Abstract
Rabbit antithymocyte globulin (rATG; thymoglobulin, Genzyme) in combination with cyclosporine, as first-line immunosuppressive therapy, was evaluated prospectively in a multicenter, European, phase 2 pilot study, in 35 patients with aplastic anemia. Results were compared with 105 age- and disease severity–matched patients from the European Blood and Marrow Transplant registry, treated with horse ATG (hATG; lymphoglobulin) and cyclosporine. The primary end point was response at 6 months. At 3 months, no patients had achieved a complete response to rATG. Partial response occurred in 11 (34%). At 6 months, complete response rate was 3% and partial response rate 37%. There were 10 deaths after rATG (28.5%) and 1 after subsequent HSCT. Infections were the main cause of death in 9 of 10 patients. The best response rate was 60% for rATG and 67% for hATG. For rATG, overall survival at 2 years was 68%, compared with 86% for hATG (P = .009). Transplant-free survival was 52% for rATG and 76% for hATG (P = .002). On multivariate analysis, rATG (hazard ratio = 3.9, P = .003) and age more than 37 years (hazard ratio = 4.7, P = .0008) were independent adverse risk factors for survival. This study was registered at www.clinicaltrials.gov as NCT00471848.
Introduction
Historically, horse antithymocyte globulin (hATG) has been the preferred animal source of ATG as first-line treatment for acquired aplastic anemia (AA) patients who are ineligible for hematopoietic stem cell transplantation (HSCT). For severe AA (SAA), the combination of ATG and cyclosporine (CSA) results in a response rate of 60% to 75% of patients, and the response is superior to using either agent alone.1-5 The addition of G-CSF to the combination of ATG and CSA has shown no significant benefit either in terms of response or survival,6-8 although it may reduce infectious complications and duration of hospital admission.6 For patients with nonsevere AA (NSAA) who are transfusion dependent, the combination of ATG and CSA is superior to CSA alone, with a higher response rate, higher blood counts, and improved disease-free survival.9
Rabbit ATG (rATG) is more commonly used for a second course after relapse or lack of response to a first course of hATG. Response to a second course for nonresponse to a first course varies from 30% to 77%10,11 and only 11% in children.12 In contrast, in patients relapsing after a first course, the response to a second course is 65%.11,13
Until 2007, there were 2 preparations of hATG, namely, lymphoglobulin (Genzyme) and ATGAM (Pfizer). The most commonly used preparation of rATG (thymoglobulin, Genzyme) uses the same immunogen as lymophoglobulin; horses or rabbits are immunized with human thymocytes obtained at the time of cardiac surgery from newborn infants. rATG is more immunosuppressive than hATG; it results in more prolonged lymphopenia,14 and it is more effective at preventing and treating acute renal allograft rejection.15 This may be related to differences in CD4+CD25+FOXP3+ Tregs numbers seen in vitro after treatment with rATG, which produces expansion of functional Tregs from normal peripheral blood mononuclear cells PB MNC in contrast to reduction in numbers after hATG (ATGAM).16
Horse ATG (lymphoglobulin) was withdrawn in 2007, resulting in the nonavailability of hATG in Europe and other countries worldwide. In contrast, hATG (ATGAM) is manufactured in the United States but is almost exclusively available in the United States. Subsequently, the use of rATG (thymoglobulin) as first-line immunosuppressive therapy (IST) has been evaluated in prospective and retrospective studies. Most of the recent studies indicate a significantly worse response rate and survival for AA patients treated with rATG in Europe and United States,17-21 but studies from Spain22 and Cleveland Clinic23 and 2 retrospective studies from Asia indicate similar response to hATG.24,25
We undertook a European study conducted by the European Blood and Marrow Transplant (EBMT) Group to assess the efficacy of rATG. The objectives of this study were (1) to assess the tolerability and efficacy of rATG (thymoglobulin) with CSA in the first-line treatment of patients with acquired SAA and patients with NSAA who are transfusion dependent; and (2) to compare the response rate of the combination of rATG and CSA from this pilot study with the response rate observed in a series of matched AA patients, treated after 1994 with the combination of hATG (lymphoglobulin) and CSA. We also examined CD4 T-cell subsets in a subset of patients to understand further the mechanism of action of rATG in AA.
Methods
Study design
This was a phase 2, nonrandomized, prospective, open-label multicenter trial of rATG (thymoglobulin) with CSA in patients with acquired AA, conducted by the EBMT Severe Aplastic Anaemia Working Party, sponsored by the EBMT (EUdraCT #2007-000902-55). The study aimed to accrue 35 patients with AA from specified EBMT centers in the United Kingdom, Germany, France, Italy, Saudi Arabia, and Switzerland. AA was defined by at least 2 of the following: hemoglobin less than 10 g/dL, platelet count less than 50 × 109/L, neutrophil count less than 1.5 × 109/L, and a hypocellular bone marrow on bone marrow biopsy.2 SAA and NSAA were defined by standard criteria.2 Patients enrolled were ineligible for HLA-identical sibling donor BMT, had not received prior IST with ATG or CSA, and included both SAA and transfusion-dependent NSAA. Other inclusion criteria were time from diagnosis to study registration less than or equal to 6 months and no prior treatment, except for hematopoietic growth factors and intravenous immunoglobulin (IVIg) with or without corticosteroids (as shown in Table 1) given for no more than 4 weeks and androgens. Patients enrolled were 16 years of age or older (≥ 18 years in Germany and Switzerland in accordance with German and Swiss law), and there was no upper age limit. Exclusion criteria were: (1) eligibility for an HLA-matched sibling donor transplant; (2) prior therapy with ATG or CSA; (3) prior therapy with hematopoietic growth factors more than 4 weeks before study enrollment; (4) diagnosis of Fanconi anemia, dyskeratosis congenita, or congenital bone marrow failure syndrome; (5) evidence of myelodysplastic disease; (6) paroxysmal nocturnal hemoglobinuria (PNH) with evidence of significant hemolysis, history of PNH-associated thrombosis, or a PNH clone more than 50% by flow cytometry; (7) diagnosis or previous history of carcinoma (except local cervical, basal cell, squamous cells, or melanoma); (8) pregnancy (eg, positive HCG test) or breastfeeding; (9) severe uncontrolled infection or unexplained fever more than 38°C; and (10) hepatic, renal, cardiac, metabolic, or other concurrent diseases of such severity that life expectancy was less than 3 months. The primary endpoint was response at 6 months after ATG treatment, and secondary end points were failure-free survival and overall survival (OS) at 2 years after ATG treatment. An independent data monitoring committee (IDMC) was established to perform an interim evaluation of side effects of rATG and response after 15 patients had been enrolled.
Patient characteristics
| Characteristic . | Value . |
|---|---|
| No. of patients | 35 |
| Median age, y (range) | 36 (17-75) |
| Sex; male/female | 22/13 |
| Disease severity (VSAA/SAA/NSAA) | 7 (20%)/19 (54%)/9 (26%) |
| Median disease duration, d, (range) | 38 (0-168) |
| PNH clone at diagnosis | 8 (23%) |
| Median clone size | |
| Red cells | 0 (0-3.4) |
| Granulocytes | 4.9 (1.0-26.0) |
| Monocytes | 7.0 (0-31.9) |
| Abnormal cytogenetic clone | 1 (trisomy 6) |
| HLA-DR15 (positive/negative/not done/unknown) | 6/10/18/1 |
| Previous treatment | 5 |
| G-CSF | 3 |
| IVIg ± corticosteroids | 2 |
| Characteristic . | Value . |
|---|---|
| No. of patients | 35 |
| Median age, y (range) | 36 (17-75) |
| Sex; male/female | 22/13 |
| Disease severity (VSAA/SAA/NSAA) | 7 (20%)/19 (54%)/9 (26%) |
| Median disease duration, d, (range) | 38 (0-168) |
| PNH clone at diagnosis | 8 (23%) |
| Median clone size | |
| Red cells | 0 (0-3.4) |
| Granulocytes | 4.9 (1.0-26.0) |
| Monocytes | 7.0 (0-31.9) |
| Abnormal cytogenetic clone | 1 (trisomy 6) |
| HLA-DR15 (positive/negative/not done/unknown) | 6/10/18/1 |
| Previous treatment | 5 |
| G-CSF | 3 |
| IVIg ± corticosteroids | 2 |
VSAA indicates very severe AA; SAA, severe AA; and NSAA, nonsevere.
Patients were compared retrospectively with matched AA patients previously treated since 1994 with hATG and CSA and reported to the EBMT. Patients were matched for age (categorized as < 20, 21-60, and 60+ years) and disease severity (nonsevere and severe). Assuming a total response of 60% with hATG and CSA, a ratio of 1:3 between the 2 groups (35 patients treated with rATG and CSA and 105 historical controls) would demonstrate a 25% difference in total response for rATG and CSA, with 80% power at a 5% level of significance.
This study was approved by the local ethics committee at each center, and all patients gave informed written consent in accordance with the Declaration of Helsinki.
Treatment protocol
Rabbit ATG dose was 1.5 vials/10 kg (3.75 mg/kg) daily for 5 days, given as an intravenous infusion over 12 to 18 hours. CSA dose was 5 mg/kg per day orally from day 1 for a minimum of 6 months, with later tailing according to individual patient response. The aim was to maintain trough whole blood CSA levels between 150 and 250 ng/mL. For prevention of serum sickness, methylprednisolone (or prednisolone) 1 to 2 mg/kg per day was given (according to individual center preference) from days 1 to 5, then halved every 5 days. An antihistamine was given before each ATG infusion (dephenhydramine or dexchlorpheniramine) as well as oral antipyretics, such as paracetamol. Red cell and platelet transfusions were given to maintain safe blood counts according to the local center's policy. Patients received prophylactic antifungal agent, antibiotics such as ciprofloxacin, or oral nonabsorbable antibiotics and antiviral drug. Response was defined according to established criteria.2
In vitro studies
PBMCs from 7 patients were analyzed at diagnosis and after rATG (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Statistical analysis
Length of survival in the 2 treatment groups was investigated using the Kaplan-Meier method; treatment groups were compared using the log-rank test. Patients receiving rATG and CSA (or hATG and CSA) were compared for total response at 6 months. The number of patients treated with rATG and CSA was 35. For the comparative study, patients were compared with historical cases treated with hATG with CSA on the EBMT Register. Patient data were analyzed with the NCSS package (NCSS). Comparisons between treatment groups were performed using χ2 test for categorical variables and nonparametric Mann-Whitney U test for variables where distributional assumptions could not be made. The end point for survival analysis was death from any cause. Cox proportional hazards model was used to test the independent effects of a series of explanatory variables after the assumption of proportional hazards had been verified. Critical level of significance was set at .05 (5%).
Results
Phase 2 pilot study
Patient characteristics.
A total of 35 patients were recruited between August 4, 2008 and September 31, 2010, from 10 centers in Europe and Saudi Arabia. Patients' characteristics are shown in Table 1. Median age was 36 years (range, 17-75 years). Numbers with very SAA (VSAA), SAA, and NSAA were 6 (17%), 20 (57%), and 9 (26%), respectively. At diagnosis, a PNH clone was detected in 8 (23%) and an abnormal cytogenetic clone (trisomy 6 in 3 of 19 metaphases) in 1 of 15 evaluable patients. Previous therapies were hemopoietic growth factors in 3 (19%), IVIg with corticosteroids in one, and IVIg alone in one patient.
Response.
Median follow-up for all patients was 397 days (range, 6-805 days). Response is summarized in Table 2. At 3 months, of 32 evaluable patients, no patient had achieved a complete response (CR), and partial response (PR) was seen in 11 (34%) patients. At 6 months, of 30 evaluable patients, 1 patient had achieved a CR (3%) and 11 (37%) had a PR, giving a total response rate of 40%. One patient relapsed at day 59 from start of ATG and died on day 390. A second patient relapsed at day 750. Subsequent events are summarized in Table 2. At last follow-up at 24 months after treatment with rATG, with or without HSCT, 2 patients were in CR, 3 in PR, 1 with no response (NR), 1 was in relapse, 8 were transplanted and 10 patients had died.
Response to rabbit ATG
| . | Mo 3 . | Mo 6 . | Mo 12 . | Mo 24 . |
|---|---|---|---|---|
| All patients | ||||
| CR | 0 | 1 | 2 | 2 |
| PR | 11 | 11 | 12 | 3 |
| NR | 20 | 18 | 4 | 1 |
| Relapse | 1 | 0 | 0 | 1 |
| Dead | 2 | 3 | 10 | 10 |
| Transplanted | 1 | 2 | 7 | 8 |
| Not reached | 0 | 0 | 0 | 10 |
| Total | 35 | 35 | 35 | 35 |
| Severe AA | ||||
| CR | 0 | 1 | 1 | 2 |
| PR | 8 | 6 | 9 | 3 |
| NR | 14 | 14 | 5 | 0 |
| Relapse | 1 | 0 | 1 | 1 |
| Dead | 2 | 3 | 7 | 8 |
| Transplanted | 1 | 2 | 3 | 4 |
| Not reached | 0 | 0 | 0 | 8 |
| Total | 26 | 26 | 26 | 26 |
| . | Mo 3 . | Mo 6 . | Mo 12 . | Mo 24 . |
|---|---|---|---|---|
| All patients | ||||
| CR | 0 | 1 | 2 | 2 |
| PR | 11 | 11 | 12 | 3 |
| NR | 20 | 18 | 4 | 1 |
| Relapse | 1 | 0 | 0 | 1 |
| Dead | 2 | 3 | 10 | 10 |
| Transplanted | 1 | 2 | 7 | 8 |
| Not reached | 0 | 0 | 0 | 10 |
| Total | 35 | 35 | 35 | 35 |
| Severe AA | ||||
| CR | 0 | 1 | 1 | 2 |
| PR | 8 | 6 | 9 | 3 |
| NR | 14 | 14 | 5 | 0 |
| Relapse | 1 | 0 | 1 | 1 |
| Dead | 2 | 3 | 7 | 8 |
| Transplanted | 1 | 2 | 3 | 4 |
| Not reached | 0 | 0 | 0 | 8 |
| Total | 26 | 26 | 26 | 26 |
CR indicates complete response; PR, partial response; and NR, no response.
For patients with SAA (n = 26), no patient had a CR by 3 months and 8 (35%) had a PR. At 6 months, there was 1 CR (5%) and 29% PR. At the 24-month follow-up, 2 patients had a CR, 3 PR, 1 patient had relapsed, 4 were transplanted, and 8 had died (Table 2).
Further treatment.
Eight patients underwent unrelated donor (UD) HSCT for NR to ATG, at 84, 153, 168, 282, 321, 322, 352, and 460 days after ATG. At the time of last follow-up (October 18, 2011), 3 patients had received a second course of ATG, at 139, 287, and 377 days after ATG, respectively.
Survival analyses.
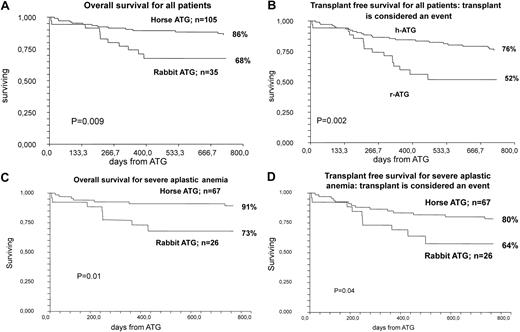
OS was 68% at 2 years, and transplant-free survival was 52%. For patients with SAA (n = 26), OS was 73% and transplant-free survival 64% (Figure 1).
Analysis of survival data. (A) OS for all patients treated with rATG and CSA compared with hATG and CSA, including patients who later received an HSCT for nonresponse to ATG. (B) Transplant-free survival for patients treated with rATG and CSA compared with hATG and CSA: transplant is considered an event. (C) OS for patients with SAA treated with rATG and and CSA compared with hATG and CSA, including patients who later received an HSCT for nonresponse to ATG. (D) Transplant-free survival for patients with SAA treated with rATG and CSA compared with hATG and CSA: transplant is considered an event.
Analysis of survival data. (A) OS for all patients treated with rATG and CSA compared with hATG and CSA, including patients who later received an HSCT for nonresponse to ATG. (B) Transplant-free survival for patients treated with rATG and CSA compared with hATG and CSA: transplant is considered an event. (C) OS for patients with SAA treated with rATG and and CSA compared with hATG and CSA, including patients who later received an HSCT for nonresponse to ATG. (D) Transplant-free survival for patients with SAA treated with rATG and CSA compared with hATG and CSA: transplant is considered an event.
Side effects and cause of death.
Complications and toxicity are listed in Tables 3 and 4. Infections occurred in 22 (63%), elevated liver function in 10 (29%), rash in 8 (23%), hemorrhage in 7 (20%), hypertension in 6 (17%), abnormal renal function in 6 (17%), arthralgia in 5 (14%), and avascular bone necrosis in 1 patient. Renal impairment occurred in 6 patients. Maximum median serum creatinine was 291μM (range, 155-430μM). At last follow-up, median serum creatinine was 130μM (range, 99-347μM). Renal impairment was considered to be secondary to sepsis in 3, CSA toxicity in 2, and unknown in 1 patient. Recovery of renal function occurred in 4 of 6 patients. There were 10 deaths after rATG, and 1 patient died after UD HSCT. Infection was the main cause of death (Table 4).
Side effects of death after rabbit ATG
| Side effects . | No. of events, n = 70 (%) . | % events . |
|---|---|---|
| Infection | 22 (31) | 31 |
| Elevated liver function tests | 10 (14) | 14 |
| Hemorrhage | 7 (10) | 10 |
| Renal impairment | 6 (9) | 9 |
| Rash | 8 (11) | 11 |
| Hypertension | 6 (9) | 9 |
| Arthralgia | 5 (7) | 7 |
| Other | ||
| Diabetes mellitus | 3 (4) | |
| Hypothyroidism | 1 | |
| Ventricular impairment | 1 | |
| Avascular bone necrosis | 1 |
| Side effects . | No. of events, n = 70 (%) . | % events . |
|---|---|---|
| Infection | 22 (31) | 31 |
| Elevated liver function tests | 10 (14) | 14 |
| Hemorrhage | 7 (10) | 10 |
| Renal impairment | 6 (9) | 9 |
| Rash | 8 (11) | 11 |
| Hypertension | 6 (9) | 9 |
| Arthralgia | 5 (7) | 7 |
| Other | ||
| Diabetes mellitus | 3 (4) | |
| Hypothyroidism | 1 | |
| Ventricular impairment | 1 | |
| Avascular bone necrosis | 1 |
Cause of death after rabbit ATG
| Cause of death (n = 11) . | Time of death (d after ATG) . | Age, y . |
|---|---|---|
| Sepsis (n = 5) | 9/210/243/326/370 | 75/65/28/23/21 |
| Pneumonia (n = 2) | 207/209 | 65/56 |
| Sepsis, renal failure, and multiorgan failure | 6 | 36 |
| Sepsis and cardiac arrest | 148 | 56 |
| Intracranial hemorrhage | 288 | 71 |
| Post-SCT invasive fungal infection | 390 | 39 |
| Cause of death (n = 11) . | Time of death (d after ATG) . | Age, y . |
|---|---|---|
| Sepsis (n = 5) | 9/210/243/326/370 | 75/65/28/23/21 |
| Pneumonia (n = 2) | 207/209 | 65/56 |
| Sepsis, renal failure, and multiorgan failure | 6 | 36 |
| Sepsis and cardiac arrest | 148 | 56 |
| Intracranial hemorrhage | 288 | 71 |
| Post-SCT invasive fungal infection | 390 | 39 |
In vitro studies of CD4+ T-cell subsets.
To further understand the mechanism of action of rATG, CD4+ T-cell subsets were analyzed in 7 patients before and after rATG. The results, summarized in the supplemental Table, show that before ATG the number of Tregs was significantly lower in AA compared with healthy age-matched controls, whereas Th1 cells and Th2 cells were higher in AA patients. After ATG, the frequency of Th2 cells was significantly reduced, whereas there was no significant change in the frequency of Tregs, Th1, and Th17 cells.
Comparison of rATG with hATG: matched pair analysis
Patient characteristics.
Patients receiving rATG were matched for age and disease severity with patients receiving hATG as first-line therapy; all patients also received CSA (Table 5). There were no significant differences between the 2 groups in terms of age, disease severity, and time interval from diagnosis to ATG treatment. Median follow-up was significantly longer in the historical hATG group.
Comparison of rATG with hATG: matched pair analysis
| . | rATG, n = 35 (%) . | hATG, n = 105 (%) . |
|---|---|---|
| Age, y | ||
| 0-20 | 4 (11%) | 12 (11%) |
| 21-60 | 24 (69%) | 72 (69%) |
| > 60 | 7 (20%) | 21 (20%) |
| Disease severity | ||
| ≤ 0.5 × 109/L neutrophils | 26 (74%) | 78 (74%) |
| > 0.5 × 109/L neutrophils | 9 (26%) | 27 (26%) |
| VSAA | 6 (17%) | 26% |
| Median interval diagnosis to treatment, d | 53 | 75 (P = 0.3) |
| Median follow-up, d (range) | 285 (6-781); P < .00001 | 1241 (21-4802) |
| . | rATG, n = 35 (%) . | hATG, n = 105 (%) . |
|---|---|---|
| Age, y | ||
| 0-20 | 4 (11%) | 12 (11%) |
| 21-60 | 24 (69%) | 72 (69%) |
| > 60 | 7 (20%) | 21 (20%) |
| Disease severity | ||
| ≤ 0.5 × 109/L neutrophils | 26 (74%) | 78 (74%) |
| > 0.5 × 109/L neutrophils | 9 (26%) | 27 (26%) |
| VSAA | 6 (17%) | 26% |
| Median interval diagnosis to treatment, d | 53 | 75 (P = 0.3) |
| Median follow-up, d (range) | 285 (6-781); P < .00001 | 1241 (21-4802) |
VSAA indicates very severe AA.
Response rate.
Response to hATG and CSA is recorded in the EBMT database at a given time, so response rates at specific time points of 3, 6, 12, and 24 months are not available. Comparison of best response rates for rATG with the 105 age- and disease-matched patients treated with hATG are shown in Table 6. The best CR rate was 23% and 44% for rATG and hATG, respectively, and PR rate 37% and 23%, respectively. The best total response for rATG was 60% compared with 67% for hATG.
Comparison of response to rATG and hATG
| . | Alive . | Dead . | Total . | % . |
|---|---|---|---|---|
| rATG | ||||
| CR | 8 | 0 | 8 | 23 |
| PR | 13 | 0 | 13 | 37 |
| NR | 3 | 9 | 12 | 34 |
| Not evaluable* | 0 | 2 | 2 | 6 |
| Total | 24 | 11 | 35 | |
| hATG | ||||
| CR | 43 | 3 | 46 | 44 |
| PR | 22 | 2 | 24 | 23 |
| NR | 21 | 14 | 35 | 33 |
| Total | 86 | 19 | 105 | |
| . | Alive . | Dead . | Total . | % . |
|---|---|---|---|---|
| rATG | ||||
| CR | 8 | 0 | 8 | 23 |
| PR | 13 | 0 | 13 | 37 |
| NR | 3 | 9 | 12 | 34 |
| Not evaluable* | 0 | 2 | 2 | 6 |
| Total | 24 | 11 | 35 | |
| hATG | ||||
| CR | 43 | 3 | 46 | 44 |
| PR | 22 | 2 | 24 | 23 |
| NR | 21 | 14 | 35 | 33 |
| Total | 86 | 19 | 105 | |
CR indicated complete response; PR, partial response; and NR, not reached.
Two patients not evaluable for response as they died on day 6 and day 9 after ATG.
Survival and mortality.
Median follow-up of all patients was 397 days (range, 6-805 days). Two-year OS after rATG was 68% compared with 86% for hATG (P = .009; Figure 1A). The transplant-free survival after rATG was 52% compared with 76% for hATG (P = .002; Figure 1B). For patients with SAA, the OS was 91% and 73% for hATG and rATG, respectively (P = .01; Figure 1C), and the transplant-free survival 80% and 64% for hATG and rATG, respectively (P = .04; Figure 1D). On multivariate analysis, the use of rATG (hazard ratio = 3.9; 95% CI, 1.5-10.1; P = .003) and age more than 37 years (hazard ratio = 4.7; 95% CI, 1.9-11.9; P = .0008) were independent adverse factors for survival. There were 11 of 35 (31%) deaths in the rATG study compared with 19 of 105 (18%) in the hATG group.
Discussion
hATG has been used in the treatment of AA since the late 1970s, with the later addition of CSA, resulting in response in two-thirds of patients.2-6 Since then, there has been a progressive improvement in survival, to approximately 80% at 5 years, although patients with severe disease and older patients (> 60 years of age) show inferior response and survival.6 After the withdrawal of hATG (lymphoglobulin), rATG has been used instead as first-line IST. It was hoped and expected that similar, or even better, response might be seen with rATG because it is more immunosuppressive than hATG, producing a lower and more prolonged lymphopenia (especially CD4+ T cells). However, our study shows inferior outcomes with rATG. A prospective, randomized study from National Institutes of Health compared hATG (ATGAM) and CSA with rATG and CSA in 120 patients. Response at 6 months for the hATG arm was 68% compared with 37% for the rATG arm (P = .001). OS at 3 years was 96% versus 76% (P = .04), respectively.17 A phase 2 study from the Cleveland Clinic of 20 SAA patients treated with rATG and CSA did not show a significant difference in response (45%) at 6 months compared with 58% among historical controls treated with hATG (ATGAM) and CSA (P = .44).23 Of 7 recent retrospective studies, 4 studies18-21 have shown worse outcomes with rATG (thymoglobulin) compared with hATG and 3 studies22,24,25 have shown similar results. Our study supports the conclusion that outcomes are worse with rATG in the United States and Europe. Although we report a similarly low response rate to rATG as in the National Institutes of Health study, with longer follow-up, comparable best response of approximately 60% was seen comparing rATG with hATG (lymphoglobulin) in the retrospective part of the study. Total response to rATG at 6 months was 40%, but a further 2 patients responded by 12 months, indicating that a later evaluation of response may be appropriate for some patients, and this may parallel the late responses seen after cyclophosphamide treatment.26 Two recent retrospective studies from Japan and South Korea, respectively, showing similar response with rATG and hATG, suggest that differences in ethnicity may play a contributing role in determining outcomes after ATG.24,25 In the Japanese study,24 the presence of glycosylphosphatidylinositol (GPI)–deficient clones predicted response to rATG. Finally, the observed inferiority of rATG may pertain to the disease of AA itself, and probably not to other indications for using the drug, such as solid organ transplantation, HSCT conditioning regimens, and treatment of GVHD.
We observed a higher number of deaths resulting from infection after rATG compared with the National Institutes of Health study. We also report a lower transplant-free survival rate of 52% compared with 76% in the National Institutes of Health study. The difference between the survival curves for rATG and hATG in this study starts to separate after 6 months, indicating that late mortality may be related to nonresponse rather than direct toxicity of rATG. However, a similar nonresponse rate was seen between the rATG and hATG groups. Another difference between the 2 studies is patient age. The mean age of patients treated with rATG in the National Institutes of Health study was 37.4 ± 2.7 years and 31.2 ± 2.6 years for those treated with hATG (ATGAM). In this study of rATG, 31% of patients were older than 50 years and 20% were older than 60 years. However, a similar proportion (30% and 21%, respectively) of patients older than 50 years and older than 60 years received hATG (lymphoglobulin) but with a lower number of deaths. Therefore, older age was not a contributory factor for the differences seen between thymoglobulin and lymphoglobulin. A further possibility may be the more intense degree of immunosuppression caused by rATG, which might be responsible for the high incidence of severe infections. Patients who received hATG were also matched for disease severity (Table 5). The National Institutes of Health study enrolled only patients with SAA, whereas our study also included patients with nonsevere disease, in whom we would expect a lower risk of infections. The IDMC reviewed response to ATG and side effects after the first 15 patients were enrolled. The study investigators requested a further IDMC review after patient 27 died on day 9 after ATG. On both occasions, the IDMC advised that there was no reason to stop the study. We also observed renal impairment in 6 (17%) patients after treatment with rATG associated with sepsis in 3 patients and not reversible in 2 patients.
The difference in response rates between rATG and hATG (lymphoglobulin) suggests a different mechanism of action in AA. The National Institutes of Health group have shown that, after ATG treatment in AA, compared with hATG (ATGAM), rATG induced a significantly lower frequency and number of CD4+ cells and a higher frequency but lower number of Tregs. Tregs are known to be reduced in AA27 and Th17 increased.28 We have recently reported that AA is characterized by clonally restricted expansion of CD4+ Th1 cells in AA.29 In this study, Tregs were decreased, whereas the Th1 and Th2 cells are increased in some AA patients compared with healthy controls. After rATG treatment, the frequency of Th2 cells is significantly reduced (supplemental Table). Further studies on larger numbers of patients are now indicated to confirm these preliminary results.
At the time of last follow-up, 8 of 13 patients who had not responded to rATG received an UD HSCT. The indication for considering UD HSCT is failure to respond to one course of IST. We observed a high mortality in patients between 6 and 12 months who had not responded to rATG, in support of early HSCT in nonresponding patients. Moreover, because outcomes after UD HSCT for children with SAA are now similar to outcomes after matched sibling donor HSCT,30 UD HSCT is being considered as first-line treatment for SAA patients who lack a matched sibling donor, instead of prior treatment with a course of ATG.31 In light of the poor survival after treatment with rATG shown by this and the National Institutes of Health study,17 first-line UD HSCT may also be considered in young adults, as OS survival after UD HSCT is approximately 75% to 83%,32,33 which is superior to survival after IST using rATG. Another alternative therapeutic option is to consider using alemtuzumab. Responses have been reported in several small series for untreated patients and nonresponders to ATG.34-36 A large prospective study from the National Institutes of Health showed 37% response for refractory SAA and 56% for relapsed SAA. Patients with untreated SAA were randomized to alemtuzumab as part of the third arm of the prospective randomized study from the National Institutes of Health, the other 2 arms being hATG and rATG, respectively, as reported by Scheinberg et al.17 The third arm using alemtuzumab was closed prematurely because of 3 early deaths and a response rate of only 19%.37
In conclusion, this study shows that the combination of rATG and CSA results in low response at 3 and 6 months, similar later response compared with hATG, and worse survival compared with hATG and CSA. Because of the high risk of infections and the results of a recent randomized trial,6 G-CSF may be considered when using rATG as first-line therapy with CSA. Unrelated donor transplantation for young adults would also be a clinical option.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Britta Hochsmann for helping to activate the study and recruit patients in Germany, and Louise Wallis at the Royal Bournemouth Hospital, United Kingdom.
This study was supported by Genzyme Therapeutics (an unrestricted grant for data acquisition). The Cambridge National Institute for Health Research Biomedical Research Center supported the study at Addenbrooke's Hospital.
Authorship
Contribution: J.C.M. was the principal investigator for this study and wrote the paper; J.C.M., J.R.P., A.T., H.S., G.S., and A. Bacigalupo designed the study and performed investigations; J.C.M., A. Bacigalupo, H.S., A.T., A.M.R., J.R.P., M.A., C.D., S.B.K., A. Barrois, T.H.B., A.J.W., A.F., P.S., R.O., A.R., S.K., G.J.M., and G.S. analyzed data and wrote the paper; R.O., A. Barrolg, and P.S. analyzed data; S.K. preformed the in vitro studies; J.C.M., A. Bacigalupo, A.T., J.R.P., S.B.K., A.R., A.F., A.J.W., M.A., H.A.A.-Z., T.F., M.O.E., G.J.M., and G.S. recruited patients; and P.S., A. Bacigalupo, R.O., and A. Barrois performed statistical analysis.
Conflict-of-interest disclosure: J.C.M. held a consultancy with Genzyme from May 2008 to May 2009 and from June 2009 to July 2009, and received research funding in 2010 from Genzyme. A. Bacigalupo had been on the speakers' bureau of Genzyme from 2000 to 2011 and has received research funding from Pfizer. C.D. received research funding in 2010 from Pfizer. A.M.R. received honoraria from Genzyme in 2010. H.S. received research funding in 2010/2011 from Genzyme and was a speaker in Genzyme-sponsored symposium in 2010. G.J.M. has received research funding in 2010 from Genzyme. The remaining authors declare no competing financial interests.
Correspondence: Judith C. Marsh, Department of Haematological Medicine, King's College Hospital, Denmark Hill, London SE59RS, United Kingdom; e-mail: judith.marsh@nhs.net.