Key Points
Quantitation of hematogones at engraftment is useful to predict prognosis of patients treated with allogeneic stem cell transplantation.
Abstract
Transient marrow expansion of normal B-cell precursors, termed hematogones, is occasionally observed after hematopoietic stem cell transplantation (HSCT). To understand the clinical significance of this phenomenon, we enumerated hematogones in 108 consecutive patients who received allogeneic HSCT for the treatment of hematologic malignancies, including acute myelogenous leukemia, advanced myelodysplastic syndromes, acute lymphoblastic leukemia, and non-Hodgkin lymphoma. Hematogone quantitation was performed at the time of complete donor engraftment (median day 25 and 32 in patients who received bone marrow and cord blood cell transplants, respectively). Hematogones were polyclonal B cells, and their frequencies correlated positively with blood B-cell numbers, and inversely with donors' but not recipients' age, suggesting that hematogones reflect cell-intrinsic B-cell potential of donor cells. Interestingly, patients developing hematogones that comprised > 5% of bone marrow mononuclear cells constituted a group with significantly prolonged overall survival and relapse-free survival, irrespective of their primary disease or donor cell source. In addition, patients with > 5% hematogones developed severe acute graft-versus-host diseases less frequently, which may contribute toward their improved survival. We therefore conclude that the amount of hematogones at the time of engraftment may be a useful tool in predicting the prognosis of patients treated with allogeneic HSCT.
Introduction
Hematogones are transient increases in lymphoblast-looking cells in the bone marrow.1,2 Because of the morphologic resemblance between residual leukemic clones and hematogones, expansion of hematogones during the recovery phase from chemotherapy and bone marrow transplantation occasionally causes diagnostic confusion.1-3 Phenotypic analyses have demonstrated that hematogones are normal B-cell precursors, including pro-B, pre-B, and immature B cells coexpressing CD10 and CD19.1,2 The fact that hematogones become prominent in the recovery phase after chemotherapy or hematopoietic stem cell transplantation (HSCT)1-6 suggests that they could reflect active B-cell reconstitution. They are also sometimes seen in steady-state hematopoiesis, especially in healthy infants and young people.2,4,7,8 Previous work demonstrated that in the recovery phase after chemotherapy, the percentage of hematogones in the bone marrow was inversely correlated with patients' age.1 However, it is unclear whether the age-associated decline in hematogones frequency reflects cell-intrinsic defects of hematopoietic stem cell activity or cell-extrinsic defects such as aging of the bone marrow microenvironment.
Recent reports have shown that hematogone expansion correlates with favorable outcomes in acute myelogenous leukemia (AML) patients treated with chemotherapy5 or cord blood transplantation (CBT).6 However, the precise number or frequency above which hematogones correlate with clinical significance has not been clarified. Previous reports1,5,6 have reported hematogone frequency relative to bone marrow mononuclear cells (MNCs), total nuclear cells (TNCs), and frequencies of B-cell precursors, and as a result, hematogone expansion has been described with frequencies ranging from > 0% to 0.9%.1,5,6
To better understand the etiology and clinical significance of hematogones, we measured percentages of B-cell precursors in the bone marrow via flow cytometry in 108 consecutive patients with hematologic malignancies, including AML, advanced myelodysplastic syndromes (MDS), acute lymphoblastic leukemia (ALL), and lymphoma, who achieved successful engraftment after allogeneic HSCT at our institution. The analysis of hematogones was performed on the day of engraftment, defined as the day when circulating granulocytes reached > 0.5 × 109/L for 3 consecutive days,9-12 and the bone marrow showed complete donor-type chimerism via polymerase chain reaction (PCR) analysis. To minimize the effect of expanding granulocytes on hematogone frequencies, bone marrow MNCs were used for flow cytometric analyses in all cases. Our data suggest that the number of hematogones generally reflects cell-intrinsic B-cell potential of donor hematopoietic stem cells (HSCs) and that this declines with aging. We also found that hematogone frequencies of > 5% of total MNCs is a useful cutoff line to distinguish patient groups with significantly better overall survival (OS) or with relapse-free survival (RFS), irrespective of their primary diseases or donor cell sources. We propose that the quantitation of hematogones at engraftment may be useful to predict the prognosis of patients treated with allogeneic HSCT.
Methods
Patients
From 2005 to 2010, 134 patients with high-grade hematologic malignancies were treated with allogeneic HSCT in Kyushu University Hospital. These patients included AML cases with high risk,13 relapsed or refractory status, advanced MDS cases with intermediate-II or high risk on International Prognostic Scoring System classification,14,15 ALL cases with high risk,16 relapsed or refractory status, and relapsed non-Hodgkin lymphoma cases. Within these 134 patients, grafts were rejected in 5 cases and residual malignant cells proliferated soon after HSCT in 21 cases, without achieving successful engraftment. The remaining consecutive 108 cases, in which allogeneic HSCT was successful and complete donor-type chimerism was documented, were enrolled in this study. Fifty-nine and 49 patients received bone marrow transplantation (BMT) and CBT, respectively. Patients' characteristics are summarized in Table 1. This study was approved by the institutional review board of Kyushu University Hospital and conducted in accordance with the Declaration of Helsinki.
Patients' characteristics
| . | BMT: no. (%), [range] . | CBT: no. (%), [range] . | Total (%) . | P . |
|---|---|---|---|---|
| Recipient sex, male/female | 35 (32)/24 (22) | 26 (24)/23 (21) | NS | |
| Recipient age, y | 49.2 (mean) [20-66] | 47.3 (mean) [19-67] | NS | |
| Donor sex, male/female | 37 (34)/22 (20) | 24 (22)/25 (23) | NS | |
| Donor age, y | 36.7 (mean) [17-66] | 0 | < .001 | |
| No. of infused cell, /kg | 2.80 × 108 (mean) [0.92-4.02] | 0.28 × 108 (mean) [0.18-0.50] | < .001 | |
| Primary disease | NS | |||
| AML/advanced MDS | ||||
| CR | 14 (13) | 11 (10) | 25 (23) | |
| non-CR | 21 (19) | 14 (13) | 35 (32) | NS |
| Total | 35 (32) | 25 (23) | 60 (56) | |
| ALL | ||||
| CR | 3 (3) | 3 (3) | 6 (6) | |
| non-CR | 2 (2) | 10 (9) | 12 (11) | NS |
| Total | 5 (5) | 13 (12) | 18 (17) | |
| Lymphoma | ||||
| CR | 7 (6) | 3 (3) | 10 (9) | |
| non-CR | 12 (11) | 8 (7) | 20 (19) | NS |
| Total | 19 (18) | 11 (10) | 30 (28) | |
| Conditioning regimen | < .01 | |||
| TBI/CY | 28 (26) | 24 (22) | ||
| BU/CY | 14 (13) | 0 (0) | ||
| RIC | 17 (16) | 25 (23) | ||
| GVHD prophylaxis | < .001 | |||
| TAC + sMTX | 51 (47) | 6 (6) | ||
| CSP + sMTX | 8 (7) | 28 (26) | ||
| CSP + MMF | 0 (0) | 15 (14) | ||
| HLA disparity | < .001 | |||
| 6/6 | 39 (36) | 1 (1) | ||
| 5/6 | 20 (19) | 7 (6) | ||
| 4/6 | 0 (0) | 22 (20) | ||
| 3/6 | 0 (0) | 19 (18) | ||
| Days required for engraftment | 25 (median) [15-32] | 32 (median) [14-39] | < .01 |
| . | BMT: no. (%), [range] . | CBT: no. (%), [range] . | Total (%) . | P . |
|---|---|---|---|---|
| Recipient sex, male/female | 35 (32)/24 (22) | 26 (24)/23 (21) | NS | |
| Recipient age, y | 49.2 (mean) [20-66] | 47.3 (mean) [19-67] | NS | |
| Donor sex, male/female | 37 (34)/22 (20) | 24 (22)/25 (23) | NS | |
| Donor age, y | 36.7 (mean) [17-66] | 0 | < .001 | |
| No. of infused cell, /kg | 2.80 × 108 (mean) [0.92-4.02] | 0.28 × 108 (mean) [0.18-0.50] | < .001 | |
| Primary disease | NS | |||
| AML/advanced MDS | ||||
| CR | 14 (13) | 11 (10) | 25 (23) | |
| non-CR | 21 (19) | 14 (13) | 35 (32) | NS |
| Total | 35 (32) | 25 (23) | 60 (56) | |
| ALL | ||||
| CR | 3 (3) | 3 (3) | 6 (6) | |
| non-CR | 2 (2) | 10 (9) | 12 (11) | NS |
| Total | 5 (5) | 13 (12) | 18 (17) | |
| Lymphoma | ||||
| CR | 7 (6) | 3 (3) | 10 (9) | |
| non-CR | 12 (11) | 8 (7) | 20 (19) | NS |
| Total | 19 (18) | 11 (10) | 30 (28) | |
| Conditioning regimen | < .01 | |||
| TBI/CY | 28 (26) | 24 (22) | ||
| BU/CY | 14 (13) | 0 (0) | ||
| RIC | 17 (16) | 25 (23) | ||
| GVHD prophylaxis | < .001 | |||
| TAC + sMTX | 51 (47) | 6 (6) | ||
| CSP + sMTX | 8 (7) | 28 (26) | ||
| CSP + MMF | 0 (0) | 15 (14) | ||
| HLA disparity | < .001 | |||
| 6/6 | 39 (36) | 1 (1) | ||
| 5/6 | 20 (19) | 7 (6) | ||
| 4/6 | 0 (0) | 22 (20) | ||
| 3/6 | 0 (0) | 19 (18) | ||
| Days required for engraftment | 25 (median) [15-32] | 32 (median) [14-39] | < .01 |
ALL indicates acute lymphoblastic leukemia; AML, acute myelogenous leukemia; BMT, bone marrow transplantation; BU, busulfan; CBT, cord blood transplantation; CR, complete remission; CSP, cyclosporine; CY, cyclophosphamide; GVHD, graft- versus-host disease; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; NS, not significant; RIC, reduced-intensity conditioning; sMTX, short-term methotrexate; TAC, tacrolimus; and TBI, total body irradiation.
Evaluation of remission status before HSCT
Before HSCT, patients were intensively searched for residual malignant cells to define their pretransplantation remission status. In acute leukemia or advanced MDS cases, bone marrow samples were checked first by microscopic analysis, and were subjected to multicolor flow cytometric analysis.13,17 Complete remission (CR) was diagnosed when percentages of cells of leukemia phenotype were < 0.5% in the bone marrow. Furthermore, 21 patients with acute leukemia or MDS had leukemia-specific genes such as BCR-ABL, FLT3-ITD, AML1-ETO, and MLL fusions, and PCR amplification of these genes were used to detect minimal residual disease (MRD).13 Within these 21 patients, 17 patients were diagnosed as CR based on flow cytometric analyses. CR results for these 17 patients were also confirmed by PCR. In lymphoma patients, remission status was defined by evaluating the involvement of lymphoid organs using FDG-PET CT scan and/or MRI methods, and was also defined by evaluating the involvement of bone marrow by flow cytometry, as previously described.18
Transplantation procedures
Patients' characteristics were not statistically different between BMT and CBT recipient groups in terms of sex, age, and primary disease (Table 1). Conditioning regimen consisted of total body irradiation/cyclophosphamide (CY) for 28 BMT and 24 CBT recipients, busulfan (BU)/CY for 14 BMT recipients, and fludarabine-based reduced-intensity conditioning19,20 for 17 BMT and 25 CBT recipients, respectively (Table 1). Prophylaxis for graft-versus-host disease (GVHD) was tacrolimus/short-term methotrexate (sMTX) for 51 BMT and 6 CBT recipients, cyclosporine (CSP)/ sMTX for 8 BMT and 28 CBT recipients, and CSP/mycophenolate mofetil for 15 CBT recipients (Table 1). The mean number of donor cells transplanted was 2.8 × 108/kg in BMT recipients and 0.28 × 108/kg in CBT recipients. Bone marrow units were obtained from the Japan Marrow Donor Program or related donors, and cord blood units were obtained from the Japanese Cord Blood Bank Network.
Evaluation for engraftment
The bone marrow sampling for the analysis of hematogones was performed when patients achieved successful engraftment. The standard criterion for engraftment was used according to previous studies.9-12 Blood neutrophil numbers were checked daily after transplantation, and the successful engraftment was defined when neutrophils exceeded 0.5 × 109/L for 3 consecutive days. When patients met the criteria for engraftment, host/donor microchimerism analysis was performed (see the next section). If the analysis showed complete donor type chimerism, hematogones in the bone marrow were counted by multicolor flow cytometric analysis.
Chimerism analysis
To analyze donor/recipient cell chimerism, PCR amplification of polymorphic short tandem repeats (STR) was performed to confirm engraftment of donor cells. PCR using synthesized oligonucleotide templates were performed using TAKARA Taq Reagent Kits and run in the Perkin Elmer GeneAmp PCR system 9600 or 2400. The donor-cell origin and recipient-cell origin PCR product mixture was loaded onto the 373A sequencer (Applied Biosystems) with a size marker, and the data were processed using the GeneScan software (Applied Biosystems) as described previously.21
Flow cytometry analysis and cell sorting
The bone marrow mononuclear cells were prepared by the gradient centrifugation method as previously described.22,23 Cells were stained with allophycocyanin-conjugated anti-CD34 (BD Pharmingen), biotin-conjugated anti-CD38 (Caltag Laboratories), FITC-conjugated anti-CD10 (DAKO), PE-conjugated anti-CD20 (BD Biosciences), PE-Cy7–conjugated anti-CD19 (BioLegend), and Cy5-PE–conjugated lineage (Lin) mixture (anti-CD3, -CD4, -CD8 (BD Pharmingen) -CD11b (Caltag Laboratories), -CD14, and -CD56 (Beckman Coulter).22-25 Streptavidin-conjugated Cy7-allophycocyanin (BD Pharmingen) was used for visualization of biotinylated antibodies. For analysis of mature B cells, peripheral blood (PB) cells were stained with FITC-conjugated anti-CD10 (DAKO), PE-conjugated anti-CD20 (BD Biosciences), PE-Cy7–conjugated anti-CD19 (BioLegend), and Cy5-PE–conjugated Lin mixture. Available PB cells at day 90 after HSCT could be obtained from 64 patients and evaluated. Cells were analyzed by using a FACSAria (BD Biosciences) or FACSCanto (BD Biosciences). Cell sorting was performed on a 5-color FACSAria (BD Biosciences). To minimize contamination, cells were collected after the second round of sorting using sorting gates identical to those used in the first-round sorting. Definition of hematogones is a series of normal B-lymphoid precursors, including CD34+CD38+CD10+CD19+Lin− pro-B cells, CD34−/loCD38+CD10+CD19+ pre-B cells, and CD34−CD38+CD10+CD19+ CD20+ immature B cells26-28 in bone marrow MNCs. Isotype controls were used to define the cutoff of positivity of each antigen on a FACS.
PCR analysis of IGH gene rearrangement
Statistical analysis
Relationships of percentages of hematogones with age, the day of engraftment, and numbers of circulating B lymphocytes were analyzed with the Spearman rank correlation analysis. Comparison between 2 groups or condition was tested with the Mann-Whitney U test. The categorical variables were analyzed with the 2-tailed χ2 test. Survival was plotted with Kaplan-Meier curves, taking the interval from date of HSCT to death/relapse or last contact. Comparisons between each group were performed with the log-rank test and the Cox proportional hazards model. Univariate analysis was performed with logistic or exact logistic regression, and the parameters that present P < .20 were reevaluated by multivariate analysis.31 Multivariate analysis was performed with logistic regression applying Firth's bias reduction. A P value < .05 was considered to be statistically significant.
Results
Hematogones that appeared at the time of engraftment are polyclonal B-cell precursors
One hundred eight consecutive cases treated with successful allogeneic BMT or CBT were enrolled in this study. Hematogones in the bone marrow were counted on the day of engraftment by multicolor flow cytometric analysis. The successful engraftment was judged when neutrophils exceeded > 0.5 × 109/L for 3 consecutive days.9-12 At this phase, it is critical to exclude residual leukemic cells or host-derived B-cell precursors from a cell fraction of hematogones. To this end, polymorphic STR was amplified to test the host/donor microchimerism, and only when patients' bone marrow consisted of 100% donor-derived cells, the analysis of hematogones was performed. The complete donor-type chimerism verifies that host-derived normal hematopoietic cells and malignant leukemic cells have been eliminated below the sensitivity of FACS,32-34 and therefore that phenotypically defined hematogones in these patients on FACS were donor-derived normal cells.
Hematogones were morphologically blastic cells (Figure 1A), and were identified by surface phenotype, according to the definition of pro-B, pre-B, and/or immature B cells that coexpress CD10 and CD19 on their cell surface1,26-28 (Figure 1B). To minimize the effect of granulocytes on hematogone frequencies, we used MNCs instead of TNCs in our analysis. The frequencies of hematogones in MNCs are usually higher than those in TNCs (not shown), as reported previously.1
Detection of hematogones after allogeneic HSCT. (A) Typical appearance of hematogones in the bone marrow after HSCT (Giemsa staining ×1000; OLYMPUS BH-2 microscope [Olympus]; ACT-2U imaging software [Nikon]; 27°C). (B) Evaluation of hematogones on a 5-color FACS. Hematogones are defined as MNCs coexpressing CD10 and CD19 in the bone marrow at engraftment. They include CD34+CD38+CD10+CD19+Lin− pro-B cells, CD34−/loCD38+CD10+CD19+ pre-B cells, and CD34−CD38+CD10+CD19+CD20+ immature B cells. (C) Percentage of hematogones in the bone marrow MNCs in patients who received BMT and CBT. CBT recipients presented much higher frequency of hematogones compared with BMT recipients (P < .001). Solid bars indicate the median percentage of hematogones for each recipient; MNC, mononuclear cells; BMT, bone marrow transplantation; and CBT, cord blood transplantation. (D) The relationship between the day of engraftment and percentages of hematogones. There was no relationship between these parameters. (E) IGH rearrangement analysis of purified hematogones. B-cell precursors were polyclonal in all 106 recipients analyzed.
Detection of hematogones after allogeneic HSCT. (A) Typical appearance of hematogones in the bone marrow after HSCT (Giemsa staining ×1000; OLYMPUS BH-2 microscope [Olympus]; ACT-2U imaging software [Nikon]; 27°C). (B) Evaluation of hematogones on a 5-color FACS. Hematogones are defined as MNCs coexpressing CD10 and CD19 in the bone marrow at engraftment. They include CD34+CD38+CD10+CD19+Lin− pro-B cells, CD34−/loCD38+CD10+CD19+ pre-B cells, and CD34−CD38+CD10+CD19+CD20+ immature B cells. (C) Percentage of hematogones in the bone marrow MNCs in patients who received BMT and CBT. CBT recipients presented much higher frequency of hematogones compared with BMT recipients (P < .001). Solid bars indicate the median percentage of hematogones for each recipient; MNC, mononuclear cells; BMT, bone marrow transplantation; and CBT, cord blood transplantation. (D) The relationship between the day of engraftment and percentages of hematogones. There was no relationship between these parameters. (E) IGH rearrangement analysis of purified hematogones. B-cell precursors were polyclonal in all 106 recipients analyzed.
The time median to engraftment was 25 and 32 days in patients treated with BMT and CBT, respectively (Table 1). The time required for engraftment appears to be consistent with previous reports.34-39 Percentages of B-cell precursors within the bone marrow MNCs at the time of complete donor-type engraftment were significantly higher in CBT recipients than in BMT recipients (6.37% vs 1.75%; P < .001; Figure 1C). There was no significant relationship between the day of engraftment (the day of sampling) and the frequency of hematogones (Figure 1D).
In 106 of 108 patients who had > 0.1% of B-cell precursors in the bone marrow MNCs, B-cell precursors were purified by a multicolor FACS and were subjected to IGH rearrangement analysis. In all of these patients, B-cell precursors were polyclonal based on the rearrangement analysis of the IGH genes (Figure 1E).
Hematogones generally represent B-cell recovery potential of the graft and their emergence is related to age of donors but not recipients
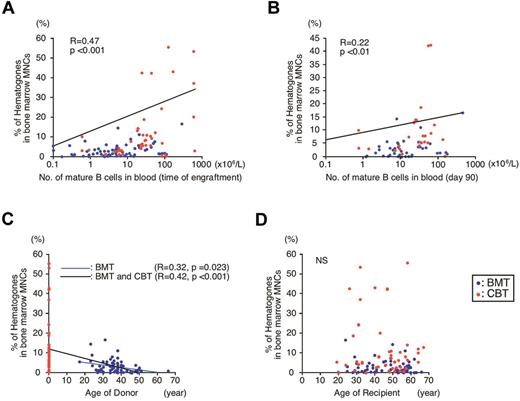
Because hematogones are normal B-cell precursors, we tested whether the presence of a high number of them could reflect the active B-cell recovery after HSCT. FACS analysis of circulating blood cells revealed that the frequency of bone marrow B-cell precursors was significantly correlated with the number of blood B cells at the time of engraftment (R = 0.47, P < .001; Figure 2A), and with those even on day 90 after HSCT (R = 0.22, P < .01; Figure 2B). These results suggest that expansion of hematogones reflects not only enhanced B-cell reconstitution potential of the graft, but also prolonged B cell–producing capability of donor HSCs.
Analysis of hematogones, and the correlation of their frequency compared with blood B-cell numbers and age of donors. (A) A relationship between frequencies of hematogones and blood B cells at engraftment (P < .001). (B) A relationship between frequencies of hematogones at engraftment and blood B cells on day 90 (P < .01). (C) A relationship between frequency of hematogones and donor's age in patients who received BMT (blue line, P = .023), and in all recipients treated with either BMT or CBT (black line, P < .001). (D) No significant relationship was observed between frequency of hematogones and recipient age. NS indicates not significant.
Analysis of hematogones, and the correlation of their frequency compared with blood B-cell numbers and age of donors. (A) A relationship between frequencies of hematogones and blood B cells at engraftment (P < .001). (B) A relationship between frequencies of hematogones at engraftment and blood B cells on day 90 (P < .01). (C) A relationship between frequency of hematogones and donor's age in patients who received BMT (blue line, P = .023), and in all recipients treated with either BMT or CBT (black line, P < .001). (D) No significant relationship was observed between frequency of hematogones and recipient age. NS indicates not significant.
The age of BMT donors ranged from 17 to 66 years old (median, 37 years; Table 1). Interestingly, there was a significant inversed correlation between donor age and percentage of bone marrow hematogones in patients treated with BMT (R = 0.32, P = .02; Figure 2C blue line). When the age of CBT donors were defined as 0-year old, the significant inversed correlation between age and hematogone numbers was also found in all patients entered in this study (R = 0.42, P < .001; Figure 2C black line). In contrast, recipients' age and hematogone numbers did not show any relationship (Figure 2D). Furthermore, as shown in Table 2, the time of engraftment was not affected by primary diseases of patients, or by their remission status at the time of HSCT. Thus, although the patients who fail to achieve CR are usually treated with higher total doses of chemotherapeutic drugs because of their refractory disorders, it did not affect the day of engraftment or the day of hematogone analysis for this study. These data strongly suggest that the number of hematogones after HSCT generally reflects the cell-intrinsic B-cell recovery potential of donor HSCs, which may decline by aging.
Time required for engraftment in patients grouped by their primary disease or complication of infection or acute GVHD
| Disease . | Remission status . | Time required for engraftment, d . | |||||
|---|---|---|---|---|---|---|---|
| BMT . | CBT . | ||||||
| No. . | Mean, d . | P . | No. . | Mean, d . | P . | ||
| AML and advanced MDS | CR/non-CR | 14/21 | 27.0/24.4 | .11 | 11/14 | 31.5/31.8 | .37 |
| ALL | CR/non-CR | 3/2 | 23.7/29.5 | .35 | 3/10 | 34.3/32.1 | .45 |
| Lymphoma | CR/non-CR | 7/12 | 26.1/22.8 | .10 | 3/8 | 33.0/29.3 | .44 |
| Overall | .10 | Overall | .53 | ||||
| After HSCT | |||||||
| Infections | Yes/No | 38/21 | 25.3/24.8 | .66 | 22/27 | 30.8/32.2 | .61 |
| Acute GVHD | Grade II-IV/Grade 0-I | 26/33 | 24.7/25.3 | .25 | 14/35 | 32.3/31.3 | .47 |
| Disease . | Remission status . | Time required for engraftment, d . | |||||
|---|---|---|---|---|---|---|---|
| BMT . | CBT . | ||||||
| No. . | Mean, d . | P . | No. . | Mean, d . | P . | ||
| AML and advanced MDS | CR/non-CR | 14/21 | 27.0/24.4 | .11 | 11/14 | 31.5/31.8 | .37 |
| ALL | CR/non-CR | 3/2 | 23.7/29.5 | .35 | 3/10 | 34.3/32.1 | .45 |
| Lymphoma | CR/non-CR | 7/12 | 26.1/22.8 | .10 | 3/8 | 33.0/29.3 | .44 |
| Overall | .10 | Overall | .53 | ||||
| After HSCT | |||||||
| Infections | Yes/No | 38/21 | 25.3/24.8 | .66 | 22/27 | 30.8/32.2 | .61 |
| Acute GVHD | Grade II-IV/Grade 0-I | 26/33 | 24.7/25.3 | .25 | 14/35 | 32.3/31.3 | .47 |
ALL indicates acute lymphoblastic leukemia; AML, acute myelogenous leukemia; BMT, bone marrow transplantation; CBT, cord blood transplantation; CR, complete remission; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; and MDS, myelodysplastic syndrome.
The emergence of hematogones up to > 5% of MNCs in the bone marrow represents a good prognosis for patients treated with allogeneic HSCT
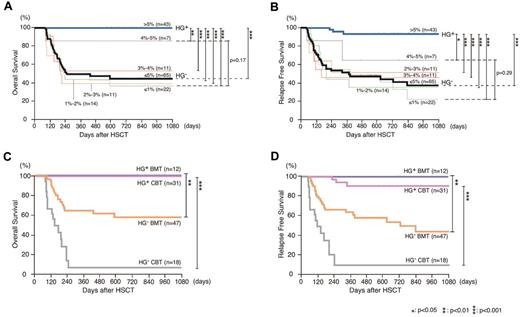
It should be critical to draw a line of hematogone numbers to distinguish a group of patients with clinical significance. Therefore, we first compared the OS and RFS among patient subgroups with ≤ 1%, 1%-2%, 2%-3%, 3%-4%, 4%-5%, or > 5% of hematogones in our study (Figure 3A-B). Strikingly, patients who developed hematogones up to > 5% of MNCs showed significantly better 3-year OS (100%) and RFS (93.3%), compared with any other group. Patient groups with ≤ 1%, 1%-2%, 2%-3%, and 3%-4% of hematogones showed similar 3-year OS and RFS that were 37%-53% and 22%-51%, respectively. Interestingly, patients with 4%-5% hematogones appeared to show intermediate levels of OS (86%) and RFS (64%), although this is not statistically better than those in patients with ≤ 1% hematogones (Figure 3A-B). Based on these results, we hypothesized that the development of > 5% of hematogones might be critical to distinguish a patient group with favorable prognosis.
Patients whose hematogones comprised > 5% bone marrow MNCs constitute a group with significantly improved survival, irrespective of HSC sources. (A-B) The Kaplan-Meier estimates of (A) OS and (B) RFS among patient subgroups with ≤ 1% (gray line), 1%-2% (green line), 2%-3% (orange line), 3%-4% (red line), 4%-5% (purple line), ≤ 5% (black bold line), or > 5% (blue bold line) hematogones in the bone marrow MNCs. Forty-three patients who developed > 5% MNC hematogones (HG+) showed significantly better 3-year OS and RFS, compared with any of each group (P < .01 and P < .05, respectively), as well as to 65 patients with ≤ 5% MNCs hematogones (HG−; P < .001 for both). (C-D) The Kaplan-Meier estimates of (C) OS and (D) RFS in HG+ and HG− groups that received transplants with BMT or CBT. The improved OS and RFS were seen in HG+ groups regardless of the source of HSC. HG+ indicates patients developed hematogones (> 5% of bone marrow MNCs); HG− indicates patients who failed to develop hematogones (≤ 5% of bone marrow MNCs).
Patients whose hematogones comprised > 5% bone marrow MNCs constitute a group with significantly improved survival, irrespective of HSC sources. (A-B) The Kaplan-Meier estimates of (A) OS and (B) RFS among patient subgroups with ≤ 1% (gray line), 1%-2% (green line), 2%-3% (orange line), 3%-4% (red line), 4%-5% (purple line), ≤ 5% (black bold line), or > 5% (blue bold line) hematogones in the bone marrow MNCs. Forty-three patients who developed > 5% MNC hematogones (HG+) showed significantly better 3-year OS and RFS, compared with any of each group (P < .01 and P < .05, respectively), as well as to 65 patients with ≤ 5% MNCs hematogones (HG−; P < .001 for both). (C-D) The Kaplan-Meier estimates of (C) OS and (D) RFS in HG+ and HG− groups that received transplants with BMT or CBT. The improved OS and RFS were seen in HG+ groups regardless of the source of HSC. HG+ indicates patients developed hematogones (> 5% of bone marrow MNCs); HG− indicates patients who failed to develop hematogones (≤ 5% of bone marrow MNCs).
According to this criteria, 43 patients developed > 5% MNCs of hematogones (HG+) and the remaining 65 patients had ≤ 5% MNCs of hematogones (HG−). As shown in Figure 3A, in HG+ patients, 3-year OS was 100%, whereas in HG− patients, it was 45% (P < .001). The favorable OS in HG+ groups is at least because of the less frequent disease relapse. As shown in Figure 3B, significant association was observed between the presence of hematogones and 3-year RFS after HSCT: 3-year RFS was 93% and 37% in HG+ and HG− patients, respectively (P < .001). The association between the presence of > 5% hematogones and favorable OS and RFS was also seen when the analysis was performed in patient subgroups that received either BMT or CBT (Figure 3C-D). These data strongly suggest that the emergence of hematogones is a useful predictor of favorable outcomes at least in terms of OS and RFS, irrespective of donor cell source.
The emergence of hematogones (> 5% of MNCs) marks favorable outcomes for allogeneic HSCT especially in patients who failed to achieve complete remission, irrespective of primary malignant disease
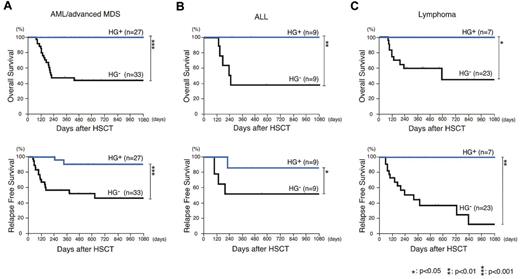
We then analyzed whether the good prognosis designated by the emergence of hematogones is dependent on the primary malignant disorder. The OS and RFS were analyzed in each patient group with AML/advanced MDS, ALL, or non-Hodgkin lymphoma. As shown in Figure 4, HG+ patients always showed significantly better OS and RFS compared with HG− patients, in any of these patients groups suffering from different primary diseases.
Patients who developed > 5% hematogones as a fraction of their MNCs constitute a group with significantly improved survival, irrespective of their primary disease. The Kaplan-Meier estimates of OS and RFS in HG+ and HG− patients differentiated with their primary disease. In each group of patients with (A) AML or advanced MDS, (B) ALL, and (C) lymphoma, HG+ groups showed significantly better OS and RFS, compared with the HG− group (P < .001 for both).
Patients who developed > 5% hematogones as a fraction of their MNCs constitute a group with significantly improved survival, irrespective of their primary disease. The Kaplan-Meier estimates of OS and RFS in HG+ and HG− patients differentiated with their primary disease. In each group of patients with (A) AML or advanced MDS, (B) ALL, and (C) lymphoma, HG+ groups showed significantly better OS and RFS, compared with the HG− group (P < .001 for both).
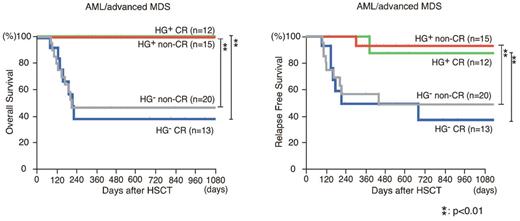
It is well known that the achievement of CR at the time of transplantation favorably affects the prognosis after allogeneic HSCT.13 Interestingly, in AML/advanced MDS patients, the HG+ group showed significantly prolonged OS and RFS compared with the HG− group, irrespective of their remission status at HSCT (Figure 5). The similar analysis was performed in ALL and lymphoma patient groups (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Although each group contained only a limited number of patients, statistically significant prolonged OS and RFS were also seen in patients who did not achieve CR at HSCT in both the ALL and the lymphoma patient groups.
The presence of hematogones marks a group with good prognosis in AML/advanced MDS patients. The Kaplan-Meier estimates of OS and RFS in HG+ and HG− patients in AML or advanced MDS differentiated with their remission status before HSCT. Significantly better OS and RFS were seen in HG+ groups irrespective of their remission status.
The presence of hematogones marks a group with good prognosis in AML/advanced MDS patients. The Kaplan-Meier estimates of OS and RFS in HG+ and HG− patients in AML or advanced MDS differentiated with their remission status before HSCT. Significantly better OS and RFS were seen in HG+ groups irrespective of their remission status.
Thus, the appearance of hematogones might mark favorable OS and RFS regardless of their primary malignancy.
Expansion of hematogones is frequently observed in patients who did not develop infection or severe acute GVHD
In this study, all 43 HG+ patients are currently alive, although primary diseases have relapsed in 3 patients. In contrast, 32 of 65 HG− patients have died. The causes of death in these 32 HG− patients are shown in Table 3. Twenty-six patients died of their refractory primary disease, and 6 patients died of TRM, including acute GVHD (2 patients) and viral infections (4 patients). Of the 26 patients who died of primary disease, 24 developed both acute GVHD and infections.
Cause of death in patients with less than 5% hematogones
| Primary disease . | Cause of death, no. (%) . | |||||
|---|---|---|---|---|---|---|
| Relapse of primary disease . | Transplantation-related mortality . | |||||
| Infection . | Acute GVHD (Grade II-IV) . | Total . | Infection . | Acute GVHD (Grade II-IV) . | Total . | |
| AML and advanced MDS | 14/33 (42.4) | 14/33 (42.4) | 15/33 (45.4) | 2/33 (6.1) | 0/33 (0) | 2/33 (6.1) |
| ALL | 3/9 (33.3) | 3/9 (33.3) | 3/9 (33.3) | 1/9 (11.1) | 1/9 (11.1) | 2/9 (22.2) |
| Lymphoma | 7/23 (30.4) | 7/23 (30.4) | 8/23 (34.8) | 1/23 (4.3) | 1/23 (4.3) | 2/23 (8.7) |
| Total | 24/65 (36.9) | 24/65 (36.9) | 26/65 (40) | 4/65 (6.2) | 2/65 (3.1) | 6/65 (9.2) |
| Primary disease . | Cause of death, no. (%) . | |||||
|---|---|---|---|---|---|---|
| Relapse of primary disease . | Transplantation-related mortality . | |||||
| Infection . | Acute GVHD (Grade II-IV) . | Total . | Infection . | Acute GVHD (Grade II-IV) . | Total . | |
| AML and advanced MDS | 14/33 (42.4) | 14/33 (42.4) | 15/33 (45.4) | 2/33 (6.1) | 0/33 (0) | 2/33 (6.1) |
| ALL | 3/9 (33.3) | 3/9 (33.3) | 3/9 (33.3) | 1/9 (11.1) | 1/9 (11.1) | 2/9 (22.2) |
| Lymphoma | 7/23 (30.4) | 7/23 (30.4) | 8/23 (34.8) | 1/23 (4.3) | 1/23 (4.3) | 2/23 (8.7) |
| Total | 24/65 (36.9) | 24/65 (36.9) | 26/65 (40) | 4/65 (6.2) | 2/65 (3.1) | 6/65 (9.2) |
ALL indicates acute lymphoblastic leukemia; AML, acute myelogenous leukemia; GVHD, graft-versus-host disease; and MDS, myelodysplastic syndrome.
We analyzed the relationship between the emergence of documented hematogones in the bone marrow, and variables including sex of donor/recipient, days required for engraftment, primary diseases, times of intensive chemotherapy before HSCT, remission status, conditioning regimen, documented infectious disease, and episode of acute/chronic GVHD by using univariate and multivariate analysis. These analyses were performed in patient groups treated with BMT and CBT, respectively.
There were no correlations found between the emergence of > 5% of hematogones and clinical factors such as the day of engraftment, primary disease, times of intensive chemotherapies, and remission status, in either univariate or multivariate analyses. As shown in Table 4, in univariate analysis, a hematogone increase up to > 5% of MNCs was found more frequently in patients without viral infection (such as cytomegalovirus, human herpesvirus 6, and adenovirus; BMT: P = .03; CBT: P < .01), and those did not develop severe acute GVHD of grade II-IV (BMT: P < .01; CBT: P < .01). Time required for engraftment did not differ between patient groups with or without infections, or acute GVHD (Table 2). These data appear to be compatible with the analysis of causes of death in HG− patients (Table 3). On the other hand, in multivariate analysis, severe acute GVHD of grade II-IV, but not infection was the significant risk factor for emergence of hematogones (BMT: P = .03; CBT: P = .04; Table 4). Based on these analyses, the emergence of hematogones heralds less frequent development of severe acute GVHD.
Risk factors for development of more than 5% MNCs of hematogone based on univariate and multivariate analyses
| . | BMT . | CBT . | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate . | Multivariate . | Univariate . | Multivariate . | |||||
| Odds ratio (95% CI) . | P . | Odds ratio (95% CI) . | P . | Odds ratio (95% CI) . | P . | Odds ratio (95% CI) . | P . | |
| Infections, Yes/No | 0.19 (0.05-0.74) | .03 | 0.42 (0.02-1.58) | .23 | 0.16 (0.04-0.57) | < .01 | 0.37 (0.02-2.81) | .10 |
| Acute GVHD, Grade II-IV/0-I | 0.09 (0.01-0.73) | < .01 | 0.04 (0.00-0.85) | .03 | 0.12 (0.03-0.48) | < .01 | 0.04 (0.00-0.80) | .04 |
| . | BMT . | CBT . | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate . | Multivariate . | Univariate . | Multivariate . | |||||
| Odds ratio (95% CI) . | P . | Odds ratio (95% CI) . | P . | Odds ratio (95% CI) . | P . | Odds ratio (95% CI) . | P . | |
| Infections, Yes/No | 0.19 (0.05-0.74) | .03 | 0.42 (0.02-1.58) | .23 | 0.16 (0.04-0.57) | < .01 | 0.37 (0.02-2.81) | .10 |
| Acute GVHD, Grade II-IV/0-I | 0.09 (0.01-0.73) | < .01 | 0.04 (0.00-0.85) | .03 | 0.12 (0.03-0.48) | < .01 | 0.04 (0.00-0.80) | .04 |
BMT indicates bone marrow transplantation; CBT, cord blood transplantation; CI, confidence interval; GVHD, graft-versus-host disease; and MNC, mononuclear cell.
Discussion
Hematogones are immature B-cell precursors that reside mainly in the bone marrow of every normal individual,1,2,27,40 and their numbers could reflect activity of normal B lymphopoiesis. Hematogones are occasionally seen in large numbers in healthy people, especially in infants and young children.2,4,7,8 Interestingly, recent reports have suggested that the presence of detectable numbers of hematogones at the recovery phase from myelosuppression reflects better prognosis of patients with AML treated with chemotherapy5 or CBT,6 although the underlying mechanism of this phenomenon is unclear. The increase of hematogones may reflect eradication of leukemic cells that could inhibit normal hematopoiesis,1,5 or rapid immune reconstitution that could suppress infection and severe acute GVHD in an allogeneic HSCT setting.6
In these reports, the presence of hematogones was documented when they were detectable at a low frequency: ≥ 0.01% of TNCs at a recovery phase5 or > 0% and > 0.9% of MNCs on day 21 and 100, respectively.6 In contrast, the patient cohort in our study received allogeneic HSCT, and the majority (106 of 108 cases) of patients had > 0.1% of hematogones at engraftment by our multicolor flow cytometric analysis (Figure 1C). Therefore, it was critical to set an appropriate threshold value and timing of sampling to decide a clinically meaningful increase of hematogones in an allogeneic HSCT setting. Furthermore, previous studies were performed only in patients with AML,5,6 but not in patients with lymphoid neoplasms, presumably because it was difficult to discriminate a small number of neoplastic lymphoid cells from hematogones.6
To accurately enumerate hematogones in patients with various clinical backgrounds and with different donor cell sources, we performed the analysis on the day when patients met the clinical criteria of engraftment9-12 and displayed complete donor-type chimerism. The confirmation for donor-type chimerism allowed us to avoid miscounting neoplastic lymphoid cells as hematogones. Because these samples should be free from host-derived cells, we included patients with lymphoid malignancies in our study. We rigorously measured the frequencies of hematogones within bone marrow MNCs by 6-color flow cytometric analysis.
In our study, donor-derived hematogones were polyclonal, based on IGH rearrangement analysis in all cases, and therefore the presence of hematogones should be a snapshot of normal B lymphopoiesis at the recovery phase. In fact, the frequencies of hematogones at engraftment were correlated with circulating B-cell numbers at least until day 90 (Figure 2). Importantly, we here show that the frequencies of hematogones were correlated with donors' age, but not with recipients' age, suggesting the age-dependent decline of B-cell potential of donor HSCs. This is compatible with previous mouse studies in which younger HSCs are capable of producing more abundant B cells.41-43
According to our criteria, the engraftment was seen on days 25 and 32 (median) in BMT and CBT groups (Figure 1D), respectively, consistent with previous studies.35-39,44 Within each BMT or CBT group, the engraftment day was not significantly altered by the patients' primary disease or remission status at transplantation (Table 2). The bone marrow sampling for hematogone analysis was performed on the day of engraftment. As shown in Table 3, the timing of sampling (= the day of engraftment) was not significantly related to emergence of hematogones in univariate and multivariate analyses. Interestingly, however, our data suggest that when hematogones reach > 5% of MNCs at engraftment, it has a profound clinical impact on patients' OS and RFS (Figure 3A-B). It is of note that the emergence of documented hematogones was not related to times of intensive chemotherapies or remission status of patients before transplantation in both univariate and multivariate analyses (Table 3). This result suggests that the emergence of hematogones was not affected by the potential damage of host microenvironment through multiple chemotherapies.
The improvement of OS and RFS in HG+ patients was seen in all patient groups: those suffering from AML/advanced MDS, ALL, or lymphoma (Figure 4). Furthermore, this effect became more evident when patients who had failed to achieve CR before transplantation were analyzed (Figure 5). In this case, the appearance of hematogones clearly marks a subgroup with favorable OS and RFS, irrespective of their primary diseases. In contrast, in patients who had achieved CR before transplantation, prolonged OS and RFS were found only in patients with AML/advanced MDS, but not in patients with ALL or lymphoma (Figure 5). A larger study including higher number of patients should be performed to clarify the impact of hematogones on HSCT results in CR patients with lymphoid malignancies.
The analyses of risk factors for the appearance of > 5% MNCs of hematogones revealed that in both BMT and CBT patients, the less frequent occurrences of severe acute GVHD and infections were significantly correlated in univariate analyses, whereas the less frequent severe acute GVHD was the only risk factor in multivariate analyses (Table 3). As shown in Table 4, all 32 deaths occurred only in HG− patients, and 24 of these 32 patients developed both severe acute GVHD and infection before the relapse of the disease. In these patients, doses of immunosuppressive drugs were escalated to control acute GVHD, which might cause development of infections as well as recurrence of primary disease.6,45,46 It is therefore possible that less frequent development of severe acute GVHD in HG+ patients is one of the reasons for their better OS and RFS.
The rapid reconstitution of the immune system represented by a high number of hematogones should be able to prevent infection.6,45 In turn, successful prevention of acute GVHD could result in proliferation of hematogones because acute GVHD itself may suppress hematopoietic recovery by targeting the bone marrow HSC niche47 or by attacking directly B-lymphoid cells.46 In addition, the fact that improvement of RFS is associated with the expansion of hematogones suggests an interesting possibility that B cells play a role in the graft-versus-leukemia effect,48 although this is still controversial.41 Also in turn, it is possible that the successful eradication of neoplastic cells from the bone marrow by HSCT results simply in rapid expansion of hematogones.
Thus, our data suggest that the expansion of hematogones is a useful indicator to discriminate a patient group with improved OS and RFS after allogeneic HSCT. Based on rigorous evaluation of frequencies of hematogones after HSCT, we propose that 5% of MNCs is a threshold value for a clinically valuable increase of hematogones. The prognostic value of this definition should be tested by future studies in larger groups of patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the medical and nursing staff working on the Fukuoka Blood and Marrow Transplantation Group for providing patient information, and D. Dalma-Weiszhausz for critically reviewing the manuscript.
This work was supported, in part, by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (K.A., T.M.).
Authorship
Contribution: T.S. and T.M. coordinated the project, designed and performed the transplantation and experiments, analyzed the data, and wrote the manuscript; Y.K., Y.M., K. Kamezaki, K. Takenaka, H.I., K.N., T.T, and K. Kato performed the transplantation and provided technical advice; K. Takase, H.H., A.N., Y.I., T.K., and T.E. provided patient information, clinical samples, and technical advice; and K.A. designed the experiments, reviewed the data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Koichi Akashi, Department of Medicine and Biosystemic Sciences, Kyushu University Graduate School of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-0054, Japan; e-mail: akashi@med.kyushu-u.ac.jp.

![Figure 1. Detection of hematogones after allogeneic HSCT. (A) Typical appearance of hematogones in the bone marrow after HSCT (Giemsa staining ×1000; OLYMPUS BH-2 microscope [Olympus]; ACT-2U imaging software [Nikon]; 27°C). (B) Evaluation of hematogones on a 5-color FACS. Hematogones are defined as MNCs coexpressing CD10 and CD19 in the bone marrow at engraftment. They include CD34+CD38+CD10+CD19+Lin− pro-B cells, CD34−/loCD38+CD10+CD19+ pre-B cells, and CD34−CD38+CD10+CD19+CD20+ immature B cells. (C) Percentage of hematogones in the bone marrow MNCs in patients who received BMT and CBT. CBT recipients presented much higher frequency of hematogones compared with BMT recipients (P < .001). Solid bars indicate the median percentage of hematogones for each recipient; MNC, mononuclear cells; BMT, bone marrow transplantation; and CBT, cord blood transplantation. (D) The relationship between the day of engraftment and percentages of hematogones. There was no relationship between these parameters. (E) IGH rearrangement analysis of purified hematogones. B-cell precursors were polyclonal in all 106 recipients analyzed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/5/10.1182_blood-2012-02-409607/4/m_zh89991301670001.jpeg?Expires=1769145203&Signature=rGIPk0ag4YZLTrUKOl1kwTAeUdnjQgv1oM4VQGvagH6zg8cB-TAmjWyHOD1~o0nkWyHwzUoVOFL7UU84VpGJimIS7OGU~b9YJN7~12lqsmIBOjuObD3~KCxGvydDoUjtN45nHM2L76FAFN2psrw6WONIEqRsnDBmpoBqAitMaHwcwqEuyFgMPBty2z1BxjDS8ocZVjS91fsgs3kHPR5Ulm84Cwy3Rw9uAezsZEpYGyqC6Oug8j20EnehZUW-CO88Zw7qOsmF1qf4iOaJjmfvoI5XaQQVdBxsiZ3U31t6-12YmGL01hrrxkVGUIp9kawKb0w1DJd6k9PO25rEWO-PkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal