinase 3, cathepsin G, elastase, and granzyme B.66-70 Susceptibility to cleavage is also influenced by conformational changes in VWF.69,70 The cleavage sites for proteinase 3, cathepsin G, and elastase are situated at or near the ADAMTS13 cleavage site,69 whereas sites for granzyme B70 are located in the A1 domain and at the A2 and A3 domain boundary. The presence of proteolytic fragments of VWF in Adamts13−/− mice further supports the notion that ADAMTS13-independent processes also regulate VWF size.71 That said, the (patho)physiologic role of these proteases remains questionable because VWF fragments are still observed in neutropenic patients and after depletion of neutrophils in Adamts13−/− mice.71 Moreover, proteolysis of VWF strings by enzymes other than ADAMTS13 has only been reported for granzyme B,70 and used concentrations were appreciably higher than those likely to be found in plasma. Whether proteases, such as proteinase 3, cathepsin G, elastase, and granzyme B, are relevant for VWF string proteolysis in vivo, perhaps after local accumulation at sites of inflammation remains to be studied.
What is the physiologic role of VWF strings?
Although our understanding of VWF strings increased appreciably in recent years, many important questions remain unanswered. Perhaps the biggest question centers around whether VWF strings attached to endothelial cells play a specific functional role in (patho)physiologic processes. We discuss the consequences of VWF string-blood cell interactions in different pathologic settings in which interactions between the activated endothelium and blood cells have been identified, and the potential relevance of such interactions in the vascular system in the presence/absence of ADAMTS13.
A role of VWF strings in disease pathology seems likely when ADAMTS13 activity is absent (eg, in TTP). However, although persisting platelet-decorated VWF strings are observed on the surface of activated microvenules in Adamts13−/− mice where they can promote thrombus formation, these VWF strings are not observed in Adamts13−/− arterioles.39,41,42,59 As arterial, rather than venous, thrombi contribute to the pathology of TTP, it might seem unlikely that VWF strings are responsible for TTP-associated microangiopathy. This is in stark contrast with the role of circulating UL-VWF in TTP, which can spontaneously interact with platelets and therefore precipitate the formation of microthrombi that block arterioles and capillaries in humans,36,37 mice41,72 and baboons,73 causing organ failure and death.
Although the role of VWF and UL-VWF has been clearly demonstrated in arterial thrombosis,74 the absence of platelet-decorated VWF strings in damaged arterioles of Adamts13−/− mice41,42,59 suggests that VWF strings, per se, might not contribute to arterial thrombosis, at least in mice. Recently, it was demonstrated in a mouse model that deep vein thrombosis is dependent on VWF.75 Flow restriction in the inferior vena cava induced VWF-dependent platelet adhesion and thrombus formation, but a specific role for VWF strings in this model was not examined.
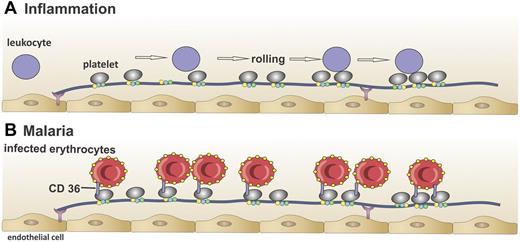
VWF strings have been suggested to contribute to inflammation. In vitro experiments have shown that, in the absence of ADAMTS13, platelet-decorated VWF strings can slow down leukocyte rolling on endothelial cells (Figure 3A).51 ADAMTS13 deficiency also increases basal leukocyte rolling in vivo and promotes leukocyte adhesion and extravasation under inflammatory conditions. This proinflammatory effect is dependent on VWF and platelets, as almost no leukocyte tethering was observed in VWF−/− mice or when platelets were absent.76 Accordingly, a strong, protective anti-inflammatory effect of ADAMTS13 was recently observed in a mouse model of acute myocardial infarction.77,78 A plausible mechanism for this is that ADAMTS13 attenuates inflammatory processes by removing the leukocyte-capturing VWF strings from the endothelial surface. Importantly, though, ADAMTS13 levels tend (if anything) to be decreased, rather than absent, in thrombotic79 and inflammatory diseases.80-84 Therefore, whether partially reduced ADAMTS13 levels are sufficient to enable platelet-decorated VWF strings to survive long enough to mediate inflammatory diseases remains a key unanswered question.
Interaction of platelet-decorated VWF strings with leukocytes and infected red blood cells in vitro. (A) Leukocytes (purple circles) interact with platelet-decorated VWF strings in vitro in the absence of ADAMTS13,51 which results in rolling of leukocytes over the VWF string, suggesting that VWF strings in the absence of ADAMTS13 can contribute to inflammation. (B) Red blood cells infected with the malaria parasite P falciparum (red circles) adhere to platelet-decorated VWF strings via the platelet receptor CD36 in vitro in the absence of ADAMTS13.85 This mechanism might explain binding of infected red blood cells to the brain endothelium in cerebral malaria.
Interaction of platelet-decorated VWF strings with leukocytes and infected red blood cells in vitro. (A) Leukocytes (purple circles) interact with platelet-decorated VWF strings in vitro in the absence of ADAMTS13,51 which results in rolling of leukocytes over the VWF string, suggesting that VWF strings in the absence of ADAMTS13 can contribute to inflammation. (B) Red blood cells infected with the malaria parasite P falciparum (red circles) adhere to platelet-decorated VWF strings via the platelet receptor CD36 in vitro in the absence of ADAMTS13.85 This mechanism might explain binding of infected red blood cells to the brain endothelium in cerebral malaria.
In cerebral malaria, Plasmodium falciparum–infected erythrocytes adhere to the activated endothelium, which leads to their sequestration in the capillaries and postcapillary venules of the brain. Adherence to the vascular endothelium is normally considered to occur via an interaction between the parasite-encoded protein, P falciparum erythrocyte membrane protein 1, and the endothelial counter-receptor CD36.85 However, CD36 is not expressed at high levels in the brain microcirculation, suggesting that adhesion might occur via a different mechanism. Bridges et al elegantly demonstrated that platelet-decorated VWF strings could potentially play an important role in the adhesion of infected erythrocytes to endothelial cells lacking CD36.86 In their in vitro model, platelets bound to the VWF strings provided the CD36 receptors required for infected erythrocyte binding to brain endothelium (Figure 3B). In this way, complexes of red blood cells, platelets, and VWF strings attached to the endothelium might contribute to parasite sequestration and the associated microangiopathy. Again, although in malaria levels of VWF and UL-VWF are increased87,88 and plasma ADAMTS13 activity is often reduced,89 it remains to be determined whether string survival time in these patients is long enough to support this mechanism.
Plasma UL-VWF also accumulates in sickle cell disease (SCD) patients.90,91 As UL-VWF can bind spontaneously both to platelets and to sickle red blood cells, it may have the propensity to aggravate clinical manifestations of SCD, such as aggregate formation and red blood cell lysis.92-94 VWF-dependent sickle cell adhesion to stimulated rat venous endothelium, followed by vaso-obstruction, has been reported.93 However, these experiments were once again performed in the absence of ADAMTS13. It is possible that the inhibitory effect of elevated free plasma hemoglobin on ADAMTS13-mediated proteolysis of UL-VWF and VWF strings in SCD patients95,96 might be sufficient to prolong the lifetime of VWF strings long enough to induce vaso-occlusion, but specific analyses that address this contention have not yet been performed. Moreover, it should be considered that ADAMST13 activity is not decreased in SCD patients90 and also the direct binding of sickle cells to VWF strings has not been demonstrated as yet, allowing only speculation to a pathophysiologic role of VWF strings in SCD.
In conclusion, VWF strings remain bound to the surface of endothelial cells in the absence of ADAMTS13, and it is clear that they have intriguing characteristics that distinguish them from normal circulating plasma VWF molecules. Extraordinarily long VWF strings can be observed in vitro, although they have not yet been detected in vivo. Although VWF strings have open A1 domains, potentially allowing firm platelet adhesion, such strings rapidly disappear under normal physiologic conditions via cleavage by ADAMTS13. This seems a logical protective process as these strings are probably prothrombotic in nature. The fate of these platelet-decorated VWF strings entering circulation remains to be determined, but a rapid clearance from the circulation might be expected. Elucidation of unsolved questions (eg, why some of the strings bind platelets and others do not, why VWF strings persist under arterial conditions in vitro but not in vivo, and how VWF strings are anchored to endothelial cells) will further increase our understanding of the unique characteristics of VWF strings. More studies are also needed to unravel the role of VWF strings in different disease pathologies. Although VWF strings are long-lived in Adamts13−/− mice on stimulation of endothelium, it is currently not known whether strings survive long enough in the presence of ADAMTS13 to participate in processes, such as inflammatory leukocyte adhesion or malaria parasite sequestration.
Since their discovery, explaining the composition, characteristics, and relevance of VWF strings has been a puzzling process. Future research that provides more definitive answers into their biochemical characteristics, and (patho)physiologic roles will undoubtedly add to our understanding of these intriguing features of the vascular system.
Acknowledgments
The authors thank Louis Deforche (IWT 111507) for help with preparation of the figures.
K.D.C. was supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (PhD grant 83328, IWT Vlaanderen). S.F.D.M. was supported by the Research Foundation Flanders (Fonds voor Wetenschappelijk Onderzoek Vlaanderen; postdoctoral fellowship). Publication and part of the work described here were supported by Fonds voor Wetenschappelijk Onderzoek Vlaanderen (grant G.0607.09).
Authorship
Contribution: K.D.C., S.F.D.M., and K.V. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karen Vanhoorelbeke, Laboratory for Thrombosis Research, IRF Life Sciences, KU Leuven, KULAK, E Sabbelaan 53, B-8500 Kortrijk, Belgium; e-mail: karen.vanhoorelbeke@kuleuven-kulak.be.