Key Points
Efficacy of transplanting adequately dosed 1- or 2-cord blood units.
Abstract
Cell dose is a major limitation for umbilical cord blood (UCB) transplantation because units containing a minimum of 2.5 × 107 total nucleated cells (TNC)/kilogram patient body weight are frequently not available. The transplantation of 2 partially HLA-matched UCB units has been adopted as a simple approach for increasing the TNC. We sought to determine whether the relative safety and efficacy of this approach was comparable with a single UCB transplantation. Included are adults with acute leukemia who received transplants with 1 (n = 106) or 2 (n = 303) UCB units. All UCB units for single UCB transplantations contained TNC ≥ 2.5 × 107/kg. For double UCB transplantations, the total TNC for units 1 and 2 were > 2.5 × 107/kg but in approximately half of these transplantations, 1 of the 2 units contained < 2.5 × 107 TNC/kg. Adjusting for factors associated with outcomes, risks of neutrophil recovery (odds ratio 0.83, P = .59), transplantation-related mortality (hazard ratio [HR] 0.91, P = .63), relapse (HR 0.90, P = .64), and overall mortality (HR 0.93, P = .62) was similar after double UCB and adequate dose single UCB transplantations. These data support double UCB unit transplantation for acute leukemia when an adequately dosed single UCB unit is not available thereby extending access to nearly all patients.
Introduction
Umbilical cord blood (UCB) is now widely used as a source of hematopoietic stem cells for transplantation in patients who lack a suitable related or unrelated adult volunteer donor. While HLA-matching requirements restrict the availability of unrelated adult donors, the total nucleated cell (TNC) dose of single UCB units has limited its use in older or heavier patients.1 The minimum acceptable pre-cryopreservation TNC of a unit is 2.5 × 107/kg patient body weight. Transplantation of UCB with cell doses below this threshold has been associated with slow hematopoietic recovery, poor engraftment, and high transplantation-related mortality (TRM).
Transplantation of 2 partially HLA-matched UCB units (double UCB) has been studied as one potential strategy for overcoming the cell-dose limitation. Multiple reports confirm high rates of engraftment and promising survival outcomes after double UCB transplantation.2-8 Reports from a single institution also suggest a higher risk of grade 2 acute graft-versus-host disease (GVHD) and lower risk of relapse after double UCB transplantation in patients with acute leukemia.9,10 Data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) suggest increasing numbers of double UCB transplantations being performed and now account for approximately 80% of UCB transplantations for adults in the United States. The decision to infuse 2 UCB units varies between institutions.11-13 Using data reported to registries, we performed a retrospective analysis to determine the relative risks and benefits of transplanting 2 UCB units compared with adequately dosed 1 UCB unit.
Methods
Collection of data
Data were obtained from the CIBMTR (N = 330) or the National Cord Blood Program, New York Blood Center (N = 79). The CIBMTR is a working group of more than 450 transplant centers worldwide that provide detailed demographic, treatment, and outcome data on consecutive transplantations at their institutions to a Statistical Center at the Medical College of Wisconsin. Transplantations facilitated by the National Cord Blood Program are reported to that Cord Blood Bank. Patients provided written informed consent in accordance with the Declaration of Helsinki. The institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Inclusion criteria
Patients aged 16 years or older with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) who received transplants with 1 or 2 UCB units after 2001 were eligible. Recipients of prior autologous (n = 1) or allogeneic (n = 5) transplantations were excluded. Single UCB units contained a minimum cryopreserved TNC dose of 2.5 × 107/kg. TNC for double UCB transplantations was treated as sum of units 1 and 2. The sum TNC was in excess of 2.5 × 107/kg for all double UCB transplantations, but individual units contained TNC < 2.5 × 107/kg in approximately half of these transplantations. HLA match between the UCB unit and patient was determined using antigen-level typing at HLA-A and -B and allele level at -DRB1. For double UCB transplantations, the assignment of donor-recipient HLA match was based on the greatest HLA mismatch of the 2 units; however, in most cases (65%), the number of antigens mismatched was the same for both units. Interunit HLA-matching requirements varied between institutions.
End points
Neutrophil recovery was defined as achieving an absolute neutrophil count of ≥ 0.5 × 109/L for 3 consecutive days and donor chimerism (> 90% within the first 3 months from transplantation). Platelet recovery was defined as achieving 20 × 109/L without transfusions for 7 days. Incidences of grade 2-4 acute and chronic GVHD were based on reports using standard criteria.14,15 TRM was defined as time to death not related to leukemia recurrence and relapse was defined as leukemia recurrence based on morphologic evaluation. Leukemia-free survival (LFS) was defined as survival in a state of continuous complete remission and treatment failure, as relapse or death (inverse of LFS). Overall mortality was defined as death from any cause.
Statistical analysis
Differences among the groups were examined using the χ2 statistic for categorical variables. The probabilities of LFS and overall survival were calculated with Kaplan-Meier estimator.16 Probabilities of neutrophil and platelet recovery, acute and chronic GVHD, TRM, and relapse were calculated with the cumulative incidence estimator.16 For all analyses, data on patients without an event was censored at last follow-up.
Cox proportional hazard regression models were constructed for acute and chronic GVHD, TRM, relapse, treatment failure, and overall mortality.17 Logistic regression models were built for neutrophil recovery at day 42 and for platelet recovery at 6 months using the pseudo-value approach.18 Multivariate models were built with the use of stepwise forward selection, using a P ≤ .05 to include variables in the model. Proportional-hazards assumption was tested for each variable individually and any violations were appropriately adjusted for by stratifying the final regression models for the affected variables. Results are expressed as relative hazard ratio (HR) or odds ratio (OR) with 95% confidence interval (CI).
The primary objective was to evaluate transplantation outcomes after double UCB and single UCB unit transplantation and the variable for number of UCB units infused was held in all steps of model building. We explored for an infused TNC dose that may be associated with survival advantage separately for single and double UCB transplantations and found none. We also tested for an effect of transplant center on overall survival using the score test of Commenges and Andersen resulting in a P value of .06.19 Consequently, all multivariate models were adjusted for the effect of transplant center using the marginal approach which adjust for dependence between outcomes at center using a robust covariance estimator.20 In addition to the variable for number of UCB units infused, other variables considered in model building for neutrophil and platelet recovery, acute and chronic GVHD, TRM, relapse, treatment failure (inverse of LFS), and overall mortality included: age (16-20 years vs 21-39 years vs ≥ 40 years), recipient cytomegalovirus serology (positive vs negative), disease (ALL vs AML), disease status at transplantation: first complete remission (CR) vs second or third CR vs not in remission, conditioning regimen (myeloablative with total body irradiation [TBI] vs myeloablative without TBI vs reduced-intensity with TBI 200 cGy, cyclophosphamide, and fludarabine vs non-TBI reduced-intensity with alkylating agent and fludarabine), GVHD prophylaxis (cyclosporine vs tacrolimus), infused TNC (< 2.5 vs 2.5-3.0 vs > 3.0-4.0 vs > 4.0), and year of transplantation (2002-2004 vs 2005-2009). All P values are 2-sided. Analyses were done with SAS software (Version 9.1).
Results
Patients, disease, and transplantation characteristics
The characteristics of patients, their diseases, and transplantations are shown in Table 1. Relatively few adults had an adequate single UCB unit (N = 106) with the majority (N = 303, 75%) receiving 2 partially HLA-matched units. While patient cytomegalovirus (CMV) serostatus and leukemia type were similar between groups, double UCB transplant recipients were more likely to be heavier (median weight 76 kg vs 62 kg), older (43 years vs 33 years), in remission (84% vs 70%), conditioned with a dose-reduced regimen (34% vs 23%), and treated with mycophenolate mofetil (MMF) in combination with cyclosporine A or tacrolimus (86% vs 57%). The predominant myeloablative conditioning regimen was total body irradiation (TBI) + cyclophosphamide and the reduced-intensity regimen, TBI 200 cGy + cyclophosphamide + fludarabine. The use of antithymocyte globulin (ATG) varied with conditioning regimen intensity; approximately 25% of recipients of myeloablative regimens received ATG compared with approximately 40% of recipients who received reduced-intensity regimens (footnote to Table 1). The median pre-cryopreserved TNC for double UCB transplantation was 4.6 × 107/kg and for single UCB unit transplantation, 3.5 × 107/kg. The corresponding median-infused TNC doses were 3.8 × 107/kg and 2.8 × 107/kg. In addition, recipients of 2 units were more likely to be HLA-mismatched at 2 loci (4 of 6 HLA match) compared with recipients of single UCB unit transplantations. While the number of transplantations with a single UCB unit remained stable over the years, double UCB transplantations were more frequent after 2004.
Patient, disease, and transplantation characteristics
| Characteristics . | No. (%) . | P . | |
|---|---|---|---|
| Double UCB Transplantation . | Single UCB Transplantation . | ||
| No. of patients | 303 | 106 | |
| No. of transplantation teams | 45 | 47 | |
| Median recipient age, y (range) | 43 (16-71) | 33 (16-69) | < .0001 |
| 16-20 | 32 (11) | 27 (25) | |
| 21-30 | 53 (17) | 20 (19) | |
| 31-40 | 48 (16) | 21 (20) | |
| 41-50 | 51 (17) | 15 (14) | |
| 51-60 | 76 (25) | 14 (13) | |
| 61-71 | 43 (14) | 9 (8) | |
| Recipient CMV serostatus transplantation | .52 | ||
| Negative | 93 (31) | 35 (33) | |
| Positive | 193 (64) | 68 (64) | |
| Not reported | 17 (6) | 3 (3) | |
| Disease | .34 | ||
| Acute myeloid leukemia | 215 (71) | 70 (66) | |
| Acute lymphoblastic leukemia | 88 (29) | 36 (34) | |
| Disease status | .006 | ||
| First complete remission | 135 (45) | 38 (36) | |
| Second complete remission | 100 (33) | 31 (29) | |
| Third complete remission | 20 (7) | 5 (5) | |
| Relapse | 48 (16) | 32 (30) | |
| Donor-recipient HLA match | .07 | ||
| 6/6 | 13 (4) | 10 (9) | |
| 5/6 | 80 (26) | 33 (31) | |
| 4/6 | 210 (69) | 63 (59) | |
| Conditioning regimen | < .0001 | ||
| Myeloablative | |||
| TBI + cyclophosphamide* | 94 (31) | 49 (46) | |
| TBI + other agents** | 22 (7) | 10 (9) | |
| Busulfan + cyclophosphamide*** | 7 (2) | 8 (8) | |
| Busulfan + other agents**** | 16 (5) | 10 (9) | |
| Reduced intensity | |||
| TBI + cyclophosphamide + fludarabine† | 119 (39) | 20 (19) | |
| Busulfan + fludarabine†† | 5 (2) | 2 (2) | |
| Melphalan + fludarabine††† | 35 (12) | 5 (5) | |
| Cyclophosphamide + fludarabine†††† | 5 (2) | 2 (2) | |
| GVHD prophylaxis | < .0001 | ||
| Tacrolimus alone or with steroid | 8 (3) | 10 (9) | |
| Tacrolimus + methotrexate | 6 (2) | 9 (8) | |
| Tacrolimus + mycophenolate mofetil | 85 (28) | 20 (19) | |
| Cyclosporine alone or with steroid | 8 (3) | 19 (8) | |
| Cyclosporine + methotrexate | 1 (< 1) | 6 (6) | |
| Cyclosporine + mycophenolate mofetil | 176 (58) | 40 (38) | |
| Mycophenolate mofetil + steroid | 1 (< 1) | ||
| Not reported | 18 (6) | 2 (2) | |
| TNC cryopreserved, ×107/kg (sum of units) | < .0001 | ||
| Median dose (range) | 4.5 (2.6-15) | 3.5 (2.5-8.6) | |
| 2.5-3.0 | 18 (6) | 26 (28) | |
| 3.0-4.0 | 77 (25) | 47 (43) | |
| > 4.0 | 202 (67) | 33 (30) | |
| Not reported | 6 (2) | ||
| TNC infused, ×107/kg (sum of units) | < .0001 | ||
| Median dose (range) | 3.7 (1.8-15.8) | 2.8 (1.0-7.5) | |
| < 2.5 | 28 (9) | 30 (28) | |
| 2.5-3.0 | 49 (16) | 35 (33) | |
| 3.0-4.0 | 108 (36) | 21 (20) | |
| > 4.0 | 111 (37) | 20 (19) | |
| Not reported | 6 (2) | ||
| Year of transplantation | < .0001 | ||
| 2002-2004 | 40 (14) | 32 (30) | |
| 2005-2009 | 263 (86) | 74 (70) | |
| Median follow-up, mo (range) | 23 (3-74) | 17 (3-62) | |
| Characteristics . | No. (%) . | P . | |
|---|---|---|---|
| Double UCB Transplantation . | Single UCB Transplantation . | ||
| No. of patients | 303 | 106 | |
| No. of transplantation teams | 45 | 47 | |
| Median recipient age, y (range) | 43 (16-71) | 33 (16-69) | < .0001 |
| 16-20 | 32 (11) | 27 (25) | |
| 21-30 | 53 (17) | 20 (19) | |
| 31-40 | 48 (16) | 21 (20) | |
| 41-50 | 51 (17) | 15 (14) | |
| 51-60 | 76 (25) | 14 (13) | |
| 61-71 | 43 (14) | 9 (8) | |
| Recipient CMV serostatus transplantation | .52 | ||
| Negative | 93 (31) | 35 (33) | |
| Positive | 193 (64) | 68 (64) | |
| Not reported | 17 (6) | 3 (3) | |
| Disease | .34 | ||
| Acute myeloid leukemia | 215 (71) | 70 (66) | |
| Acute lymphoblastic leukemia | 88 (29) | 36 (34) | |
| Disease status | .006 | ||
| First complete remission | 135 (45) | 38 (36) | |
| Second complete remission | 100 (33) | 31 (29) | |
| Third complete remission | 20 (7) | 5 (5) | |
| Relapse | 48 (16) | 32 (30) | |
| Donor-recipient HLA match | .07 | ||
| 6/6 | 13 (4) | 10 (9) | |
| 5/6 | 80 (26) | 33 (31) | |
| 4/6 | 210 (69) | 63 (59) | |
| Conditioning regimen | < .0001 | ||
| Myeloablative | |||
| TBI + cyclophosphamide* | 94 (31) | 49 (46) | |
| TBI + other agents** | 22 (7) | 10 (9) | |
| Busulfan + cyclophosphamide*** | 7 (2) | 8 (8) | |
| Busulfan + other agents**** | 16 (5) | 10 (9) | |
| Reduced intensity | |||
| TBI + cyclophosphamide + fludarabine† | 119 (39) | 20 (19) | |
| Busulfan + fludarabine†† | 5 (2) | 2 (2) | |
| Melphalan + fludarabine††† | 35 (12) | 5 (5) | |
| Cyclophosphamide + fludarabine†††† | 5 (2) | 2 (2) | |
| GVHD prophylaxis | < .0001 | ||
| Tacrolimus alone or with steroid | 8 (3) | 10 (9) | |
| Tacrolimus + methotrexate | 6 (2) | 9 (8) | |
| Tacrolimus + mycophenolate mofetil | 85 (28) | 20 (19) | |
| Cyclosporine alone or with steroid | 8 (3) | 19 (8) | |
| Cyclosporine + methotrexate | 1 (< 1) | 6 (6) | |
| Cyclosporine + mycophenolate mofetil | 176 (58) | 40 (38) | |
| Mycophenolate mofetil + steroid | 1 (< 1) | ||
| Not reported | 18 (6) | 2 (2) | |
| TNC cryopreserved, ×107/kg (sum of units) | < .0001 | ||
| Median dose (range) | 4.5 (2.6-15) | 3.5 (2.5-8.6) | |
| 2.5-3.0 | 18 (6) | 26 (28) | |
| 3.0-4.0 | 77 (25) | 47 (43) | |
| > 4.0 | 202 (67) | 33 (30) | |
| Not reported | 6 (2) | ||
| TNC infused, ×107/kg (sum of units) | < .0001 | ||
| Median dose (range) | 3.7 (1.8-15.8) | 2.8 (1.0-7.5) | |
| < 2.5 | 28 (9) | 30 (28) | |
| 2.5-3.0 | 49 (16) | 35 (33) | |
| 3.0-4.0 | 108 (36) | 21 (20) | |
| > 4.0 | 111 (37) | 20 (19) | |
| Not reported | 6 (2) | ||
| Year of transplantation | < .0001 | ||
| 2002-2004 | 40 (14) | 32 (30) | |
| 2005-2009 | 263 (86) | 74 (70) | |
| Median follow-up, mo (range) | 23 (3-74) | 17 (3-62) | |
TBI indicates total body irradiation; TNC, total nucleated cell dose; and UCB, umbilical cord blood.
N = 4 double UCB recipients and N = 13 single UCB recipients received ATG.
N = 4 double UCB recipients and N = 4 single UCB recipients received ATG.
N = 3 double UCB recipients and N = 6 single UCB recipients received ATG.
N = 9 double UCB recipients and N = 6 single UCB recipients received ATG.
N = 35 double UCB recipients and N = 4 single UCB recipients received ATG; TBI dose = 200 cGy
N = 2 double UCB recipients and N = 1 single UCB recipients received ATG.
N = 30 double UCB recipients and N = 4 single UCB recipients received ATG.
N = 2 double UCB recipients and N = 1 single UCB recipients received ATG.
Seventy transplant centers in the United States contributed patients; 22 centers performed both single and double UCB transplantations, 25 centers performed only single UCB transplantations, and 23 centers performed only double UCB transplantations. Approximately 70% of centers contributed < 5 transplantations, 25% contributed 5-10 transplantations, and the remaining 5%, > 10 transplantations. The median follow-up of surviving patients after double UCB transplantation was 23 months and 17 months after single UCB transplantation.
Neutrophil and platelet recovery
The median time to neutrophil recovery was 20 days after double or adequately dosed single UCB transplantation. The probabilities of neutrophil recovery by day 42 were 78% (95% CI, 72-83) after double and 81% (95% CI, 74-88) after adequate single UCB transplantations (P = .83). In multivariate analysis, the likelihood of neutrophil recovery after double relative to adequate single UCB transplantation was similar (OR 0.83, 95% CI 0.41-1.67, P = .59). Disease status influenced neutrophil recovery independent of the number of UCB units infused. Compared with patients who received transplants in CR, those receiving transplants in relapse were less likely to recover neutrophils (OR 0.44, 95% CI 0.21-0.89, P = .02).
There were no significant differences in the likelihood of platelet recovery at 6 months between the 2 groups (OR 0.96, 95% CI 0.69-1.33, P = .81). The median time to recovery after double UCB transplantation was 55 days vs 46 days after an adequate dose single. The corresponding probabilities of recovery at 6 months were 68% (95% CI, 62-74) and 63% (95% CI, 53-72; P = .34). Disease status at transplantation and transplantation-conditioning regimen were associated with platelet recovery. Compared with transplantations in CR, recovery was lower for patients who received transplants in relapse (OR 0.49, 95% CI 0.32-0.77, P = .002). Compared with myeloablative TBI regimens, the likelihood of platelet recovery was higher after reduced-intensity TBI-containing (OR 1.87, 95% CI 1.33-2.62, P = .0003) and non-TBI regimens (OR 1.64, 95% CI 1.04-2.61, P = .04).
Acute and chronic GVHD
In multivariate analysis, risks of grade 2-4 acute GVHD was significantly higher in recipients of double UCB compared with single UCB transplantations during the period 2002-2004 (HR 6.14, 95% CI 2.54-14.87, P < .001). In the later period (2005-2009), grade 2-4 acute GVHD risks were not significantly different in recipients of double and single UCB transplantations (HR 1.69, 95% CI 0.68-4.18, P = .30). The day-100 probabilities of grade 2-4 acute GVHD after double and single UCB transplantations in 2002-2004 were 58% (95% CI, 42-71) and 18% (95% CI, 8-32), respectively, P < .001. The corresponding probabilities after double and single UCB transplantations in 2005-2009 were 31% (95% CI, 25-37) and 27% (95% CI, 18-37), respectively. There were no differences in the risks of grade 3-4 acute GVHD after double and single UCB transplantation (HR 0.88, 95% CI 0.51-1.51, P = .64). The day-100 probabilities of grade 3-4 acute GVHD after double and single UCB transplantation were 18% (95% CI, 11-26) and 14% (95% CI, 10-19), respectively. Similarly, there were no differences in chronic GVHD risks after double and single UCB transplantation (HR 1.33, 95% CI 0.80-2.19, P = .27). The 2-year probabilities of chronic GVHD were 31% (95% CI, 26-37) and 24% (95% CI, 15-34) after double and single UCB transplantation, respectively.
Transplantation-related mortality and relapse
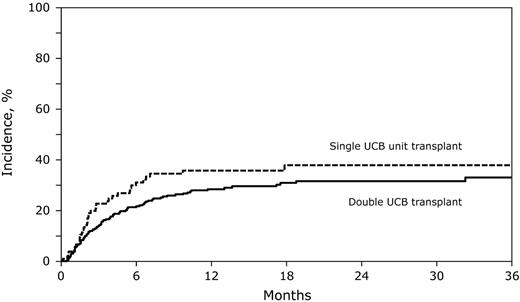
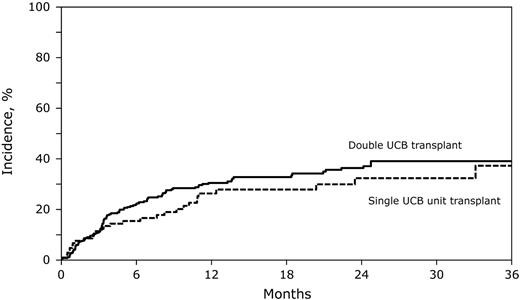
In multivariate analysis, there were no significant differences in risks of TRM and relapse after double and single UCB transplantation (Table 2). The 2-year probabilities of TRM after double and single UCB transplantation were similar (Figure 1), although there was a trend toward a lower 6-month probability of TRM after double UCB transplantation (21% [95% CI, 17-26] vs 31% [95% CI, 22-40], P = .06). The 2-year probabilities of relapse were similar after double and single UCB transplantation (Figure 2). Other factors associated with higher relapse risks were older age, not in first remission at transplantation and use of non–TBI-containing myeloablative or reduced-intensity transplantation-conditioning regimens (Table 2).
Risks of TRM, relapse, treatment failure, and overall mortality
| Variable . | Hazard ratio (95% confidence interval) . | P . |
|---|---|---|
| TRM* | ||
| Double UCB vs single UCB | 0.91 (0.65-1.27) | .56 |
| Relapse | ||
| Double UCB vs single UCB | 0.93 (0.55-1.56) | .78 |
| Disease status at transplantation | ||
| Second/third vs first CR | 1.33 (1.01-1.76) | .05 |
| Relapse vs first CR | 3.56 (2.69-4.72) | < .0001 |
| Age, y | ||
| 21-40 vs 16-20 | 2.73 (1.51-4.92) | .001 |
| > 40 vs 16-20 | 2.59 (1.32-5.12) | .006 |
| Conditioning regimen | ||
| Non-TBI MAC vs TBI MAC | 2.69 (1.68-4.32) | < .0001 |
| Non-TBI RIC vs TBI MAC | 1.79 (1.06-3.03) | .03 |
| TBI RIC vs TBI MAC | 3.14 (2.13-4.63) | < .0001 |
| Treatment failure (inverse of LFS) | ||
| Double UCB vs single UCB | 0.97 (0.77-1.22) | .80 |
| Disease status at transplantation | ||
| Second/third vs first CR | 1.27 (1.04-1.55) | .02 |
| Relapse vs first CR | 2.22 (1.78-2.76) | < .0001 |
| Overall mortality | ||
| Double UCB vs single UCB | 0.93 (0.65-1.33) | .67 |
| Disease status at transplantation | ||
| Second/third vs first CR | 1.34 (1.05-1.70) | .02 |
| Relapse vs first CR | 2.28 (1.83-2.86) | < .0001 |
| Variable . | Hazard ratio (95% confidence interval) . | P . |
|---|---|---|
| TRM* | ||
| Double UCB vs single UCB | 0.91 (0.65-1.27) | .56 |
| Relapse | ||
| Double UCB vs single UCB | 0.93 (0.55-1.56) | .78 |
| Disease status at transplantation | ||
| Second/third vs first CR | 1.33 (1.01-1.76) | .05 |
| Relapse vs first CR | 3.56 (2.69-4.72) | < .0001 |
| Age, y | ||
| 21-40 vs 16-20 | 2.73 (1.51-4.92) | .001 |
| > 40 vs 16-20 | 2.59 (1.32-5.12) | .006 |
| Conditioning regimen | ||
| Non-TBI MAC vs TBI MAC | 2.69 (1.68-4.32) | < .0001 |
| Non-TBI RIC vs TBI MAC | 1.79 (1.06-3.03) | .03 |
| TBI RIC vs TBI MAC | 3.14 (2.13-4.63) | < .0001 |
| Treatment failure (inverse of LFS) | ||
| Double UCB vs single UCB | 0.97 (0.77-1.22) | .80 |
| Disease status at transplantation | ||
| Second/third vs first CR | 1.27 (1.04-1.55) | .02 |
| Relapse vs first CR | 2.22 (1.78-2.76) | < .0001 |
| Overall mortality | ||
| Double UCB vs single UCB | 0.93 (0.65-1.33) | .67 |
| Disease status at transplantation | ||
| Second/third vs first CR | 1.34 (1.05-1.70) | .02 |
| Relapse vs first CR | 2.28 (1.83-2.86) | < .0001 |
CR indicates complete remission; LFS, leukemia-free survival; MAC, myleoablative conditioning; RIC, reduced-intensity conditioning; TBI, total body irradiation; TRM, transplantation-related mortality; and UCB, umbilical cord blood.
Stratified for transplantation-conditioning regimen and GVHD prophylaxis.
The cumulative incidence of TRM after double UCB and adequately dosed single UCB unit transplantation. The 2-year cumulative incidence of TRM after double UCB transplantations was 32% (95% CI, 26-37) compared with 38% (95% CI, 28-48) after single UCB transplantation.
The cumulative incidence of TRM after double UCB and adequately dosed single UCB unit transplantation. The 2-year cumulative incidence of TRM after double UCB transplantations was 32% (95% CI, 26-37) compared with 38% (95% CI, 28-48) after single UCB transplantation.
The cumulative incidence of relapse after double UCB and adequately dosed single UCB unit transplantation. The 2-year cumulative incidence of relapse after double UCB transplantations was 36% (95% CI, 30-42) compared with 32% (95% CI, 22-43) after single UCB transplantation.
The cumulative incidence of relapse after double UCB and adequately dosed single UCB unit transplantation. The 2-year cumulative incidence of relapse after double UCB transplantations was 36% (95% CI, 30-42) compared with 32% (95% CI, 22-43) after single UCB transplantation.
Leukemia-free and overall survival
The 2-year probability of LFS after double UCB transplantation was 32% (95% CI, 26-39) and similar to that observed in recipients of an adequate single UCB unit, 30% (95% CI, 20-41), P = .72. In multivariate analysis, there were no significant differences in risks of treatment failure between the 2 groups (Table 2). The only factor associated with treatment failure was disease status; treatment failure was higher in patients who were not in remission at transplantation and in second or third CR relative to those in first remission at transplantation.
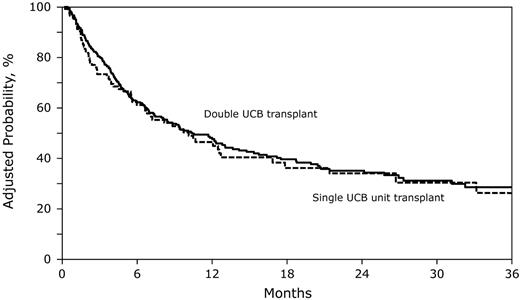
Similarly, the risk of overall mortality was similar in recipients of double and an adequate single UCB transplantation (Table 2; Figure 3). As seen for treatment failure, mortality risks were higher for patients who received transplants in relapse and second or third CR relative to those who received transplants in first remission. The most common causes of death in the 2 treatment groups were recurrent leukemia, infection, and organ failure.
The adjusted probability of overall survival after double UCB and adequately dosed single UCB unit transplantation. The 2-year probability of overall survival after double UCB transplantations was 35% (95% CI, 29-42) compared with 33% (95% CI, 22-44), P = .66) after single UCB transplantation, adjusted for disease status at transplantation.
The adjusted probability of overall survival after double UCB and adequately dosed single UCB unit transplantation. The 2-year probability of overall survival after double UCB transplantations was 35% (95% CI, 29-42) compared with 33% (95% CI, 22-44), P = .66) after single UCB transplantation, adjusted for disease status at transplantation.
Subset analysis
In 2 separate multivariate analyses, we examined for significant differences in TRM, relapse, treatment failure, and overall mortality after double UCB and single UCB transplantations by conditioning regimen intensity. As shown in Table 3 and consistent with the main analysis, the risks of TRM, relapse, treatment failure, and overall mortality are not significantly different after double UCB and single UCB transplantations whether the transplantation-conditioning regimen is myeloablative or reduced intensity.
Results of multivariate analysis for transplantation-related mortality, relapse, leukemia-free, and overall survival by transplantation conditioning regimen
| Outcome . | Hazard ratio (95% confidence interval) . | P . |
|---|---|---|
| Myeloablative conditioning regimen | ||
| Transplantation-related mortality | ||
| Double UCB vs single UCB | 0.81 (0.52-1.28) | .37 |
| Relapse* | ||
| Double UCB vs single UCB | 0.94 (0.51-1.73) | .84 |
| Treatment failure (inverse of LFS)† | ||
| Double UCB vs single UCB | 0.88 (0.61-1.26) | .48 |
| Overall mortality† | ||
| Double UCB vs single UCB | 0.88 (0.61-1.27) | .51 |
| Reduced-intensity conditioning regimen | ||
| Transplantation-related mortality | ||
| Double UCB vs single UCB | 1.49 (0.51-4.36) | .46 |
| Relapse* | ||
| Double UCB vs single UCB | 0.88 (0.47-1.63) | .67 |
| Treatment failure (inverse of LFS)† | ||
| Double UCB vs single UCB | 1.08 (0.64-1.82) | .78 |
| Overall mortality† | ||
| Double UCB vs single UCB | 1.01 (0.57-1.77) | .98 |
| Outcome . | Hazard ratio (95% confidence interval) . | P . |
|---|---|---|
| Myeloablative conditioning regimen | ||
| Transplantation-related mortality | ||
| Double UCB vs single UCB | 0.81 (0.52-1.28) | .37 |
| Relapse* | ||
| Double UCB vs single UCB | 0.94 (0.51-1.73) | .84 |
| Treatment failure (inverse of LFS)† | ||
| Double UCB vs single UCB | 0.88 (0.61-1.26) | .48 |
| Overall mortality† | ||
| Double UCB vs single UCB | 0.88 (0.61-1.27) | .51 |
| Reduced-intensity conditioning regimen | ||
| Transplantation-related mortality | ||
| Double UCB vs single UCB | 1.49 (0.51-4.36) | .46 |
| Relapse* | ||
| Double UCB vs single UCB | 0.88 (0.47-1.63) | .67 |
| Treatment failure (inverse of LFS)† | ||
| Double UCB vs single UCB | 1.08 (0.64-1.82) | .78 |
| Overall mortality† | ||
| Double UCB vs single UCB | 1.01 (0.57-1.77) | .98 |
LFS indicates leukemia-free survival; and UCB, umbilical cord blood
Model adjusted for age and disease status.
Model adjusted for disease status.
We tested carefully for an effect of total infused TNC on transplantation outcomes, we found none. To further explore for an effect of TNC, recipients of double UCB transplantations were assigned to 3 groups: group 1, infused TNC of both units was < 2.5 × 107/kg (n = 142); group 2, infused TNC of unit 1 was < 2.5 × 107/kg and unit 2 ≥ 2.5 × 107/kg (n = 96); and group 3, infused TNC of both units was ≥ 2.5 × 107/kg (n = 59). These groups were compared with recipients of single UCB transplantations. The 2-year probabilities of overall survival, adjusted for disease status at transplantation, were 34% (95% CI, 24-45) after single UCB and 37% (95% CI, 27-46), 36% (95% CI, 25-46), and 33% (95% CI, 21-46) after group 1, 2, and 3 double UCB transplantations, respectively (P = .97; Figure 4). The corresponding probabilities of TRM were 38% (95% CI, 28-48), 35% (95% CI, 26-44), 29% (95% CI, 20-39), and 26% (95% CI, 15-39; P = .40).
The adjusted probability of overall survival after double UCB transplantation (infused TNC of both units was < 2.5 × 107/kg [group 1], infused TNC of unit 1 was < 2.5 × 107/kg and unit 2, ≥ 2.5 × 107/kg [group 2] and infused TNC of both units was ≥ 2.5 × 107/kg [group 3]) and adequately dosed single UCB unit transplantation. The 2-year probabilities of overall survival were 37% (95% CI, 27-46), 36% (95% CI, 25-46), and 33% (95% CI, 21-46) after group 1, 2, and 3 double UCB transplantations, respectively, and 34% (95% CI, 24-45) after single UCB transplantation, adjusted for disease status at transplantation.
The adjusted probability of overall survival after double UCB transplantation (infused TNC of both units was < 2.5 × 107/kg [group 1], infused TNC of unit 1 was < 2.5 × 107/kg and unit 2, ≥ 2.5 × 107/kg [group 2] and infused TNC of both units was ≥ 2.5 × 107/kg [group 3]) and adequately dosed single UCB unit transplantation. The 2-year probabilities of overall survival were 37% (95% CI, 27-46), 36% (95% CI, 25-46), and 33% (95% CI, 21-46) after group 1, 2, and 3 double UCB transplantations, respectively, and 34% (95% CI, 24-45) after single UCB transplantation, adjusted for disease status at transplantation.
Discussion
Numerous reports have shown that cell dose is a critical determinant of hematopoietic recovery, TRM, and overall survival after UCB transplantation.1,13,21,22 The concept of transplanting 2 partially HLA-matched UCB units to overcome the cell-dose barrier was pioneered at the University of Minnesota2-4 and adopted by others to extend the application of UCB transplantation to adults. To date, there are several reports from single centers2-8 and one multicenter phase 2 clinical trial23 that demonstrate the potential effectiveness of double UCB transplantation for adults with hematologic malignancies. However, the purpose of this study was to address the fundamental question of whether the infusion of 2 partially HLA-matched UCB units could effectively “create” an adequately dosed graft for patients lacking access to a single UCB unit containing ≥ 2.5 × 107 TNC/kg. To answer this question, we compared transplantation outcomes in patients who received transplants with 2 UCB units either as a result of center practice or when an adequate single UCB unit could not be identified. The results of this retrospective registry study support the efficacy of double UCB transplantation as it is associated with rates of neutrophil recovery, TRM, relapse, LFS, and OS that are similar to those observed in recipients of a single UCB unit with an adequate cell dose.
In contrast to what has been reported by others,10,24 we did not observe significant differences in relapse risks after single and double UCB transplantations. Verneris and colleagues report lower relapse risks in patients with AML or ALL who received transplants in first or second CR after double UCB compared with single UCB transplantation.10 Rocha and colleagues report lower relapse risks after double UCB compared with single UCB transplantations for patients in first but not second CR.24 However, neither report10,24 demonstrated differences in overall or leukemia-free survival. The observed differences may be explained by differences in patient, disease, and transplantation characteristics of the study populations. The results from a recently completed multicenter phase 3 trial that randomized children with acute leukemia to single or double UCB transplantation with a uniform transplantation conditioning and GVHD prophylaxis regimen and the ongoing multicenter randomized trial in children and adults younger than 35 years of age may provide a more definitive conclusion on the effect of double UCB transplantations on relapse and LFS.
We observed higher risks of grade 2 acute GVHD after double UCB transplantations in the period 2002-2004 which is consistent with that reported by the Minnesota group.7 Almost all patients in the current analysis who received double UCB transplantations in 2002-2004 received transplants at that institution and only 10% of patents received ATG. In the later period, double UCB transplantations occurred at multiple centers and a third of patients received ATG. We hypothesize the observed higher rate of grade 2 acute GVHD in 2002-2004 can be attributed to use of non-ATG–containing regimens. Practice varies between transplant centers and transplantation periods. We controlled for these factors by considering transplantation period and the effect of transplant centers in the final analyses.
We explored for a threshold dose of TNC on survival and found none. The relatively narrow range of TNC for adult transplantations regardless of whether 1 or 2 UCB units were infused prevented us from identifying a threshold dose above which there would be a survival advantage. Moreover, the infused TNC for single unit UCB transplantations was relatively high and that for double UCB transplantations significantly higher compared with single UCB transplantations (3.8 × 107/kg vs 2.8 × 107/kg, P < .0001). Published reports suggest a minimum pre-cryopreserved TNC dose (2.5-3.0 × 107/kg) is needed to achieve hematopoietic recovery and lower mortality but the data does not suggest progressive increase in TNC improves hematopoietic recovery or survival.13,21,22
We confirm infusing 2 UCB units to overcome the cell-dose barrier extends hematopoietic stem cell transplantation to a substantial number of adults who would benefit from this treatment procedure. Although we performed a carefully controlled analysis considering known risk factors for transplantation outcome comparing treatments, the comparative effectiveness of transplanting 1 versus 2 UCB units should ideally be tested in a prospective, randomized manner. Reports on single UCB transplantations primarily in children and adolescents suggest higher TRM is associated with HLA mismatch. We were not able to study for an effect of HLA mismatch on outcomes in the current analysis. There are too many donor-recipient pairs to explore for an HLA effect, and most transplantations are mismatched at 2 HLA loci making it impossible to explore for differences between 0, 1, and 2 HLA-matched transplantations. The variability of CD34+ cell measurements across laboratories prohibited us from exploring the effect of this characteristic on transplantation outcomes. Furthermore, detailed analyses of the engrafting UCB unit is also beyond the scope of the current analysis.6,25 Biologic studies of the engrafting unit26,27 and ongoing graft manipulation strategies28 to improve hematopoietic recovery are likely to further improve on the current success of double UCB transplantation. Results from the 2 recent cord blood expansion phase 2 trials28,29 suggest this strategy may offer additional benefit above transplantation of 2 cord blood units but this is not definitive and will be studied in a prospective randomized trial that is opening shortly. An important determinant of survival after transplantation of double or single UCB unit was disease status at transplantation. Having established that UCB is a suitable alternative30 and that transplantation of 2 UCB units is a safe and efficacious way to extend transplantation to the many adults with leukemia who lack a single UCB with an adequate TNC, early referral for transplantation will likely improve survival.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Public Health Service grant (U24-CA76518) from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases, Health Resources and Services Administration (HHSH234200637015C), and the Office of Naval Research, Department of Navy to the National Marrow Donor Program (N00014-10-01-0204).
Opinions, findings, and conclusions or recommendations expressed herein are those of the authors and do not reflect the views of the Office of Naval Research or the National Marrow Donor Program.
National Institutes of Health
Authorship
Contribution: A.S., C.G.B., M.E., J.L.-R., M.J.L., J.E.W., and E.J.S. conceived and designed the study; M.E., J.L.-R., and F.K. collected, assembled, and analyzed data; and all authors provided study materials, interpreted data, wrote the manuscript, and gave final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Elizabeth J. Shpall, The MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: eshpall@mdanderson.org.
References
Author notes
A.S. and C.G.B. are co–first authors.
J.E.W. and E.J.S. are co–senior authors.

![Figure 4. The adjusted probability of overall survival after double UCB transplantation (infused TNC of both units was < 2.5 × 107/kg [group 1], infused TNC of unit 1 was < 2.5 × 107/kg and unit 2, ≥ 2.5 × 107/kg [group 2] and infused TNC of both units was ≥ 2.5 × 107/kg [group 3]) and adequately dosed single UCB unit transplantation. The 2-year probabilities of overall survival were 37% (95% CI, 27-46), 36% (95% CI, 25-46), and 33% (95% CI, 21-46) after group 1, 2, and 3 double UCB transplantations, respectively, and 34% (95% CI, 24-45) after single UCB transplantation, adjusted for disease status at transplantation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/5/10.1182_blood-2012-08-449108/4/m_zh89991301710004.jpeg?Expires=1769118820&Signature=5B1Dx3lxEgny1WJ~RtK45kKg1H9Pn3U8N61gHwpUBoeGvs99eiFeJsQ7f14vjsIOW-5Azw3r0N-MaLApdipYlJ7v42glG0vq98e~fULYHmCXAPxna0aOlvOTssEvR07sFXsswuJhQNGDTjB6EoNKe1nFd5EIClv9qjHYLOa3N18ekCZeZS8eGuaPZu2YKia0~zIstNh7ryX42Iaq6pI2Q4k8x12Uh8GPEF8qPJkq6T4DocMJoYgPTAcUZC8eQZSJAwI3dqKHFL1u5lc48Rh7QLdrypoCsZPWI101FYsYX63ZAozXNFdLs3VKmAPb~vuQzvzfovWFiLekXno4gxvP8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)