Key Points
Blockade of VWF-A1 by ALX-0081 induces reperfusion of a thrombus-occluded middle cerebral artery without provoking cerebral bleeding.
The interaction between GPIb and VWF is not only essential for platelet adhesion but also for initial thrombus stabilization.
Abstract
Thrombolytic therapy is the cornerstone of treatment of acute atherothrombotic ischemic stroke but is associated with brain hemorrhage; antiplatelet therapy has limited efficacy and is still associated with intracranial bleeding. Therefore, new antithrombotic approaches with a better efficacy/safety ratio are required. We have assessed the effect of ALX-0081, a Nanobody against the A1 domain of von Willebrand factor (VWF) that blocks VWF binding to GPIb, of the thrombolytic agent recombinant tissue plasminogen activator (rtPA), and of the GPIIb/IIIa antagonist tirofiban, in a middle cerebral artery (MCA) thrombosis model in guinea pigs. Drugs were administered before, immediately after, or 15 or 60 minutes after the total occlusion of the MCA. ALX-0081 prevented MCA thrombosis and induced reperfusion when given immediately after and 15 minutes after complete occlusion and reduced brain damage without inducing hemorrhage, whereas tirofiban prevented thrombosis but did not induce reperfusion and induced striking brain hemorrhage. rtPA also induced reperfusion when given 60 minutes after occlusion but provoked brain hemorrhage. Skin bleeding time was not modified or was moderately prolonged by ALX-0081, whereas tirofiban and rtPA prolonged it. The inhibition of the GPIb–VWF axis in guinea pigs prevents cerebral artery thrombosis and induces early reperfusion without provoking intracerebral bleeding thus reducing brain infarct area.
Introduction
Platelet adhesion to a damaged blood vessel is the initial trigger for thrombosis. When subendothelium is exposed to flowing blood, platelets rapidly adhere to the surface and arrest and aggregate to stop bleeding. In vessels with high flow, such as arteries, the interaction between subendothelium-attached von Willebrand factor (VWF) and its main platelet receptor glycoprotein (GP) Ib-IX-V is required to slow down platelets and allow their α2β1 and GP VI receptors to bind collagen, resulting in GPIIb/IIIa activation, fibrinogen binding, and platelet aggregation.1,2 Inhibition of platelet activation is a crucial strategy for the control of ischemic cardiovascular disease,3-5 but a limitation of currently used platelet inhibitors is that they not only prevent thrombosis in injured arteries but they also prevent normal hemostasis, thus increasing bleeding.6 An increased bleeding risk is particularly ominous when treating ischemic cerebrovascular disease because of the harmful consequences of intracranial hemorrhage;7 therefore, ischemic cerebrovascular disorders represent a clinical setting in which an antiplatelet regimen with a lower bleeding risk would be of special value.8,9
Antiplatelet therapy with aspirin, dypiridamole, clopidogrel, or their combination is the mainstay for secondary prevention of stroke, but its effectiveness for acute stroke is limited and is associated with increased intracranial bleeding.10 Intravenous GPIIb/IIIa inhibitors, effective in acute coronary syndromes,11 have given disappointing results in acute ischemic stroke: a recent phase 3 trial with abciximab was stopped prematurely because of increased intracranial hemorrhage and mortality and lack of efficacy.12 Therefore GPIIb/IIIa inhibitors cannot be recommended for acute stroke.13
The thrombolytic agent recombinant tissue plasminogen activator (rtPA), given within 3 to 4.5 hours from the beginning of symptoms, is currently the only antithrombotic therapy for acute ischemic stroke approved by the US Food and Drug Administration.14,15 Treatment with rtPA improves clinical outcome15 but at the cost of increased intracranial hemorrhage.15-17
Several observations suggest that VWF has a special pathogenic role in ischemic stroke.18 In the prospective Rotterdam Study, the risk of ischemic stroke increased with rising levels of VWF in plasma,19 and in the case-control SAHLSIS Study, VWF levels were found to be increased in ischemic stroke, both in the acute phase and 3 months after the event.20
The inhibition of the GPIb-IX-V–vWF interaction may theoretically prevent pathologic thrombosis but may not have much influence on physiologic hemostasis, given the preeminent role of GPIb–VWF in platelet activation under high shear conditions21 and may thus represent a particularly promising target for the treatment of ischemic stroke.
Recently, rADAMTS13 was shown to act as a thrombolytic drug in vivo. Similar to application of rtPA, application of rADAMTS13 onto thrombus-occluded dorsal skinfold chamber venules induced reperfusion and dramatically increased the rate of recanalization within the first hour following treatment, suggesting that early thrombolysis can be attained by blocking the activity of VWF.22
ALX-0081 is a bivalent construct consisting of two identical humanized Nanobodies directed against the A1 domain of VWF that specifically inhibits the interaction between human platelet GPIb and VWF under high shear conditions (ie, those observed in stenotic arteries). ALX-0081 was shown to prevent arterial thrombosis in a Folts femoral artery model in baboons and to reverse thrombocytopenia and microangiopathic anemia in a baboon model of thrombotic thrombocytopenic purpura without concomitantly increasing bleeding; it also showed a broader therapeutic window compared with clopidogrel or abciximab.23,24
The aim of this study was to evaluate the ability of ALX-0081 to prevent and/or to reperfuse middle cerebral artery (MCA) thrombosis in guinea pigs compared with that of the thrombolytic agent rtPA and of the GPIIb/IIIa inhibitor tirofiban and to evaluate their safety.
Materials and methods
Drugs
Nanobodies against VWF were isolated from a llama immunized with recombinant human VWF A1 domain and subsequently humanized, resulting in the monovalent VWF-binding Nanobody PMP12A2h1. ALX-0081 (28 kDa) consists of 2 PMP12A2h1 building blocks linked to each other by a 3-alanine linker.23 ALX-0081 was diluted in vehicle buffer (200 mg/L KCl, 509 mg/L KH3PO4, 8 g/L NaCl, 1750 mg/L Na2HPO4, 0.2 M glycine, 0.05% Tween-80 [pH 7.1]) to the appropriate concentrations. Recombinant human ADAMTS13 (rhADAMTS13) was purchased from R&D Systems (Minneapolis, MN) and diluted in phosphate-buffered saline (PBS). rtPA was purchased from Boehringer Ingelheim, Ingelheim, Germany; tirofiban (Aggrastat) was purchased from Merck, Darmstad, Germany.
Pharmacokinetic/pharmacodynamic studies
Male Dunkin-Hartley guinea pigs (Charles River Laboratories Italia; Calco, Como, Italy), weighing between 350 and 400 g, were anesthetized with ketamine (50 mg/kg intraperitoneally) and xylazine (5 mg/kg intraperitoneally), and a heparinized catheter (PE50, Intramedic Polyethylene Tubing; Beckton Dickinson, Franklin Lakes, NJ) was inserted into the carotid artery for blood sampling.
ALX-0081 was administered as an intravenous bolus followed by two subcutaneous injections using these 3 dosing regimens: (1) 0.1 mg/kg intravenously + 0.4 mg/kg subcutaneously at time 0 followed by 0.75 mg/kg subcutaneously 6 hours later (1.25 mg/kg in total); (2) 0.2 mg/kg intravenously + 0.8 mg/kg subcutaneously at time 0 followed by 1.5 mg/kg subcutaneously 6 hours later (2.5 mg/kg in total); or (3) 0.4 mg/kg intravenously + 1.6 mg/kg subcutaneously at time 0 followed by 3 mg/kg subcutaneously 6 hours later (5 mg/kg in total).
ALX-0081 plasma concentration was determined by an enzyme-linked immunosorbent assay that used a biotinylated bivalent anti–ALX-0081 Nanobody captured onto a neutravidin-coated microtiter plate. Test samples were diluted in assay diluent supplemented with 10% guinea pig plasma and added to the coated plate. Complexes between ALX-0081 and VWF in the test samples were detected by using a rabbit anti-guinea pig VWF antibody, and secondary detection was carried out by using a goat anti-rabbit horseradish peroxidase–labeled antibody and revealed by a 3,3′,5,5′-tetramethylbenzidine substrate. The lower limit was 30 ng/mL and the upper limit was 350 ng/mL.
Ristocetin Cofactor (RiCof) activity assays were performed by using a Chronolog 4-channel aggregometer (Havertown, PA), with Aggrolink software for data analysis. Analyses were performed at 37°C at a constant stirring speed of 1200 rpm, as described.25
Binding of VWF to guinea pig platelets induced by ristocetin as well as binding of fibrinogen to platelets stimulated with adenosine diphosphate was assessed by flow cytometry, as previously reported25-27 (described in supplemental Methods).
Template bleeding time
The skin template bleeding time was measured as previously described,28 at baseline and 30 minutes after drug administration in animals kept in a 21°C constant temperature room. Briefly, a standardized cut was inflicted to the ventral surface of the foot of anesthetized guinea pigs by using a template (Surgicutt; ITC, Edison, NJ). The blood emerging from the cut was blotted every 15 seconds with filter paper until the arrest of bleeding, and the total bleeding time was annotated.
Platelet count
Platelet adhesion to a collagen-coated surface under flow
Stroke models
MCA thrombosis was photochemically induced as previously reported27 (described in supplemental Methods). In some experiments, transient MCA occlusion was mechanically induced (described in supplemental Methods).
Treatment protocols
Drugs were administered immediately before the induction of damage (preinjury protocol) or at various intervals after the complete thrombotic occlusion of the MCA (postinjury protocols): at the moment of the total occlusion (immediate postinjury protocol), 15 minutes after the total occlusion (early postinjury protocol), or 60 minutes after the total occlusion (delayed postinjury protocol).
For ALX-0081, a dosing regimen that gave sustained inhibition of RiCof for 24 hours without the need to use infusion pumps was devised on the basis of the results of a previous pharmacokinetic (PK)/pharmacodynamics (PD) study in guinea pigs. ALX-0081 was administered at 5 mg/kg total dose (0.4 mg/kg intravenously + 1.6 mg/kg subcutaneously at time 0 followed by 3 mg/kg subcutaneously 6 hours later) or 8.5 mg/kg total dose (0.7 mg/kg intravenously + 2.8 mg/kg subcutaneously at time 0 and 5 mg/kg subcutaneously 6 hours later).
The GPIIb/IIIa receptor antagonist tirofiban (Merck) was used as a comparator at a dose of 20 µg/kg intravenous bolus + 20 µg/kg intravenously infused over 2 hours.32
The thrombolytic agent rtPA was used at two regimens: 0.032 mg/kg intravenously (bolus) + 0.576 mg/kg intravenously (infusion over 30 minutes) and 0.1 mg/kg intravenously (bolus) + 0.9 mg/kg intravenously (infusion over 30 minutes).33 rhADAMTS13 was administered intravenously at 4 µg/kg.34 Control animals were administered the same volume of vehicle buffer at the same time intervals as the tested drugs.
Combinations of ALX-0081 (0.4 mg/kg intravenous bolus + 1.6 mg/kg subcutaneously at time 0 and 3 mg/kg subcutaneously 6 hours later) and rtPA (0.032 mg/kg intravenous bolus + 0.576 mg/kg as an intravenous infusion over 30 minutes) and combinations of ALX-0081 (0.4 mg/kg intravenous bolus + 1.6 mg/kg subcutaneously at time 0 and 3 mg/kg subcutaneously 6 hours later) and rhADAMTS13 (1µg intravenous bolus at time 0) were also studied in a postinjury setting.
Brain damage
Guinea pigs were euthanized 24 hours after the end of photoirradiation by using an overdose of anesthetic. Brains were removed after 10 minutes of cardiac perfusion with PBS and stained with 1% 2,3,5-triphenyltetrazolium chloride (Sigma, Milan, Italy) to visualize the damaged area (described in supplemental Methods).
In some experiments, brains were removed after 10-minute perfusion with 4% paraformaldehyde in PBS, embedded in paraffin, cut in 4-µm sections with a microtome, and stained to visualize the presence of thrombus-occluded brain vessels35 (described in supplemental Methods). To evaluate brain hemorrhage, hemoglobin (Hb) content in brain tissue was measured by using the Drabkin method36 (described in supplemental Methods).
All animal studies were approved by the Committee on Ethics of Animal Experiments of the University of Perugia and by the Italian Ministry of Public Health (Authorization No. 231/2010).
Blood clotting tests
Guinea pig blood was drawn from the jugular vein 24 hours after treatment in sodium citrate 3.8% (1:10 [v/v]) and centrifuged at 1000 × g for 15 minutes at room temperature; the supernatant plasma was stored at −80°C until assay. Activated partial thromboplastin time (aPTT), prothrombin time (PT), and fibrinogen levels were determined by light scattering photometry (Koagulab MJ; Ortho-Clinical Diagnostics, Milan, Italy) by using reagents from Instrumentation Laboratory (Milan, Italy).35 VWF antigen was evaluated by enzyme-linked immunosorbent assay (Asserachrom VWF:Ag; Diagnostica Stago, Milan, Italy).
Statistical analysis
Data were analyzed by one-way analysis of variance, followed by the Newman-Keuls multiple comparison test between all groups. All analyses were performed by GraphPad Prism 4.0 for Windows software (GraphPad Software, San Diego, CA; www.graphpad.com). Data are expressed as arithmetic means ± standard deviation. A P value of less than .05 was considered statistically significant.
Results
PK/PD
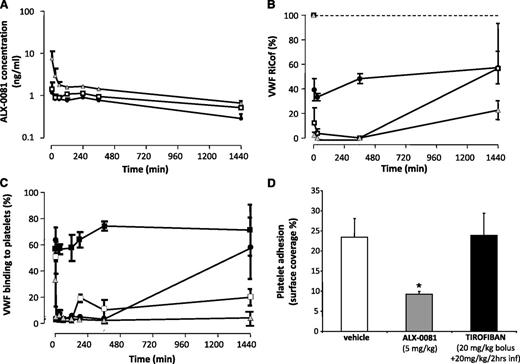
The plasma concentration-time profile after ALX-0081 administration is shown in Figure 1A. In the highest dose group, PK show an initial phase characterized by a fast decline of total ALX-0081 plasma levels, reflecting the fast renal clearance of free ALX-0081, followed by a second slow decline, reflecting the clearance of ALX-0081 bound to VWF. VWF–RiCof activity was almost totally suppressed 5 minutes after the administration of ALX-0081 at all doses, and complete inhibition was sustained for up to 6 hours. An 80% inhibition was still evident at 24 hours with the highest dose (Figure 1B). The binding of vWF to platelets induced by ristocetin was completely suppressed immediately after ALX-0081 administration, and it remained suppressed for 24 hours with the highest dose (Figure 1C). VWF antigen and aPTT were unaffected by ALX-0081 administration (Table 1).
PK/PD studies. (A) ALX-0081 plasma concentrations. (B) VWF-RiCof activity. (C) Ristocetin-induced vWF binding to platelets as assessed by flow cytometry. ALX-0081 doses: (●) 1.25 mg/kg; (□) 2.5 mg/kg; (shaded triangle) 5 mg/kg; (▪) vehicle. (D) Platelet adhesion on a collagen-coated surface under high shear stress (3000 sec−1) expressed as percentage of the surface covered by platelets. *P < .05 vs vehicle and tirofiban; n = 5 per group.
PK/PD studies. (A) ALX-0081 plasma concentrations. (B) VWF-RiCof activity. (C) Ristocetin-induced vWF binding to platelets as assessed by flow cytometry. ALX-0081 doses: (●) 1.25 mg/kg; (□) 2.5 mg/kg; (shaded triangle) 5 mg/kg; (▪) vehicle. (D) Platelet adhesion on a collagen-coated surface under high shear stress (3000 sec−1) expressed as percentage of the surface covered by platelets. *P < .05 vs vehicle and tirofiban; n = 5 per group.
Blood clotting parameters
| Assay . | vehicle . | ALX-0081 (5 mg/kg) . | rtPA Low dose . | ALX-0081+rtPA . |
|---|---|---|---|---|
| Activated partial thromboplastin time (sec) | 31.5 ± 11.9 | 31.8 ± 4.4 | 35.9 ± 7.2 | 33.0 ± 6.3 |
| Prothrombin time (sec) | 30.9 ± 1.1 | 32.7 ± 5.1 | 31.5 ± 4.6 | 32.1 ± 3.7 |
| Fibrinogen level (mg/dl) | 108.5 ± 12.9 | ND | 119.5 ± 15.7 | 121.3 ± 13.7 |
| VWF Ag (%) | ||||
| Time (min) | ||||
| 0 (Baseline) | 35.0 ± 4.7 | |||
| 5 | 30.0 ± 0.96 | |||
| 30 | 33.4 ± 9.4 | |||
| 60 | 38.9 ± 9.9 | |||
| 1440 | 41.8 ± 2.6 |
| Assay . | vehicle . | ALX-0081 (5 mg/kg) . | rtPA Low dose . | ALX-0081+rtPA . |
|---|---|---|---|---|
| Activated partial thromboplastin time (sec) | 31.5 ± 11.9 | 31.8 ± 4.4 | 35.9 ± 7.2 | 33.0 ± 6.3 |
| Prothrombin time (sec) | 30.9 ± 1.1 | 32.7 ± 5.1 | 31.5 ± 4.6 | 32.1 ± 3.7 |
| Fibrinogen level (mg/dl) | 108.5 ± 12.9 | ND | 119.5 ± 15.7 | 121.3 ± 13.7 |
| VWF Ag (%) | ||||
| Time (min) | ||||
| 0 (Baseline) | 35.0 ± 4.7 | |||
| 5 | 30.0 ± 0.96 | |||
| 30 | 33.4 ± 9.4 | |||
| 60 | 38.9 ± 9.9 | |||
| 1440 | 41.8 ± 2.6 |
Data represents means ± SD (n=5).
ND, not determined.
Platelet adhesion to a collagen-coated surface at high shear rate (3000 sec−1) was strongly inhibited in samples taken 24 hours after ALX-0081 administration, but it was unaffected in animals treated with tirofiban (Figure 1D). Treatment with rtPA did not modify aPTT, PT, or fibrinogen compared with vehicle (Table 1).
MCA thrombosis
Preinjury protocol
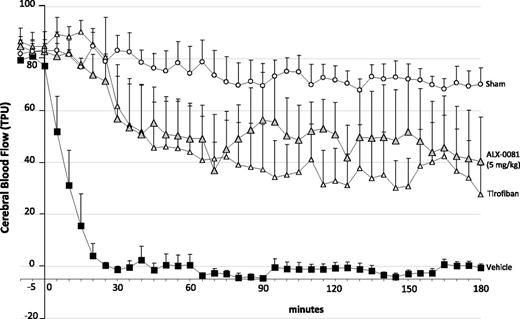
Photochemical damage led to complete occlusion of the MCA in 19 ± 6 minutes. ALX-0081 administered before injury completely prevented the occlusion of the MCA, and GPIIb/IIIa blockade by tirofiban also completely prevented the occlusion of the MCA (Figure 2).
Cerebral blood flow continuously recorded after photochemically induced MCA damage: effect of the different drugs administered immediately before photochemical damage. (Shaded triangle) ALX-0081 5 mg/kg, (△) tirofiban (20 µg/kg bolus + 20 µg/kg over a 2-hour infusion), (▪) vehicle, (○) sham guinea pigs (animals that underwent MCA transillumination but without Rose bengal infusion). n = 5 per group (n = 3 in sham group).
Cerebral blood flow continuously recorded after photochemically induced MCA damage: effect of the different drugs administered immediately before photochemical damage. (Shaded triangle) ALX-0081 5 mg/kg, (△) tirofiban (20 µg/kg bolus + 20 µg/kg over a 2-hour infusion), (▪) vehicle, (○) sham guinea pigs (animals that underwent MCA transillumination but without Rose bengal infusion). n = 5 per group (n = 3 in sham group).
Postinjury protocols
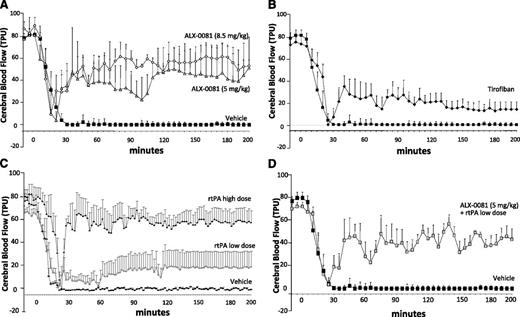
When ALX-0081 was administered immediately after the complete occlusion of MCA (immediate postinjury protocol), reperfusion was observed; this was rapid and almost complete with the high dose (8.5 mg/kg total dose), with blood flow returning to ∼72% of baseline, and somewhat less complete with the low dose (5 mg/kg total dose), with blood flow returning to ∼50% of baseline (Figure 3A). Tirofiban administered immediately after complete occlusion induced only minimal reperfusion (Figure 3B). On the contrary, rtPA administered immediately after complete occlusion induced a rapid and complete reperfusion at the highest dose tested, whereas it was almost inactive at the lowest dose (Figure 3C). The combination of a low rtPA dose (0.032 mg/kg bolus + 0.576 mg/kg over 30 minutes of infusion) with ALX-0081 (5 mg/kg total dose) induced reperfusion to the same extent as ALX-0081 alone, with blood flow returning to ∼49% of baseline (Figure 3D).
Cerebral blood flow continuously recorded after photochemically induced MCA damage: effect of the different drugs administered immediately after the induction of damage. (A) ALX-0081 5 mg/kg (shaded triangle) and 8.5 mg/kg (♢); (B) tirofiban 20 µg/kg bolus + 20 µg/kg over a 2-hour infusion (♦); (C) rtPA low dose of 0.032 mg intravenous bolus + 0.576 mg/kg over 30 minutes (○) and high dose of 0.1 mg intravenous bolus + 0.9 mg/kg over 30 minutes (●); (D) ALX-0081 5 mg/kg plus low-dose rtPA at 0.032 mg intravenous bolus + 0.576 mg/kg over 30 minutes (shaded square). (A-D) Vehicle (▪). Area over the curve (AOC) has been calculated for each treatment by using GraphPad Prism 4.0 for Windows software. (A) AOC: control = 14 487 ± 509; ALX-0081 5 mg/kg = 7445 ± 1216; ALX-0081 8.5 mg/kg = 4048 ± 542. (B) AOC: control = 14 487 ± 509; tirofiban = 10 507 ± 805. (C) AOC: control = 14 487 ± 509; rtPA low dose = 12 242 ± 1776; rtPA high dose = 5251 ± 949. (D) AOC: control = 14 487 ± 509; ALX-0081 + rtPA low dose = 7285 ± 1272. n = 5 per group.
Cerebral blood flow continuously recorded after photochemically induced MCA damage: effect of the different drugs administered immediately after the induction of damage. (A) ALX-0081 5 mg/kg (shaded triangle) and 8.5 mg/kg (♢); (B) tirofiban 20 µg/kg bolus + 20 µg/kg over a 2-hour infusion (♦); (C) rtPA low dose of 0.032 mg intravenous bolus + 0.576 mg/kg over 30 minutes (○) and high dose of 0.1 mg intravenous bolus + 0.9 mg/kg over 30 minutes (●); (D) ALX-0081 5 mg/kg plus low-dose rtPA at 0.032 mg intravenous bolus + 0.576 mg/kg over 30 minutes (shaded square). (A-D) Vehicle (▪). Area over the curve (AOC) has been calculated for each treatment by using GraphPad Prism 4.0 for Windows software. (A) AOC: control = 14 487 ± 509; ALX-0081 5 mg/kg = 7445 ± 1216; ALX-0081 8.5 mg/kg = 4048 ± 542. (B) AOC: control = 14 487 ± 509; tirofiban = 10 507 ± 805. (C) AOC: control = 14 487 ± 509; rtPA low dose = 12 242 ± 1776; rtPA high dose = 5251 ± 949. (D) AOC: control = 14 487 ± 509; ALX-0081 + rtPA low dose = 7285 ± 1272. n = 5 per group.
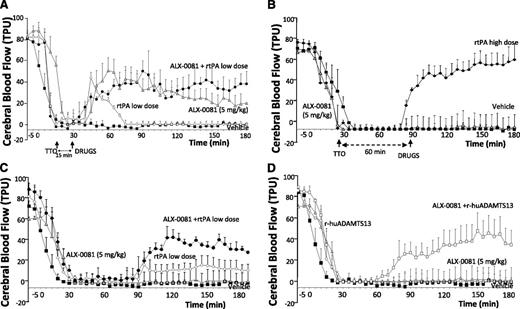
When ALX-0081 was administered 15 minutes after the complete occlusion of the MCA (early postinjury protocol), reperfusion was achieved within 10 minutes; 50% of baseline blood flow was reestablished in all the animals tested (Figure 4A), although a subsequent trend to a progressive reduction of blood flow was seen. A similar effect was observed when ALX-0081 was administered in combination with a low dose of rtPA; 49% of baseline blood flow was restored (Figure 4A), and a low dose of rtPA alone induced only a mild and transient MCA reperfusion (Figure 4A).
Blood flow continuously recorded after photochemically induced MCA damage: effect of drugs administered 15 minutes and 1 hour after MCA occlusion. ALX-0081 at 5 mg/kg (shaded triangle), rtPA high dose at 0.1 mg/kg intravenous bolus + 0.9 mg/kg over 30 minutes (♦), rtPA low dose at 0.032 mg intravenous bolus + 0.576 mg/kg over 30 minutes (♢), ALX-0081 + rtPA low dose (●), rhADAMTS13 at 1 µg/body (○), ALX-0081 + rhADAMTS13 (□). (A-D) Vehicle (▪). AOC has been calculated for each treatment by GraphPad Prism 4.0 for Windows software. (A) AOC: control = 15 124 ± 1142, rtPA low dose = 14 087 ± 1558, ALX-0081 = 7946 ± 1132, ALX-0081 + rtPA = 10 200 ± 953. (B) AOC: control = 15 124 ± 1142, ALX-0081 = 14 418 ± 1140, rtPA = 9845 ± 859. (C) AOC: control = 15 124 ± 1142, rtPA low dose = 9854 ± 794, rtPA low dose + ALX-0081 = 12 350 ± 1126. (D) AOC: control = 15 191 ± 1521, rhADAMTS13 = 14 660 ± 968, rhADAMTS13 + ALX-0081 = 11 240 ± 1032. n = 5 per group.
Blood flow continuously recorded after photochemically induced MCA damage: effect of drugs administered 15 minutes and 1 hour after MCA occlusion. ALX-0081 at 5 mg/kg (shaded triangle), rtPA high dose at 0.1 mg/kg intravenous bolus + 0.9 mg/kg over 30 minutes (♦), rtPA low dose at 0.032 mg intravenous bolus + 0.576 mg/kg over 30 minutes (♢), ALX-0081 + rtPA low dose (●), rhADAMTS13 at 1 µg/body (○), ALX-0081 + rhADAMTS13 (□). (A-D) Vehicle (▪). AOC has been calculated for each treatment by GraphPad Prism 4.0 for Windows software. (A) AOC: control = 15 124 ± 1142, rtPA low dose = 14 087 ± 1558, ALX-0081 = 7946 ± 1132, ALX-0081 + rtPA = 10 200 ± 953. (B) AOC: control = 15 124 ± 1142, ALX-0081 = 14 418 ± 1140, rtPA = 9845 ± 859. (C) AOC: control = 15 124 ± 1142, rtPA low dose = 9854 ± 794, rtPA low dose + ALX-0081 = 12 350 ± 1126. (D) AOC: control = 15 191 ± 1521, rhADAMTS13 = 14 660 ± 968, rhADAMTS13 + ALX-0081 = 11 240 ± 1032. n = 5 per group.
ALX-0081 (5 mg/kg total dose) administered 60 minutes after the complete occlusion of the MCA (delayed postinjury protocol) no longer induced reperfusion, but rtPA (0.1 mg/kg intravenously + 0.9 mg/kg given over a 30-minute infusion) still induced almost complete (∼80%) reperfusion (Figure 4B).
When ALX-0081 (5 mg/kg) was administered together with a low dose of rtPA (0.032 mg/kg intravenously + 0.576 mg/kg given over a 30-minute infusion), 42% reperfusion was observed 1 hour after thrombosis (Figure 4C). Similarly, the administration of rhADAMTS13 (1µg) in association with ALX-0081 (5 mg/kg) given 60 minutes after thrombosis partially restored MCA blood flow (53% reperfusion) (Figure 4D).
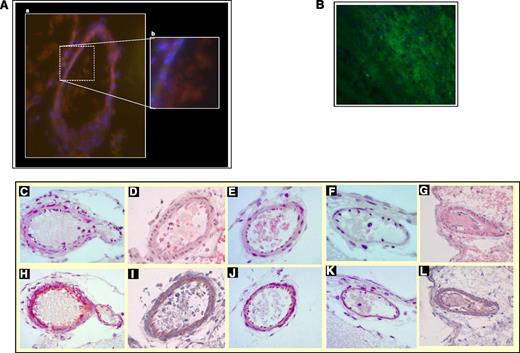
In vehicle-treated guinea pigs, MCA thrombi collected immediately after total artery occlusion were mainly formed by platelets at immunofluorescence, with small amounts of interposed fibrin (Figure 5A).
MCA thrombosis. (A) Representative images of an occluding MCA thrombus formed in vehicle-treated guinea pigs at immunofluorescence. Sections of frozen brains were cut and stained for a platelet marker (CD41, red) and fibrinogen/fibrin (green) and were counterstained for nuclei (blue): thrombus is mainly formed by platelets with only minor presence of fibrin (A, Magnification ×20; B, Magnification ×100). (B) Positive control for fibrin: a thrombus obtained in the stenosis-induced deep venous thrombosis model was longitudinally cut and stained for fibrinogen/fibrin (green) and counterstained for nuclei (blue). Magnification ×100. A Zeiss Axiovert 200 inverted fluorescence microscope (objectives: Zeiss Plan-Apochromate 10×/0.45 or 63×/1.4) connected to a monochrome camera (AxioCam MRm) was used for imaging. Colors for fluorescent channels were assigned by using Axiovision software (Axio Vs 40, Version 4.6.3.0). (C-L) Representative histologic micrographs of MCA thrombus of guinea pigs treated with vehicle and ALX-0081 at 5 mg/kg after photochemical-induced thrombosis. Slides were stained with hematoxylin and eosin for cellular components (top row), or phosphotungstic acid for fibrin (bottom row). (C,H) Vehicle-treated guinea pigs (brains collected immediately after the complete occlusion of MCA). (D,I) Vehicle-treated guinea pigs (brains collected 1 hour after the complete occlusion of MCA). (E,J) ALX-0081 administered at the moment of complete occlusion. (F,K) ALX-0081 administered 15 minutes after occlusion. (G,L) ALX-0081 administered 60 minutes after occlusion. Sections were analyzed by optical microscopy (Leica; Wetzler, Germany) using ×40 magnification.
MCA thrombosis. (A) Representative images of an occluding MCA thrombus formed in vehicle-treated guinea pigs at immunofluorescence. Sections of frozen brains were cut and stained for a platelet marker (CD41, red) and fibrinogen/fibrin (green) and were counterstained for nuclei (blue): thrombus is mainly formed by platelets with only minor presence of fibrin (A, Magnification ×20; B, Magnification ×100). (B) Positive control for fibrin: a thrombus obtained in the stenosis-induced deep venous thrombosis model was longitudinally cut and stained for fibrinogen/fibrin (green) and counterstained for nuclei (blue). Magnification ×100. A Zeiss Axiovert 200 inverted fluorescence microscope (objectives: Zeiss Plan-Apochromate 10×/0.45 or 63×/1.4) connected to a monochrome camera (AxioCam MRm) was used for imaging. Colors for fluorescent channels were assigned by using Axiovision software (Axio Vs 40, Version 4.6.3.0). (C-L) Representative histologic micrographs of MCA thrombus of guinea pigs treated with vehicle and ALX-0081 at 5 mg/kg after photochemical-induced thrombosis. Slides were stained with hematoxylin and eosin for cellular components (top row), or phosphotungstic acid for fibrin (bottom row). (C,H) Vehicle-treated guinea pigs (brains collected immediately after the complete occlusion of MCA). (D,I) Vehicle-treated guinea pigs (brains collected 1 hour after the complete occlusion of MCA). (E,J) ALX-0081 administered at the moment of complete occlusion. (F,K) ALX-0081 administered 15 minutes after occlusion. (G,L) ALX-0081 administered 60 minutes after occlusion. Sections were analyzed by optical microscopy (Leica; Wetzler, Germany) using ×40 magnification.
Thrombosis of the MCA was confirmed at histology: in vehicle-treated animals, the MCA was completely occluded by a thrombus in brains collected immediately after (Figure 5C) and 60 minutes after (Figure 5D) the occlusion of the artery. The thrombus was mainly formed by platelets with only traces of fibrin (dark blue; 629 ± 70 arbitrary units) immediately after MCA occlusion (Figure 5H), while the amount of fibrin progressively increased at 15 minutes (1976.3 ± 3.13 arbitrary units) and 60 minutes (3589.5 ± 579.6 arbitrary units) after occlusion (Figure 5I). Treatment with ALX-0081 reduced MCA occlusion, as assessed at histology, by 85% and 32% when administered immediately after (Figure 5E,J) and 15 minutes after (Figure 5F,K) the complete cessation of blood flow, respectively, while it had no effect when administered 60 minutes after MCA occlusion (Figure 5G,L).
Microvascular thrombosis
In control animals, 65.7 ± 1.9% of brain vessels with a diameter between 10 and 40 µm located about 200 µm underneath the external margin of the damaged hemisphere were occluded by a thrombus. The percentage of microvascular blood vessels occluded in the contralateral hemisphere was negligible (<2% of total) and was similar in all treatment groups.
ALX-0081 (5 mg/kg, immediate postinjury protocol) greatly reduced the number of microvessels occluded (8.6% ± 3.8% of total), but rtPA had a less pronounced effect (34.2% ± 10.6% of total). ALX-0081 administered 15 minutes after total MCA occlusion (early postinjury protocol) was still effective in reducing microvascular thrombosis (35.4% ± 7.7% of total microvessels occluded vs 69.5% ± 6.3% in control animals; P < .01). The effect, although reduced, was still evident when ALX-0081 was administered 60 minutes after MCA occlusion (55.2% ± 4.9% of total microvessels occluded vs 68.8% ± 2.3% in control animals; P = .03).
ALX-0081 administered in combination with rhADAMTS13 (1 µg) 60 minutes after total MCA occlusion (delayed postinjury protocol) reduced microvascular thrombosis (37.9% ± 0.7% vs 68.8% ± 1.1%; P < .01 vs control).
In the transient MCA occlusion model, 78% ± 7.6% of microvessels were occluded by thrombi in control animals; ALX-0081 (5 mg/kg) administered 80 minutes after intraluminal thread occlusion of the MCA significantly reduced microvascular thrombosis (44% ± 8.1% of microvessels occluded; P = .0167 vs control).
Brain damage
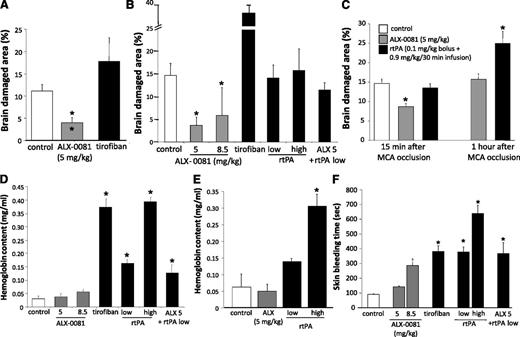
In control animals, damaged brain area was 14.6% ± 1.2% of the total brain section surface (Figure 6 and supplemental Figure 1).
Ischemic brain damage area and bleeding. (A) Preinjury protocol. (B) Immediate postinjury protocol. (C) Left: early postinjury protocol; right: delayed postinjury protocol. Guinea pigs treated with vehicle (□), ALX-0081 (shaded square), rtPA, tirofiban, and ALX- 0081 + rtPA (▪). Data are reported as percentage of damaged brain area vs total brain area. Intracerebral hemorrhage as assessed by Hb content in the damaged hemisphere vs healthy hemisphere. (D) Immediate postinjury protocol. (E) Delayed postinjury protocol; data are reported as the difference in absolute amount of Hb between control and damaged hemisphere, expressed in mg/mL (mean ± standard deviation). (F) Skin bleeding time. *Outside the column P < .05 vs vehicle; inside the column P < .05 vs tirofiban. n = 5 per group.
Ischemic brain damage area and bleeding. (A) Preinjury protocol. (B) Immediate postinjury protocol. (C) Left: early postinjury protocol; right: delayed postinjury protocol. Guinea pigs treated with vehicle (□), ALX-0081 (shaded square), rtPA, tirofiban, and ALX- 0081 + rtPA (▪). Data are reported as percentage of damaged brain area vs total brain area. Intracerebral hemorrhage as assessed by Hb content in the damaged hemisphere vs healthy hemisphere. (D) Immediate postinjury protocol. (E) Delayed postinjury protocol; data are reported as the difference in absolute amount of Hb between control and damaged hemisphere, expressed in mg/mL (mean ± standard deviation). (F) Skin bleeding time. *Outside the column P < .05 vs vehicle; inside the column P < .05 vs tirofiban. n = 5 per group.
Preinjury protocol
ALX-0081 significantly reduced brain damage area (4.0% ± 1.2% of total; P = .002 vs control); tirofiban, although effective in restoring blood flow, did not reduce brain damage (17.8% ± 5.3% of total) (Figure 6A).
Postinjury protocols
ALX-0081 administered at the time of total occlusion of the MCA (immediate postinjury protocol) significantly reduced brain damage area at both the low dose (3.6% ± 2.1% of total; P = .002 vs control) and high dose (5.8% ± 0.99% of total; P = .0014 vs control) (Figure 6B).
On the contrary, tirofiban significantly increased brain damage area (34.7% ± 6.2% of total; P < .01), and rtPA did not reduce it at either the low or the high dose (14.0% ± 1.3% and 15.7% ± 2.1% of total, respectively). Finally, the effect of the combination of low-dose rtPA and ALX-0081 on brain damage was similar to that of rtPA alone (11.4% ± 1.4% of total) and not different from controls (Figure 6B).
When drugs were administered 15 minutes after total occlusion of the MCA (early postinjury protocol), the area of brain damage was still significantly reduced in ALX-0081-treated guinea pigs, but it was not different from controls in rtPA-treated animals (Figure 6C). With the combination of rtPA and ALX-0081, brain damage was similar although not significantly reduced vs control compared with that of ALX-0081 alone (10.40% ± 3.15% of total).
When drugs were administered 60 minutes after the occlusion of the MCA (delayed postinjury protocol), ALX-0081 did not reduce the area of brain damage but rtPA increased it (15.7% ± 1.4% and 24.9% ± 3.4% of total, respectively) (Figure 6C). With the combination of rtPA and ALX-0081, brain damage was somewhat lower than that observed with rtPA alone (18.3% ± 3.76% of total; P = not significant vs control).
Bleeding
Brain hemorrhage
The absolute amount of Hb in the ischemic brain hemisphere 24 hours after MCA occlusion was slightly increased in vehicle-treated animals as compared with the amount in the contralateral, undamaged hemisphere (Figure 6D).
Postinjury protocol
ALX-0081 did not increase intracerebral hemorrhage at either the low or the high dose compared with that in vehicle-treated animals; on the contrary, both tirofiban and rtPA significantly increased it (Figure 6D). The combination of low-dose rtPA and ALX-0081 increased Hb content in the damaged hemisphere compared with ALX-0081 alone (Figure 6D). rtPA administered 15 minutes and 1 hour after the total occlusion of MCA significantly increased brain hemorrhage, especially at the highest dose (Figure 6E).
Skin bleeding time
ALX-0081 did not modify the bleeding time at the low dose and prolonged it at the high dose (Figure 6F). Tirofiban induced a larger, almost 4-fold prolongation of the skin bleeding time and rtPA also dose-dependently prolonged the bleeding time (by 3.66- and 5.5-fold at the low and high doses, respectively) (Figure 6F). The combination of ALX-0081 and rtPA prolonged the skin bleeding time to the same extent as low-dose rtPA.
Discussion
Our study shows that the inhibition of the interaction between GPIb and VWF by blockade of the A1 domain of VWF reduces MCA thrombosis and cerebral ischemia without provoking intracranial hemorrhage in guinea pigs. Interestingly, the blockade of VWF-A1 not only prevented thrombosis when administered before arterial injury, it also induced thrombus dissolution and reperfusion when given after thrombosis had occurred. It is known that the binding of GPIb to the A1 domain of VWF is crucial for the tethering of platelets to the vessel wall,36 especially in vessels with a high shear stress flow; therefore, it was expected that blocking VWF-A1 would prevent the acute thrombotic occlusion of the MCA,37,38 but it was not expected that it would induce reperfusion. Our data therefore show that the interaction of the VWF-A1 domain with platelet GPIb is not only essential for the first phases of platelet–vessel wall interaction but is also involved in the initial stabilization of an arterial thrombus.
Indeed, in vivo thrombus formation is a dynamic, multistep process, and VWF exposed on the first layers of platelets adhering to the damaged vessel wall strongly contributes to thrombus accrual by binding to GPIbα of free-flowing platelets.39 In the first phases following damage to the vessel wall, the formation of a loose platelet thrombus is continuously associated with its fragmentation, and thrombus size waxes and wanes for some time.40 It is therefore conceivable that inhibition of the interaction between the A1 domain of VWF and platelet GPIbα by a Nanobody that, because of its physical characteristics, may penetrate the gaps between adjacent platelets, facilitates platelet disaggregation and thrombus dismantling. At a later stage, when the thrombus has stabilized, tight interactions between platelet GPIIb/IIIa and its ligands have been established, and fibrin formation has occurred, VWF inhibition no longer induces thrombus dissolution. Recent data in an FeCl3-induced thrombosis model in mice showed that recombinant ADAMTS13 (the metalloproteinase which cleaves the high-molecular-weight forms of VWF) induces thrombolysis without provoking hemorrhage, whereas rtPA-induced thrombus dissolution is associated with hemorrhage.22,41 In agreement with these observations, in our model, the administration of ALX-0081 after MCA thrombosis was established produced recanalization for up to 15 minutes without hemorrhagic consequences to the brain. This effect was peculiar to the inhibition of the GPIb–VWF axis because tirofiban, a GPIIb/IIIa antagonist, induced only marginal reperfusion, and it still significantly increased intracerebral bleeding, thus confirming clinical data.12 ALX-0081 administered 1 hour after thrombosis instead, when a stable fibrin-rich thrombus had established, was no longer able to induce reperfusion. Partial reperfusion was obtained when ALX-0081 was given in combination with a low dose of rtPA (by itself inactive), thus confirming a preeminent role of fibrin in thrombus stabilization. However, the combination of ALX-0081 with rhADAMTS13 also induced reperfusion, suggesting that high-molecular-weight VWF multimers accumulating in the clot also participate in thrombus stabilization.42
It is known that the cerebral microvasculature responds rapidly to brain ischemia by upregulating adhesion molecules on endothelial cells, by releasing locally ultra-large VWF from the Weibel-Palade bodies of ischemic endothelial cells or by exposing subendothelial matrix proteins, such as collagen.43,44 The binding of platelet GPIb to subendothelial matrix-bound VWF is particularly important in the microvasculature where blood flow shear stresses are particularly elevated.45,46 Indeed, treatment with ALX-0081 reduced microvascular thrombi in the ischemic brain tissue, even when administered 1 hour after MCA occlusion. Therefore, ALX-0081 prevents cerebral ischemia by reducing platelet thrombus growth in the microvascular lumen, as has already been shown for rADAMTS13,22,43 and by reducing thrombus formation in middle-size arteries. The loss of efficacy of ALX-0081 in preventing cerebral ischemia when administered 60 minutes after an MCA thrombus had established itself seems therefore to be primarily due to the development of lysis-resistant macrovascular thrombosis.
Restoration of microvascular patency by blockading the interaction between GPIb and VWF may thus contribute substantially to the preservation of brain integrity and to fewer bleeding complications observed with ALX-0081. Indeed, although the inhibition of the GPIb–VWF axis was accompanied by a lesser prolongation of the skin bleeding time compared with that of the thrombolytic agent rtPA or with the GPIIb/IIIa blocker tirofiban, this difference was not particularly large and was not large enough to explain the sizable difference in brain hemorrhage. These results are in agreement with previous observations showing that no direct correlation exists between skin bleeding time and bleeding risk.47-49
On the basis of the above results, it is conceivable that the administration of ALX-0081 shortly after the onset of an acute stroke may induce revascularization and reduce brain damage with less hemorrhagic risk than rtPA. However, when the time interval from stroke onset increases, the inhibition of the GPIb–VWF axis may no longer be of benefit.
In conclusion, our study demonstrates that blockade of the GPIb–VWF interaction prevents cerebral artery thrombosis and induces early reperfusion, which reduces brain infarct area without inducing intracerebral bleeding in guinea pigs. These findings underline the important pathophysiological role of VWF in ischemic stroke and may open new avenues for its treatment.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Dr Silvia Giannini, Dr Anna Maria Mezzasoma, Dr Giuseppe Guglielmini, and Dr Sara Orsini (University of Perugia, Italy) for flow cytometry, vWF-RiCof, platelet adhesion studies, and statistical analysis, and Dr S. Priem and Dr S. Rossenu (Ablynx) for PK/PD analyses.
This study was supported in part by a research grant from Ablynx (Zwijnaarde, Belgium) and from Fondazione Cassa di Risparmio di Perugia (Protocol No. 2011.0137.021) to P.G.
Authorship
Contribution: P.G. and S.M. designed the study and wrote the manuscript; S.M. and M.T. performed experiments; H.U. and M.V.R. participated in study design and interpretation, supervised PK measurements, and participated in data analysis; and G.R. carried out histology.
Conflict-of-interest disclosure: H.U. and M.V.R. are employees of and own shares and/or stock options in Ablynx; P.G. has received a research grant from Ablynx. The remaining authors declare no competing financial interests.
Corresponding author: Paolo Gresele, Section of Internal and Cardiovascular Medicine, Department of Internal Medicine, University of Perugia, Via E. dal Pozzo, 06126 Perugia, Italy; e-mail: grespa@unipg.it.