Key Points
Phase I study showed that intraventricular rituximab plus methotrexate is feasible and active in the treatment of refractory CNS lymphoma.
Abstract
Recurrent CNS lymphoma continues to be associated with poor outcomes in the rituximab era. Although IV rituximab mediates superior disease control of systemic non-Hodgkin lymphoma (NHL), it fails to completely eliminate the risk of meningeal recurrence, likely due to minimal CNS penetration. Given that rituximab acts synergistically with chemotherapy, we conducted the first phase 1 study of intraventricular immunochemotherapy in patients with recurrent CNS NHL. Fourteen patients received 10 mg or 25 mg intraventricular rituximab twice weekly for 4 weeks, with rituximab administered as monotherapy during the first treatment each week and rituximab administered in combination with methotrexate (MTX) during the second treatment each week. More than 150 doses were administered without serious toxicity. In a population with high-refractory CNS NHL, 75% of patients achieved complete cytologic responses and 43% achieved an overall complete response in CSF and/or brain parenchyma. Two patients achieved a first complete response of CNS NHL with intraventricular rituximab/MTX, including 1 with CNS lymphoma refractory to high-dose systemic and intrathecal MTX plus IV rituximab. We conclude that intraventricularrituximab in combination with MTX is feasible and highly active in the treatment of drug-resistant CNS NHL that is refractory or unresponsive to IV rituximab. This trial is registered at www.clinicaltrials.gov as NCT00221325.
Introduction
A variety of data demonstrate that the blood-brain barrier impedes the efficacy of therapeutic Abs directed against malignancy within the brain and leptomeningeal compartment. Although it is well-established that the use of rituximab consistently improves outcomes in the management of systemic B-cell non-Hodgkin lymphoma (NHL), several clinical series of combination immunochemotherapy demonstrate that the addition of rituximab to CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy does not significantly decrease the rate of CNS relapse of systemic diffuse large B-cell lymphoma compared with CHOP therapy alone.1-3 These observations are in agreement with data showing that less than 1% of systemic rituximab penetrates the leptomeningeal compartment.4 Nevertheless, several studies have demonstrated that IV rituximab may induce partial (PRs) or complete response (CRs) of contrast-enhancing lesions of CNS lymphoma, suggesting selective activity in the setting of a disrupted blood-brain barrier.5 Conversely, the increased incidence of HER2+ CNS metastasis in breast cancer patients treated with trastuzumab6,7 underscores the negative impact of the blood-brain barrier on the utility of immunotherapeutic approaches for brain tumors that are based on systemic administration of large protein macromolecules.
There remains an unmet need for innovative strategies to treat relapsed primary and secondary CNS lymphoma. We recently studied the safety and activity of intraventricular rituximab monotherapy in the treatment of recurrent intraocular and CNS NHL. Rapid dissemination of rituximab throughout the craniospinal axis was demonstrated and cytologic responses were detected in 6 of 10 patients. Two patients experienced improvement in intraocular NHL and 1 exhibited resolution of brain parenchymal NHL. None of these patients received intraventricular methotrexate (MTX).8 Several other groups have also reported favorable outcomes with this approach in the treatment of CD20+ lymphoid tumors within the CNS.9-15
The major goals of this present study were to perform the first phase 1 trial of intraventricular immunochemotherapy to evaluate the safety of 2 dose levels of intraventricular rituximab, as well as its pharmacokinetics (PK) profile in combination with intraventricular MTX. Secondary goals were to obtain information regarding the efficacy of this approach in the treatment of patients withrecurrent CNS lymphoma (ie, brain parenchyma or the intraocular or leptomeningeal compartment) and to document the relationship between therapeutic responses within the brain and leptomeninges and rituximab concentration within CSF and serum.
Methods
Study design
We performed a phase 1, open label, dose-escalation study to define the safety, PK, and efficacy of intraventricular rituximab in combination with MTX in patients with recurrent/refractory/persistent CNS lymphoma. The study population consisted of 14 patients with relapsed or refractory CD20+ CNS lymphoma arising from systemic NHL or primary CNS lymphoma. None had previously received intrathecal rituximab. Eligibility required age greater than 17 years, Karnofsky performance status greater than 50%, HIV-seronegative status, an Ommaya reservoir, granulocyte concentration of at least 1000/μL, and platelet concentration of at least 50 000/μL.
No patient could receive IV rituximab within 30 days of the initiation of intraventricular rituximab. Concurrent systemic or other intrathecal therapy could not be administered and glucocorticoids were either tapered or not administered during pretreatment staging and intraventricular therapy. Other intrathecal and/or radiation therapy were to be completed at least 2 weeks before pretreatment CSF assessment and initiation of protocol intraventricular therapy. All patients signed informed consent indicating that they were aware of the investigational nature and potential risks and benefits of the study in accordance with institutional and national regulatory and review boards and the Declaration of Helsinki. The study was conducted at 3 medical centers: the University of California, San Francisco, the Dana-Farber Cancer Institute, and Massachusetts General Hospital. This study is registered at http://www.clinicaltrials.gov as NCT00221325.
Pretreatment staging included physical and ocular slit lamp examination and baseline MRI of brain and spine. Patency within the ventricular system was demonstrated in all subjects by CSF flow studies performed within 3 weeks of the initiation of intraventricular rituximab. Baseline laboratory studies included complete blood count, electrolytes, CSF cell count, differential, protein and glucose, and cytologic examination of CSF.
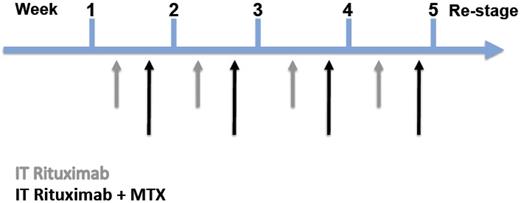
Enrollment was into 1 of 2 rituximab dose cohorts: 10 mg (dose level A) or 25 mg (dose level B) based on 3 + 3 dose escalation rules.18 Study participants received rituximab through an Ommaya reservoir on a twice-weekly basis for 1 month; the first treatment each week was rituximab as monotherapy, the second treatment each week consisted of rituximab immediately followed by the intraventricular administration of MTX (12 mg). This second weekly treatment occurred on calendar day 4 (3 days after the first weekly treatment) although a schedule deviation of 1 day from day 4 was permitted (Figure 1). Protocol design planned for 8 total doses of rituximab and 4 total doses of MTX over a 4-week period. Premedications consisted of acetaminophen 500 mg, diphenhydramine 25-50 mg IV/PO, and histamine H2 receptor antagonist (cimetidine or famotidine) IV 30 minutes before each treatment. Before injection, an equivalent volume of CSF was removed to minimize significant flux in CSF volume (on day 1 of each week, at least 6 mL of CSF was removed; on day 4 of each week, at least 12 mL of CSF was removed). Rituximab was diluted in a volume of 6 mL of preservative-free normal saline; MTX was diluted in a separate 6-mL volume of preservative-free normal saline. Treatment on day 1 consisted of intraventricular injection of rituximab, 10 mg (dose A) or 25 mg (dose B) into an Ommaya reservoir. On day 4, dose A (10 mg rituximab followed by 12 mg MTX) or dose B (25 mg rituximab followed by 12 mg MTX) was administered. Injection of each drug was performed slowly over 2-5 minutes. Manual pressure was applied to the bulb of the Ommaya device to facilitate delivery into the brain ventricles. Oral leucovorin rescue was initiated 24 hours after each intraventricular MTX administration (10 mg given 6 hours apart for a total dose of 40 mg each cycle).
Protocol schema of twice-weekly intraventricular rituximab. The first treatment each week was rituximab as monotherapy; the second treatment each week consisted of rituximab immediately followed by the intraventricular administration of MTX.
Protocol schema of twice-weekly intraventricular rituximab. The first treatment each week was rituximab as monotherapy; the second treatment each week consisted of rituximab immediately followed by the intraventricular administration of MTX.
CSF cell count, differential, and chemistries were evaluated with every intraventricular injection at intervals of twice per week; cytology was evaluated during the second injection every week. Serum laboratory studies were repeated at the time of the second injection every week.
Toxicities of intraventricular rituximab and MTX were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 scale on a continuous basis in all patients receiving at least 1 dose of intraventricular rituximab.
All patients underwent lymphoma restaging at week 5, including repeat neuroimaging of brain and spine if clinically indicated. Repeat ophthalmologic examinations were performed if intraocular NHL was demonstrated at baseline. Responses were assessed as described previously.19 Patients with at least a PR were offered a second month of combined intraventricular rituximab plus MTX. If PRs or better were sustained at week 10 of restaging, patients were offered extended dosing with every other week intraventricular rituximab.
PK sampling
Serial ventricular CSF samples for PK analysis were obtained from the Ommaya reservoir. During the first week, matched CSF and venous blood serum samples were obtained immediately before rituximab treatment and at 1 and 2 hours postdose. During the following 3 weeks, CSF and serum samples were obtained on day 1 and day 4 immediately before each rituximab dose and CSF samples were obtained 1 hour postdose. CSF samples for drug analysis were placed in cryovials for frozen storage. Blood samples were allowed to clot at room temperature for approximately 45 minutes and then centrifuged at 1300g. CSF and serum were frozen within 1 hour of collection and stored at −20°C until analysis for rituximab concentration.
Bioanalysis
CSF and serum concentrations of rituximab were determined by ELISA as described previously.4 The lower limit of quantitation for rituximab was 0.250 μg/mL for CSF and 0.500 μg/mL for serum. Samples below the limit of quantitation were denoted as less than reportable.
Analysis of FcγRIIIa polymorphisms
In brief, genomic DNA was prepared from 200 μL of venous blood using a QIAamp Blood Mini Kit (QIAGEN). TaqMan SNP real-time PCR assays (Applied Biosystems) were used to genotype FcγRIIIa 158 V/F polymorphism (C-25815666-10). Probes specific to FcγRIIIa 158 F alleles were labeled with FAM fluorescent dye and probes specific to FcγRIIIa 158 V alleles were labeled with VIC fluorescent dye. SNP real-time PCR were run in triplicate using an ABI Prism 7000 real-time PCR detection system. Every reaction plate had a triplicate nontemplate control. The FcγRIIIa genotypes were determined by allele-specific fluorescence using the allelic discrimination protocol in SDS software provided by Applied Biosystems.
Results
Patient characteristics
Fourteen patients were enrolled and baseline characteristics are summarized in Table 1. The median age was 61 years (range, 37-75) with a median Karnofsky performance status of 70 (range, 60-90). Six patients had relapsed primary CNS lymphoma and 8 had relapsed secondary CNS lymphoma, 4 of whom initially presented with extranodal localization of their tumors in the testis,2 sinus,1 and skin.1 The pathologic diagnoses were diffuse large B-cell lymphoma,11 marginal zone lymphoma,1 Burkitt lymphoma,1 and transformed follicular lymphoma.1 Patients who enrolled in this trial had experienced disease progression after a median of 5 prior treatment regimens (range, 3-10). All of the patients had tumors that had previously been treated with MTX-containing regimens. In 12 patients, MTX-containing regimens resulted in tumor progression or had yielded only a PR or stable disease (10 patients had received high-dose, systemic IV MTX and 5 had received intrathecal MTX. Four patients received both high-dose IV MTX and intrathecal MTX). Eight patients had CNS lymphomas that had failed to respond to or progressed after IV rituximab or rituximab-containing regimens.
Patient characteristics
| Patient no. . | Age, y . | Sex . | Original diagnosis . | Prior therapies . | Prior responses . |
|---|---|---|---|---|---|
| 1 | 63 | F | PCNSL (DLBCL) | MT-R | CR (4 y) |
| Etoposide-cytarabine consolidation | CR (4 y) | ||||
| HD-MTX | PR | ||||
| WBRT | CR (1 yr) | ||||
| CyberKnife radiosurgery | PR | ||||
| 2 | 64 | F | PCNSL (DLBCL) | MT-R | PD (2 mo) |
| Etoposide-cytarabine | SD | ||||
| WBRT | CR (6 mo) | ||||
| 3 | 57 | F | Secondary CNSL (marginal zone) | R-CHOP | PR (systemic disease) |
| R-CVP | PR (systemic disease) | ||||
| HD-MTX | SD in CNS | ||||
| IT MTX | SD in CNS | ||||
| IT Ara-C | SD in CNS | ||||
| 4 | 61 | M | Secondary CNSL (DLBCL, testis) | R-CHOP, XRT | CR (systemic disease, 1 mo) |
| IT-MTX | PR in CNS | ||||
| IT-Ara-C | PR in CNS | ||||
| IT-DepoCyt | PR in CNS | ||||
| 5 | 55 | M | Secondary CNSL (DLBCL, skin) | R-CHOP, XRT | CR (systemic disease, 5 y) |
| HD-MTX, R | PD in CNS | ||||
| Etoposide-cytarabine | PD in CNS | ||||
| WBRT | PR in CNS | ||||
| 6 | 61 | M | Secondary CNSL (Burkitts) | R-CHOP followed by Zevalin | CR (systemic disease, 4 y) |
| R-hyper-CVAD, Velcade | PR, CNS progression | ||||
| R-ifosfamide, VP16, etoposide | CR (systemic disease) | ||||
| IT-MTX + IT-AraC | PR in CNS | ||||
| WBRT | CR (CNS, 6 mo) | ||||
| R-TMZ | PR in CNS | ||||
| IT-Topotecan | PR in CNS | ||||
| 7 | 75 | M | Secondary CNSL (DLBCL, testis) | R-CHOP, XRT | CR (systemic disease) |
| IT MTX | PR | ||||
| HD-MTX, R | PR | ||||
| 8 | 65 | M | PCNSL (DLBCL) | HD-MTX (complicated by renal failure) | SD in CNS |
| Pemetrexed | PD | ||||
| 9 | 37 | M | PCNSL (DLBCL) | HD-MTX, procarbazine, vincristine, rituximab (R-MPV) | PR |
| HD-Ara-C | PR | ||||
| IT-Ara-C | PR | ||||
| WBRT | PR | ||||
| IT-Ara-C | PR | ||||
| 10 | 59 | F | Secondary CNSL (DLBCL, sinus) | R-CHOP, XRT | CR |
| IT-MTX | PD in CNS | ||||
| MT-R | CR in CNS | ||||
| Etoposide-cytarabine | CR (1 mo) | ||||
| 11 | 58 | F | PCNSL (DLBCL) | MT-R | CR |
| Etoposide-cytarabine | CR (3 y) | ||||
| XRT | CR | ||||
| 12 | 64 | M | Secondary CNSL (DLBCL, sinus) | R-CHOP | CR (4 mo) |
| HD-MTX + IT MTX, + IT-Ara-C + IT HC | PD | ||||
| R-DHAP | PR | ||||
| IT-DepoCyt | PR | ||||
| MT-R | SD | ||||
| IV rituximab | SD | ||||
| 13 | 65 | F | PCNSL (DLBCL) | MT-R | CR |
| Etoposide-cytarabine consolidation | CR (2 y) | ||||
| HD-MTX, R | PR | ||||
| XRT | PR | ||||
| 14 | 51 | F | Secondary CNSL (transformed follicular) | R-CHOP | CR (1 mo) |
| IT-MTX (prophylaxis) | |||||
| HD-MTX, HD-Ara-C, R | PR | ||||
| R-DHAP + IT-MTX | PR | ||||
| Cy, BCNU, VP16 (CBV-based ASCT) | CR (2 mo) |
| Patient no. . | Age, y . | Sex . | Original diagnosis . | Prior therapies . | Prior responses . |
|---|---|---|---|---|---|
| 1 | 63 | F | PCNSL (DLBCL) | MT-R | CR (4 y) |
| Etoposide-cytarabine consolidation | CR (4 y) | ||||
| HD-MTX | PR | ||||
| WBRT | CR (1 yr) | ||||
| CyberKnife radiosurgery | PR | ||||
| 2 | 64 | F | PCNSL (DLBCL) | MT-R | PD (2 mo) |
| Etoposide-cytarabine | SD | ||||
| WBRT | CR (6 mo) | ||||
| 3 | 57 | F | Secondary CNSL (marginal zone) | R-CHOP | PR (systemic disease) |
| R-CVP | PR (systemic disease) | ||||
| HD-MTX | SD in CNS | ||||
| IT MTX | SD in CNS | ||||
| IT Ara-C | SD in CNS | ||||
| 4 | 61 | M | Secondary CNSL (DLBCL, testis) | R-CHOP, XRT | CR (systemic disease, 1 mo) |
| IT-MTX | PR in CNS | ||||
| IT-Ara-C | PR in CNS | ||||
| IT-DepoCyt | PR in CNS | ||||
| 5 | 55 | M | Secondary CNSL (DLBCL, skin) | R-CHOP, XRT | CR (systemic disease, 5 y) |
| HD-MTX, R | PD in CNS | ||||
| Etoposide-cytarabine | PD in CNS | ||||
| WBRT | PR in CNS | ||||
| 6 | 61 | M | Secondary CNSL (Burkitts) | R-CHOP followed by Zevalin | CR (systemic disease, 4 y) |
| R-hyper-CVAD, Velcade | PR, CNS progression | ||||
| R-ifosfamide, VP16, etoposide | CR (systemic disease) | ||||
| IT-MTX + IT-AraC | PR in CNS | ||||
| WBRT | CR (CNS, 6 mo) | ||||
| R-TMZ | PR in CNS | ||||
| IT-Topotecan | PR in CNS | ||||
| 7 | 75 | M | Secondary CNSL (DLBCL, testis) | R-CHOP, XRT | CR (systemic disease) |
| IT MTX | PR | ||||
| HD-MTX, R | PR | ||||
| 8 | 65 | M | PCNSL (DLBCL) | HD-MTX (complicated by renal failure) | SD in CNS |
| Pemetrexed | PD | ||||
| 9 | 37 | M | PCNSL (DLBCL) | HD-MTX, procarbazine, vincristine, rituximab (R-MPV) | PR |
| HD-Ara-C | PR | ||||
| IT-Ara-C | PR | ||||
| WBRT | PR | ||||
| IT-Ara-C | PR | ||||
| 10 | 59 | F | Secondary CNSL (DLBCL, sinus) | R-CHOP, XRT | CR |
| IT-MTX | PD in CNS | ||||
| MT-R | CR in CNS | ||||
| Etoposide-cytarabine | CR (1 mo) | ||||
| 11 | 58 | F | PCNSL (DLBCL) | MT-R | CR |
| Etoposide-cytarabine | CR (3 y) | ||||
| XRT | CR | ||||
| 12 | 64 | M | Secondary CNSL (DLBCL, sinus) | R-CHOP | CR (4 mo) |
| HD-MTX + IT MTX, + IT-Ara-C + IT HC | PD | ||||
| R-DHAP | PR | ||||
| IT-DepoCyt | PR | ||||
| MT-R | SD | ||||
| IV rituximab | SD | ||||
| 13 | 65 | F | PCNSL (DLBCL) | MT-R | CR |
| Etoposide-cytarabine consolidation | CR (2 y) | ||||
| HD-MTX, R | PR | ||||
| XRT | PR | ||||
| 14 | 51 | F | Secondary CNSL (transformed follicular) | R-CHOP | CR (1 mo) |
| IT-MTX (prophylaxis) | |||||
| HD-MTX, HD-Ara-C, R | PR | ||||
| R-DHAP + IT-MTX | PR | ||||
| Cy, BCNU, VP16 (CBV-based ASCT) | CR (2 mo) |
MT-R indicates high-dose MTX (HD-MTX), temozolomide, and rituximab; PCNSL, primary CNS lymphoma; DLBCL, diffuse large B-cell lymphoma; TMZ, temozolomide; R, rituximab; Ara-C, cytarabine; XRT, irradiation; WBXRT, whole-brain irradiation; IT, intrathecal; HC, hydrocortisone; DHAP (HD-Ara-C, cisplatin, and dexamethasone); hyper-CVAD, cyclophosphamide, vincristine, doxorubicin, dexamethasone, cytarabine, and methotrexate; BCNU, carmustine; SD, stable disease; and PD, progressive disease.
Adverse events
Overall, intraventricular rituximab in combination with MTX was well tolerated. Three patients were treated at the 10-mg rituximab dose level and because there were no dose-limiting toxicities identified, the remaining 11 patients were treated at the 25-mg rituximab dose level. The most common adverse events were grade 1 paresthesias, chills, and rigors; these events were self-limited, usually resolving within 5-10 minutes. There was no evidence for significant additive toxicity when MTX was combined with rituximab for intraventricular injection and no infusion-related hypertension, the dose-limiting toxicity observed with 50-mg intraventricular rituximab.8 A total of 150 doses of intraventricular rituximab at 10 mg (42 doses) and 25 mg (108 doses) were administered to 14 patients at 3 medical centers without serious toxicity (Table 2). Given that no dose-limiting toxicity was identified, the recommended dose for this intraventricular combination is rituximab 25 mg in combination with MTX 12 mg. We did not study higher concentrations of rituximab because of potential safety issues and because tumor responses were already evident. No dose-limiting criteria were met in this study.
Grade 3 or 4 toxicities
| Patient no. . | IT rituximab dose, mg . | Doses administered, n . | Grade 3/4 adverse events . |
|---|---|---|---|
| 1 | 10 | 8 | Lymphopenia, fatigue |
| 2 | 10 | 26 | Cataract (unrelated) |
| 3 | 10 | 8 | None |
| 4 | 25 | 5 | None |
| 5 | 25 | 8 | Gait, CNIII neuropathy (possibly related) |
| 6 | 25 | 8 | Lymphopenia |
| 7 | 25 | 12 | None |
| 8 | 25 | 16 | None |
| 9 | 25 | 8 | None |
| 10 | 25 | 8 | None |
| 11 | 25 | 16 | Neutropenia |
| 12 | 25 | 22 | None |
| 13 | 25 | 8 | None |
| 14 | 25 | 4 | Muscle weakness |
| Patient no. . | IT rituximab dose, mg . | Doses administered, n . | Grade 3/4 adverse events . |
|---|---|---|---|
| 1 | 10 | 8 | Lymphopenia, fatigue |
| 2 | 10 | 26 | Cataract (unrelated) |
| 3 | 10 | 8 | None |
| 4 | 25 | 5 | None |
| 5 | 25 | 8 | Gait, CNIII neuropathy (possibly related) |
| 6 | 25 | 8 | Lymphopenia |
| 7 | 25 | 12 | None |
| 8 | 25 | 16 | None |
| 9 | 25 | 8 | None |
| 10 | 25 | 8 | None |
| 11 | 25 | 16 | Neutropenia |
| 12 | 25 | 22 | None |
| 13 | 25 | 8 | None |
| 14 | 25 | 4 | Muscle weakness |
PK parameters of intraventricular rituximab plus MTX
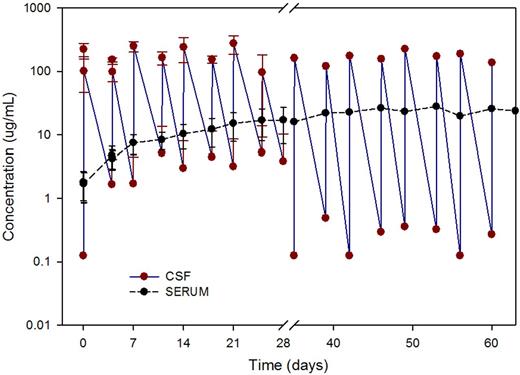
In this heavily pretreated population of patients, the median baseline rituximab concentration in the serum was 1900 ng/mL. Three patients had persistent positive CSF cytology detected at pretreatment staging despite concomitant serum rituximab concentrations of 19.8, 57.3, and 219 μg/mL (Table 3). With intraventricular administration, CSF rituximab concentrations rapidly declined after the first dose and exhibited a biphasic profile indicating distribution into at least 2 distinct biologic spaces. Serum rituximab concentrations after intraventricular injection exhibited a slow and steady increase over the course of the treatment, indicating a slow transfer from CSF to serum. The mean maximum intraventricular rituximab concentration observed 1 hour after intraventricular injection was 194 μg/mL at the 10-mg dose level (n = 3) and 580 μg/mL at the 25-mg dose level (n = 7; Figure 2).
Clinical parameters and response
| Patient no. . | IT-rituximab dose, mg . | FcγRIIIA-158 . | Baseline serum rituximab concentration, ng/mL . | Decadron dose . | IOL status . | Brain status . | CSF status . | IOL response . | Brain response . | CSF response . | Overall response in brain and CSF . | Response duration . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | V/F | < 500 | 4 mg BID | − | + | + | NA | PD | CR | PD | NA |
| 2 | 10 | V/F | 1230 | None | − | − | + | NA | NA | CR | CR | 8 months |
| 3 | 10 | F/F | < 500 | None | − | − | + | NA | NA | SD | SD | NA |
| 4 | 25 | F/F | 1540 | None | − | + | − | NA | NA | NA | Extra-CNS PD | NA |
| 5 | 25 | V/V | 1740 | 6 mg TID | − | + | + | NA | PD | SD | PD | NA |
| 6 | 25 | V/F | 57 300 | 4 mg BID | − | + | + | NA | PD | CR | PD | NA |
| 7 | 25 | F/F | 19 800 | 2 mg BID | − | − | + | NA | CR | CR | CR | 0.5 month |
| 8 | 25 | V/F | < 500 | None | − | − | + | NA | NA | CR | CR | 1 month |
| 9 | 25 | V/F | 2510 | None | − | + | − | NA | CR | NA | CR | 3 months |
| 10 | 25 | V/V | 2100 | None | + | + | + | SD | PR | CR | PR | NA |
| 11 | 25 | V/F | < 500 | None | + | + | + | SD | CR | CR | CR | 1 month |
| 12 | 25 | F/F | 219 000 | None | − | − | + | NA | NA | CR | CR | > 5.5 months |
| 13 | 25 | V/F | 2600 | 2 mg BID | − | + | + | NA | PD | CR | PD | NA |
| 14 | 25 | F/F | 3990 | None | − | + | + | NA | PD | NA | PD | NA |
| Patient no. . | IT-rituximab dose, mg . | FcγRIIIA-158 . | Baseline serum rituximab concentration, ng/mL . | Decadron dose . | IOL status . | Brain status . | CSF status . | IOL response . | Brain response . | CSF response . | Overall response in brain and CSF . | Response duration . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | V/F | < 500 | 4 mg BID | − | + | + | NA | PD | CR | PD | NA |
| 2 | 10 | V/F | 1230 | None | − | − | + | NA | NA | CR | CR | 8 months |
| 3 | 10 | F/F | < 500 | None | − | − | + | NA | NA | SD | SD | NA |
| 4 | 25 | F/F | 1540 | None | − | + | − | NA | NA | NA | Extra-CNS PD | NA |
| 5 | 25 | V/V | 1740 | 6 mg TID | − | + | + | NA | PD | SD | PD | NA |
| 6 | 25 | V/F | 57 300 | 4 mg BID | − | + | + | NA | PD | CR | PD | NA |
| 7 | 25 | F/F | 19 800 | 2 mg BID | − | − | + | NA | CR | CR | CR | 0.5 month |
| 8 | 25 | V/F | < 500 | None | − | − | + | NA | NA | CR | CR | 1 month |
| 9 | 25 | V/F | 2510 | None | − | + | − | NA | CR | NA | CR | 3 months |
| 10 | 25 | V/V | 2100 | None | + | + | + | SD | PR | CR | PR | NA |
| 11 | 25 | V/F | < 500 | None | + | + | + | SD | CR | CR | CR | 1 month |
| 12 | 25 | F/F | 219 000 | None | − | − | + | NA | NA | CR | CR | > 5.5 months |
| 13 | 25 | V/F | 2600 | 2 mg BID | − | + | + | NA | PD | CR | PD | NA |
| 14 | 25 | F/F | 3990 | None | − | + | + | NA | PD | NA | PD | NA |
IOL indicates intraocular NHL; NA, not available; SD, stable disease; and PD, progressive disease.
Rituximab concentrations in CSF and serum. Mean (± SD) CSF and serum concentrations versus nominal sampling time after 25-mg rituximab via intraventricular administration (n = 7 patients).
Rituximab concentrations in CSF and serum. Mean (± SD) CSF and serum concentrations versus nominal sampling time after 25-mg rituximab via intraventricular administration (n = 7 patients).
Evaluation of CSF rituximab concentrations at 1 and 2 hours postinjection suggested that the addition of MTX slowed the rate of rituximab egress or elimination from the intraventricular compartment: the elimination rates (first-order) were 0.88 and 0.84/h for the 10- and 25-mg dose levels, respectively, when rituximab was given alone and 0.47 and 0.36/h, respectively, when administered in combination with MTX (P < .02; Table 3 and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Response to therapy
On an intention-to-treat basis, the overall rate of CR in the leptomeningeal compartment (CSF) was 75% (9 of 12 patients with malignant cytology at pretreatment CSF evaluation; Table 4). Two patients (patients 2 and 13) had cytologic CRs to the first dose of rituximab as monotherapy, as determined by evaluation of CSF obtained on day 3 of the study, before the second treatment (rituximab plus MTX). Two patients (patients 1 and 6) exhibited a marked decrease in leptomeningeal lymphoma cells in CSF by the second day of treatment, as quantified by total cell count and differential analysis of CSF and as documented in the medical record. A median of 3 intraventricular injections of rituximab (2 as monotherapy and 1 in combination with MTX) was required to achieve complete resolution of malignant cytology in patients in this study. There was also evidence for additive or synergistic efficacy of rituximab when combined with MTX among patients with a high burden of leptomeningeal lymphoma (> 20 000 lymphoma cells/mL), as demonstrated by the rate of decline of lymphoma cells in CSF after day 3 of combination treatment manifested by patient 6 (supplemental Figure 2) and patient 14 (not shown).
Summary of rituximab CSF concentrations
| . | Wk 1 . | Wk 2 . | Wk 3 . | Wk 4 . | Wk 5 restage . | ||||
|---|---|---|---|---|---|---|---|---|---|
| d 1 . | d 4 . | d 1 . | d 4 . | d 1 . | d 4 . | d 1 . | d 4 . | ||
| 10-mg group (n = 3) | |||||||||
| Predose | LTR | 1.67 | 1.71 | 5.16 | 3 | 4.5 | 3.2 | 5.3 | 3.85 |
| 1 h postdose | 222 | 152 | 248 | 164 | 240 | 154 | 275 | 96 | |
| 2 h postdose | 100 | 98 | |||||||
| 25-mg group (n = 7) | |||||||||
| Predose | LTR | 5.6 | 1.38 | 5.5 | 1.6 | 6.2 | 4.9 | 12.1 | 2.3 |
| 1 h postdose | 757 | 412 | 807 | 392 | 754 | 380 | 705 | 413 | |
| 2 h postdose | 399 | 377 | |||||||
| . | Wk 1 . | Wk 2 . | Wk 3 . | Wk 4 . | Wk 5 restage . | ||||
|---|---|---|---|---|---|---|---|---|---|
| d 1 . | d 4 . | d 1 . | d 4 . | d 1 . | d 4 . | d 1 . | d 4 . | ||
| 10-mg group (n = 3) | |||||||||
| Predose | LTR | 1.67 | 1.71 | 5.16 | 3 | 4.5 | 3.2 | 5.3 | 3.85 |
| 1 h postdose | 222 | 152 | 248 | 164 | 240 | 154 | 275 | 96 | |
| 2 h postdose | 100 | 98 | |||||||
| 25-mg group (n = 7) | |||||||||
| Predose | LTR | 5.6 | 1.38 | 5.5 | 1.6 | 6.2 | 4.9 | 12.1 | 2.3 |
| 1 h postdose | 757 | 412 | 807 | 392 | 754 | 380 | 705 | 413 | |
| 2 h postdose | 399 | 377 | |||||||
The mean 1-h postdose concentrations were 194 ± 79 μg/mL and 580 ± 335 μg/mL for the 10 and 25 mg dose levels. The predose trough concentrations ranged from less than reportable (LTR) to 15.3 μg/mL at the 10-mg dose level and from LTR to 20.5 μg/mL at the 25-mg dose level. Coadministration with methotrexate was associated with a 50% decrease in the rate of rituximab elimination from the intraventricular compartment.
One patient (patient 12) with leptomeningeal lymphoma unresponsive to IV rituximab (20 previous infusions) and refractory to high-dose IV and intrathecal MTX, oral temozolomide, and intrathecal liposomal cytarabine obtained a first CR and remission of his CNS lymphoma with the intraventricular rituximab/MTX protocol. Subsequently, after 5 months of extended dosing with intraventricular rituximab treatment without relapse, this patient received consolidative autologous stem cell transplantation and continues to be in remission beyond 1 year. At pre-protocol treatment staging, this patient had persistently positive CSF cytology for malignant lymphoma despite detectable rituximab (1.3 μg/mL) within the ventricular CSF and a baseline serum rituximab concentration of 219 μg/mL. Two other patients participated in extended dosing without progression for 3 and 8 months, respectively. In patient 9, intraventricular rituximab/MTX also induced a first-ever CR that was maintained for 3 months. In patient 2, the 8-month remission induced by intraventricular rituximab/MTX exceeded in duration the PR achieved with induction high-dose IV MTX-based chemotherapy that included IV rituximab (< 2 months) and remission after salvage whole-brain irradiation (6 months).
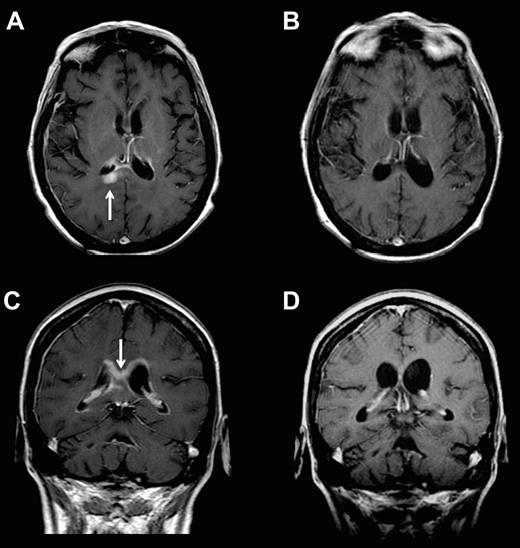
The overall CR rate in CSF and brain parenchyma was 43% and only 1 of the 6 patients who achieved cytologic and/or brain parenchymal CRs was treated with glucocorticoids during the month antecedent to pretreatment staging or during the course of protocol therapy. Complete regressions of parenchymal lesions within the temporal lobe and cerebral cortex and 1 PR within the corpus callosum were identified (Figure 3). In contrast, mixed responses were obtained in patients 1, 5, 6, and 13, in whom there was complete resolution of malignant cells in the CSF with simultaneous progression of bulky parenchymal disease that was documented by MRI at the week 5 restaging. After initial cytologic regressions during the first month of intraventricular therapy, patients 7 and 8 each developed new parenchymal lesions that were detected during the second month of the study.
Example of parenchymal response in a patient with refractory CNS lymphoma who was treated with intraventricular rituximab at 25 mg plus MTX. Panels A and C are baseline; panels B and D are after 4 weeks of therapy.
Example of parenchymal response in a patient with refractory CNS lymphoma who was treated with intraventricular rituximab at 25 mg plus MTX. Panels A and C are baseline; panels B and D are after 4 weeks of therapy.
Two of 14 patients were unable to complete the planned 4 weeks of therapy due to early disease progression: patient 4 exhibited NHL progression outside of the CNS at 3 weeks and thus was not available for CNS response and patient 14 exhibited neurologic deterioration due to lymphoma after only 2 weeks of therapy and succumbed to disease shortly thereafter (Tables 1 and 4).
Given the evidence that Ab-dependent cellular cytotoxicity contributes to the efficacy of rituximab, we evaluated the relationship between polymorphisms involving Fcγ-RIIIa (CD16) and clinical outcome among recurrent/refractory CNS lymphoma patients treated in this study. In this sample set, 2 patients (14%) had homozygous V/V (158 V/V), 7 (50%) had heterozygous V/F (158 V/F), and 5 (36%) had the low-affinity, homozygous F/F (158 F/F) allele (Table 3). Although the distribution of polymorphisms at position 158 of Fcγ-RIIIa in this population of relapsed/refractory CNS lymphoma patients was nearly identical to the distribution described in other clinical series,20 we did not observe a relationship between Fcγ-RIIIa V/F polymorphism and therapeutic resistance to intraventricular rituximab/MTX; however, our sample size was small.
Conclusions
This is the first prospective phase 1 trial of the feasibility and safety of intraventricular immunochemotherapy in the treatment of brain tumors. Combined intraventricular rituximab and MTX was well tolerated at both the 10- and 25-mg rituximab dose levels and did not produce arachnoiditis, a significant complication of intraventricular chemotherapy.21 An accumulation of evidence demonstrates a positive correlation between higher rituximab concentrations and improved disease control in systemic lymphoma.22-24 Intraventricular rituximab plus MTX therapy yielded a 75% rate of CSF cytologic CRs and a 43% rate of overall CRs in the CSF and/or brain parenchyma in a population with highly refractory primary and secondary CNS lymphoma. Although the total number of patients enrolled in this early-stage clinical trial is small, primary and secondary CNS lymphoma fulfill the quantitative criteria for an orphan disease, making it very difficult to conduct large-scale trials. Despite these limitations, our results are encouraging and offer considerable promise for future investigations to improve outcomes in CNS lymphoma and related leptomeningeal diseases.
Only 1 of the 6 patients who achieved a CR in brain and CSF at restaging after 1 month of intraventricular rituximab/MTX had been treated with glucocorticoids during protocol staging or therapy; notably, this patient (patient 7) had the shortest response duration, only 0.5 months (Table 4). This argues against systemic glucocorticoids contributing to the tumor responses evident in this trial. We believe that our study provides the first evidence that increased dose intensity of rituximab and MTX within the leptomeningeal space overcomes drug resistance in recurrent meningeal lymphoma, an important site of relapse relevant to both primary and secondary CNS lymphoma. These data are therefore pertinent to the development of novel immunochemotherapeutic strategies to be used in the treatment and the prophylaxis of CNS lymphoma and perhaps also for the neoplastic meningeal infiltration from a variety of common malignancies. Prophylactic strategies will of course be dependent on intrathecal administration via lumbar puncture, rather than intraventricular administration, as described in other studies.8,25
It is notable that 3 recurrent CNS lymphoma patients treated in this study at the 25-mg dose level experienced the complete or partial resolution of intraparenchymal brain lesions involving the corpus callosum and basal ganglia without glucocorticoid administration. Previously, these structures have been assumed to be poorly accessible via intra-CSF delivery (Figure 2). These results replicate the experience of the phase 1 trial of intraventricular rituximab monotherapy in which brain parenchymal responses to intraventricular immunotherapy were observed. Our PK findings are in agreement with a population PK model of intraventricular rituximab, based on our results in cynomolgus monkeys,4 which predicts a 3-compartment model with distinct distribution rate constants for rituximab distribution from CSF to serum and from CSF to brain tissue. Consistent with these observations are data showing that rituximab selectively penetrates full-thickness retina after intravitreal injection.26 It is also noteworthy that several recent preclinical studies have demonstrated that the intraventricular administration of neurotrophic proteins induces neurogenesis in deep-brain structures including the striatum, substantia nigra, thalamus, and dentate gyrus.27-31
In the present study, we also observed a reproducible delay in rituximab elimination from the lateral ventricle when administered in combination with MTX, which is suggestive of drug-induced perturbation of CSF flow or of a drug-drug interaction between mAb and small molecule. This finding may have significant implications for the implementation of other Ab/small molecule combinations. Further studies are needed to define the PK disposition within the neuroaxis of therapeutic proteins such as rituximab or other Abs injected within the brain ventricular system as well as to further evaluate the basis for delayed CSF elimination of rituximab when administered in combination with MTX.
Given that the majority of responding CNS lymphoma patients ultimately exhibit resistance to intraventricular rituximab, usually within 1-3 months, we infer that its short half-life in CSF may be a contributing factor to the emergence of resistant clones. By extrapolation, we hypothesize that innovations that yield higher sustained concentrations of rituximab in CSF will improve efficacy, both by enhancing drug delivery to deep-brain parenchyma and by minimizing the intervals during which the concentration of the agent is subtherapeutic. Another approach is to consider the combined administration of intraventricular plus IV rituximab as a strategy to minimize fluctuations in trough CSF concentrations of rituximab, exploit disruptions in the blood-brain barrier, and simultaneously provide dose-intensive therapy to the leptomeningeal compartment. Given its favorable toxicity profile and demonstration of potent antilymphoma efficacy within the CSF compartment in a highly refractory population, intraventricular rituximab in combination with MTX should be considered as a strategy to rapidly cytoreduce refractory leptomeningeal CD20+ NHL. The addition of MTX to rituximab may be particularly active in the setting of high burden of leptomeningeal disease; for example, lymphoma cell count in the CSF of greater than 20 000 cells/mL. The addition of MTX to rituximab may also be an attractive option if intrathecal rituximab therapy yields only a PR. This approach will be applied in a successor study in development that evaluates lenalidomide in combination with intraventricular plus IV rituximab.
Presented in abstract form at the 53rd Annual Meeting of the American Society of Hematology, San Diego, CA, December 13, 2011.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this study and Sherri Bush, Elaine Cooperstein, and Paula Fiermonte for their contributions.
This research was supported by the Leukemia & Lymphoma Society, a University of California, San Francisco Brain Tumor Specialized Programs of Research Excellence grant, Gabrielle's Angel Foundation, and the National Institutes of Health (grant R01CA139-83-01A1 to J.L.R.).
National Institutes of Health
Authorship
Contribution: J.L.R. designed and performed the research, analyzed the data, and wrote the manuscript; J.L., J.H., G.A., C.K., C.L., and S.C. analyzed the data and wrote the manuscript; L.C., R.A., and H.K. performed the research and wrote the manuscript; J.D., E.G., and T.B. performed the research, analyzed the data, and wrote the manuscript; and P.M., M.A.S., and L.E.D. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: T.B. was a consultant to Genentech/Roche. The remaining authors declare no competing financial interests.
Correspondence: James L. Rubenstein, MD, PhD, University of California, San Francisco Division of Hematology/Oncology, M1282 Box 1270, San Francisco, CA 94143; e-mail: jamesr@medicine.ucsf.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal