To the editor:
Immunosuppressive therapy (IST) with horse antithymocyte globulin (h-ATG) and cyclosporine (CSA) is an effective therapy for aplastic anemia (AA), resulting in a response rate of 60%-70% and an excellent survival for responders. However, lymphoglobulin (h-ATG; Genzyme) was withdrawn from the market in 2007. Because of the unavailability of h-ATG in most countries, the evaluation of rabbit ATG (r-ATG) as first-line therapy was stimulated. Marsh et al recently showed a low response to IST at 6 months (37%) in 35 patients who were given r-ATG (thymoglobulin; Genzyme).1 The overall survival at 2 years was significantly lower (68%) compared with age- and disease-matched patients given h-ATG (n = 105, 86% P = .009). Furthermore, in a randomized controlled trial, Scheinberg et al showed an inferior response (37% vs 68% at 6 months, P < .001) and a decreased survival (76% vs 96%, 2 years, P = .04) after r-ATG (thymoglobulin) compared with h-ATG (ATGAM; Pfizer).2 In none of the published series r-ATG was found to be superior to h-ATG.3,4 However, these series did not focus on outcome of children, so we compared both ATGs in children with AA.
Children (< 18 years of age) with severe AA diagnosed in Germany, Austria, and Switzerland and registered in the SAA-94 study between November 1993 and August 2011 received IST if no matched sibling donor was available.5 IST initially included h-ATG (lymphoglobulin 15 mg/kg/d for 8 days) and CSA as described previously.5 G-CSF was given if the neutrophil count was < 0.5 × 109/L. Because of the unavailability of h-ATG, 32 patients received r-ATG (thymoglobulin 3.75 mg/kg/d for 5 days) since 2007. Approval for the study was obtained from the institutional review board of the Ludwig-Maximilians University of Munich. Written informed consent was provided by the parents of each patient.
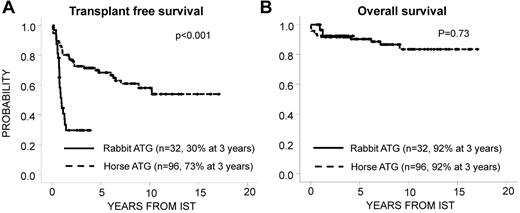
Patients receiving r-ATG (n = 32) were matched for age (categorized as < 10 or ≥ 10 years) and disease severity (very severe or severe) with patients receiving h-ATG (n = 96). The median age of patients in the r-ATG group was 9.7 years; 10 patients had severe disease and 22 patients had very severe disease. The median follow-up times were 2.1 years (r-ATG) and 6.9 years (h-ATG; P < .001). Response was defined as described previously.5 The response rate at 6 months was 34% for r-ATG and 65% for h-ATG (P = .003). Although Marsh et al observed several late responses after r-ATG resulting in similar best response rates for r-ATG and h-ATG,1 we observed only 2 late responses after r-ATG and a lower transplantation-free survival rate after IST with r-ATG compared with h-ATG (Figure 1A).1 However, in contrast to previous studies, because of successful second-line hematopoietic stem cell transplantation (HSCT), the overall survival for both r-ATG and h-ATG was excellent (Figure 1B). The increased risk of death from infection after r-ATG that was observed by Marsh et al was not confirmed in our study.1 These results demonstrate that in children with AA, IST with r-ATG is less effective than h-ATG. However, the majority of nonresponders could be rescued by HSCT. We conclude that, whenever possible, IST for treatment of children with AA should include h-ATG (ATGAM) and that, in the light of the improved results of unrelated HSCT, this procedure should be offered to all nonresponders at 6 months.6,7 Even earlier HSCT should be considered in patients with prolonged severe neutropenia (0.2 × 109/L) to avoid fatal infections.
Transplantation-free and overall survival. Transplantation-free survival (A) and overall survival (B) after IST with r-ATG and CSA compared with h-ATG and CSA. Second-line HSCT was considered as an event in the analysis of transplantation-free survival. HSCT was performed in 20 patients at a median of 258 days (range, 57-504) after IST in the r-ATG group and in 29 patients with a median of 625 days (range, 27-3718) after IST in the h-ATG group.
Transplantation-free and overall survival. Transplantation-free survival (A) and overall survival (B) after IST with r-ATG and CSA compared with h-ATG and CSA. Second-line HSCT was considered as an event in the analysis of transplantation-free survival. HSCT was performed in 20 patients at a median of 258 days (range, 57-504) after IST in the r-ATG group and in 29 patients with a median of 625 days (range, 27-3718) after IST in the h-ATG group.
Authorship
Acknowledgments: The authors thank Sonja Behrendt, Carla Haid, and Stephanie Gehring for excellent support in data management.
Contribution: A.Y. designed the study, collected and analyzed the data, and wrote the manuscript; and C.M.N., M.M.F., and B.S. designed the study, collected the data, and critically revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ayami Yoshimi, MD, PhD, Division of Paediatric Hematology and Oncology, Department of Pediatrics and Adolescent Medicine, University of Freiburg, Mathildenstrasse 1, 79106 Freiburg, Germany; e-mail: ayami.yoshimi@uniklinik-freiburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal