Key Points
Antibodies against factor VIII show distinct characteristics in healthy individuals and different cohorts of hemophilia A patients.
IgG4 antibodies against FVIII are only found in patients with inhibitors but not in healthy individuals or patients without inhibitors.
Abstract
Neutralizing antibodies against factor VIII (FVIII) remain the major complication in the replacement therapy of hemophilia A patients. To better understand the evolution of these antibodies it is important to generate comprehensive datasets which include both neutralizing and nonneutralizing antibodies, their isotypes, and IgG subclasses. We developed sensitive ELISAs to analyze FVIII-binding antibodies in different cohorts of hemophilia A patients and in healthy individuals. Our data reveal the prevalence of FVIII-binding antibodies among healthy individuals (n = 600) to be as high as 19%, with a prevalence of antibody titers ≥ 1:80 of 2%. The prevalence of FVIII-binding antibodies was 34% (5% for titers ≥ 1:80) in patients without FVIII inhibitors (n = 77), 39% (4% for titers ≥ 1:80) in patients after successful immune tolerance induction therapy (n = 23), and 100% (n = 20, all titers ≥ 1:80) in patients with FVIII inhibitors. We found significant differences for IgG subclasses of FVIII-binding antibodies between the different study cohorts. IgG4 and IgG1 were the most abundant IgG subclasses in patients with FVIII inhibitors. Strikingly, IgG4 was completely absent in patients without FVIII inhibitors and in healthy subjects. These findings point toward a distinct immune regulatory pathway responsible for the development of FVIII-specific IgG4 associated with FVIII inhibitors.
Introduction
The development of neutralizing antibodies against factor VIII (FVIII) inhibitors in approximately 25% to 30% of severe hemophilia A patients represents the most serious adverse event after replacement therapy with FVIII products.1 This problem has been known for many years, yet why some patients develop FVIII inhibitors while others do not is still far from clear.
Antibodies directed against FVIII have been found to be comprised of a polyclonal IgG response.2,3 Clinical and experimental data indicate that FVIII inhibitor development depends on CD4+ T-cell help.4-6 Interactions between B cells and CD4+ T cells not only initiate expansion and differentiation of B cells, but also trigger isotype switching and affinity maturation of antibodies.7,8
In clinical practice, antibody responses against FVIII are commonly identified as FVIII inhibitors by using the Bethesda or Nijmegen-modified Bethesda Assay.9 Although these assays have provided important results, greatly contributing to our understanding of the loss of FVIII function seen in patients, FVIII inhibitors do not reflect the whole picture of FVIII-specific antibody responses. More recently, several assay platforms for the detection of total FVIII-binding antibodies were presented which were based on classic ELISA formats, immunoblotting or bead-based formats.10-13 Studies using these platforms clearly showed that neutralizing antibodies represent only part of the overall antibody spectrum directed against FVIII. As expected, there are binding antibodies with specificity to FVIII that do not neutralize the protein and, therefore, are not detectable using a Bethesda assay. In addition, circulating antibodies against FVIII are found in a proportion of patients without FVIII inhibitors and even in some healthy individuals.12 So far, the biologic significance of FVIII-binding antibodies in patients without FVIII inhibitors and in healthy individuals has not been elucidated. Studies in other diseases indicated that nonneutralizing binding antibodies against therapeutic proteins may alter the protein‘s pharmacokinetic or pharmacodynamic profiles.14,15 Furthermore, recent studies of self-reactive antibodies in healthy individuals indicated that these antibodies might be involved in the maintenance of immune homeostasis.16,17
Gilles et al and others reported that the antibody response to FVIII in patients is not isotypically restricted and involves all IgG subclasses with a preponderance of IgG1 and IgG4.18 Only limited information is available on the characteristics of antibodies against FVIII which are found in patients without FVIII inhibitors and in healthy individuals. Most studies that have been published have incorporated affinity purification steps associated with acid treatment of the antibody preparation19-21 which might have created artificial multireactivity that would not be seen in vivo.22,23 Therefore, results of previous studies have to be interpreted with caution.
We present a comprehensive set of data on antibodies against FVIII, including their Ig isotypes and IgG subclasses, found in healthy individuals and in hemophilia A patients with and without FVIII inhibitors. We established and validated ELISA-based assays that were set up according to current regulatory guidelines for the assessment of the immunogenicity of protein therapeutics.24,25 Using these assays, we analyzed plasma samples from 600 healthy individuals stratified by age, sex, and geography as well as samples from 77 hemophilia A patients without FVIII inhibitors, 20 hemophilia A patients with inhibitors, and 23 hemophilia A patients who had undergone successful immune tolerance induction therapy (ITI). In addition, we included plasma samples from 9 acquired hemophilia A patients for comparison.
Our results estimate the prevalence of FVIII-binding antibodies in the healthy population and in different populations of patients with hemophilia A, with separate calculations for antibody titers < 1:80 and antibody titers ≥ 1:80. Moreover, data on Ig isotypes and IgG subclass distribution of FVIII-binding antibodies indicate important differences between antibodies found in patients with FVIII inhibitors and antibodies found in patients without inhibitors and in healthy individuals. These differences might be indicative of different immune regulatory pathways that drive the development of antibodies against FVIII in the different study cohorts.
Methods
Human plasma samples
Samples of citrated human plasma were collected and stored at −20°C until analysis. All patient samples were received after the subjects or their guardians, respectively, gave written informed consent in accordance with the Declaration of Helsinki, and with the approval from the local ethical committees.
Healthy individuals
Plasma samples from 600 healthy donors were chosen from registered plasma donors in 6 different geographies: 100 plasma samples from centers throughout Austria and 100 samples from each of the following locations in United States: Ammon, ID; Elkhart, IN; Fargo, ND; Lakeland, FL; and Laredo, TX. This was done to achieve an equal proportion of each sex (50 females and 50 males per plasma center) and a uniform distribution of age (18-66 years) within each sex and plasma center.
Patients with hemophilia A
Plasma samples from 120 subjects with severe congenital hemophilia A (FVIII < 1%) were obtained from the Medical University in Bonn, Germany, the Medical University in Vienna, Austria, and the Institute of Hematology and Transfusion Medicine, Warsaw, Poland. Twenty samples were obtained from subjects with current FVIII inhibitors, with a median time from inhibitor diagnosis of 52 months (intraquartile range [IQR], 12-176 months) and a median historic peak inhibitor titer of 143 BU/mL (IQR, 88-213 BU/mL). Twenty-three samples were obtained from subjects after successful ITI, with a median time after successful completion of ITI of 165 months (IQR, 95-251 months) and a median historic peak inhibitor titer of 11 BU/mL (IQR, 2-22 BU/mL). The criteria of successful completion of ITI were negative Bethesda assay, FVIII recovery > 66% and FVIII half-live > 6 hours. The remaining 77 samples were obtained from subjects with no history of FVIII inhibitors after at least 100 exposure days to FVIII products without any record of transient inhibitors.
Patients with acquired hemophilia A
For comparison, 9 samples from subjects with acquired hemophilia A were included which were obtained from the Medical School in Hannover, Germany. Samples were taken after the first diagnosis with a median FVIII inhibitor titer of 35 BU/mL (IQR, 7-205 BU/mL).
Human recombinant proteins
Full-length recombinant human FVIII (FVIII) was obtained from Baxter BioScience. Refacto (Pfizer) was used as source for B-domain–deleted recombinant human FVIII (BDD FVIII).
ELISAs for the detection of binding antibodies
ELISAs for the detection of binding antibodies against FVIII were established in compliance with the most recent regulatory guidelines.24,25 Polysorp microtiter plates (Nunc) were coated with 1 μg/mL FVIII overnight at 4°C. All washing steps were done with phosphate-buffered saline (PBS; pH 7.4, Invitrogen) containing Tween (Merck). Unspecific binding sites were blocked by incubation with preselected blocking buffers for 1 hour at room temperature (RT). Afterward, plasma samples and controls were incubated for 2 hours at RT. Enzyme-conjugated secondary antibodies (see supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) were added followed by incubation for 1 hour at RT. All detection antibodies were tested and confirmed for specificity to their appropriate human Ig isotype or IgG subclass (data not shown). Appropriate substrates were added and incubated at RT in the dark. The delta optical density (DOD) for each sample was assessed using a Microplate Reader (Synergy HR BioTek Instruments) in dual mode at 405 nm (for alkaline phosphatase [AP]), 450 nm (for 3,3′,5,5′-tetramethylbenzidine [TMB]), 492 nm (for o-phenylenediamine [OPD]) measuring wavelength and 630 nm reference wavelength. DOD of each sample was corrected for blank values.
Determination of cutoffs
A predetermined cutoff was established for each assay using a statistical approach based on background signal levels of 160 healthy plasma donors as described in Jaki et al.26 The initially determined cutoff for IgG2 and IgG4 was very close to the limit of detection of the ELISA reader and outside of the linearity of the positive control curve. To account for the inherent fluctuations in the ELISA signal, the cutoffs for IgG2 and IgG4 were increased by a factor of 2 and 3, respectively. This increased the robustness of the assays while maintaining similar sensitivities.
For the assessment of IgM antibodies a “floating cut point” was established. To account for daily variation, a “floating cut-point correction factor” was used as described by Shankar et al.27
Screening assay
Each plasma sample was analyzed twice at a dilution of 1:20. The minimum dilution of 1:20 was chosen to prevent unspecific matrix effects. If the DOD of both analyses was below cutoff, the sample was deemed negative. If the DOD of both analyses was equal to or greater than the cutoff, the sample was considered positive, and subsequently analyzed for antibody titers (see “Determination of antibody titers”). In case of discrepancy, a third repetition was done which determined whether the sample was considered positive or negative.
Determination of antibody titers
The titer of a sample was defined as the highest dilution that still gave a positive signal (DOD ≥ cutoff). Appropriate positive controls and samples were diluted in geometric progression, starting at a dilution of 1:20 and continuing with 1:2 dilution steps. Each sample was analyzed at least twice. Based on the validated assay precision of ± 1 titer step (see “Validation of ELISAs for the detection of FVIII-binding antibodies” and supplemental Figure 1), a difference of 2 titer steps between the 2 analyses of the same sample was the maximal variation accepted. In approximately 10% of cases, the difference in the results of the 2 analyses of the same sample was more than 2 titer steps. In these cases, a third repetition was done.
If the difference of the 2 analyses of the same sample was 1 titer step, the higher titer was reported. Otherwise, the median titer was reported.
Confirmation of specificity
Plasma samples and controls were preincubated with recombinant human FVIII (100 μg/mL) for 1 hour at RT and subsequently assessed for antibody titers as described in “Determination of antibody titers.” The specificity of an antibody was confirmed if the competition assay showed an antibody titer that was at least 3 titer steps lower than the antibody titer detected without competition. Based on the validated assay precision, a difference of only 1 or 2 titer steps could be a reflection of the variability of the method. Thus, only plasma samples with an antibody titer ≥ 1:80 could be evaluated for specificity.
Positive and negative controls
Each assay involved a FVIII-binding human monoclonal antibody of the respective Ig isotype or IgG subclass as a positive control, all recognized the same FVIII epitope (AbD Serotec). The positive controls were spiked into a negatively screened plasma pool from healthy donors. The positive control antibodies were used to determine the sensitivity of each assay (see supplemental Table 1) and for assay validation.
The negative control was a plasma pool generated from negatively screened healthy individuals.
Validation of ELISAs for the detection of FVIII-binding antibodies
All ELISAs were validated with regard to precision (interassay and intraassay variability), specificity, linearity, and robustness. The acceptance criteria for precision of all ELISAs was ± 1 titer step (see supplemental Figure 1). Based on this assay precision, differences in antibody titers between 2 samples had to be at least 3 titer steps to be evaluated as different. Differences of only 1 or 2 titer steps could simply reflect the variability of the assays.
The mode titer of the positive controls was defined as the median titer detected during assessment of interassay precision. The validation of an assay was considered successful if the positive control did not differ more than ± 1 titer step from the mode titer, and if all negative controls (diluted 1:20 and tested in 4 parallels) tested negative. During routine analysis of plasma samples, the negative and positive controls had to meet these acceptance criteria. On change of the lot of a critical component (FVIII-coating antigen, secondary conjugated antibodies, positive and negative controls), the compliance of the new lot with the acceptance criteria during validation was verified by a requalification procedure.
Potential specificity of binding antibodies against FVIII B-domain
Positive samples with titers ≥ 1:80 against FVIII were tested for their binding to BDD FVIII using “screening assays” as described in “Screening assays.” BDD FVIII was coated at a concentration of 1 μg/mL. Samples that tested positive against FVIII but negative against BDD FVIII, were considered to potentially contain only antibodies that recognize the B-domain of FVIII. However, we would like to emphasize that this is a simplified assumption because differences in reactivity against FVIII and BDD FVIII could be because of other reasons, for example, loss of conformational epitopes because of changes in the tertiary structure of the protein.
Detection of neutralizing antibodies against FVIII
All plasma samples that contained FVIII-binding antibodies were tested for the presence of neutralizing antibodies against FVIII in clinical laboratories using a Nijmegen modification of the Bethesda assay.28
The presence of FVIII inhibitors in 3 samples obtained from healthy individuals were assessed in-house using a commercially available kit (Technoclone) because the available plasma volume was not sufficient for the routine clinical test.
Statistical analysis
The prevalence of FVIII-binding antibodies in study cohorts without FVIII inhibitors was estimated along with 95% confidence intervals (CIs) calculated according to Wilson.29
The nonparametric coefficient of correlation according to Spearman was calculated for correlation analysis of FVIII-binding antibodies and FVIII inhibitors and for correlation analysis of IgG1 and IgG4 FVIII-binding antibodies using Prism 5.0 (GraphPad Software).
For the cohort of healthy individuals, subject-specific covariates (age, sex, and geography) were used to model the observed FVIII-binding IgG1, IgG3, or IgA antibodies using a zero-inflated Poisson fixed effects regression model similar to Bonate et al.30 A fully specified model (including covariates sex, age, and geography in both components) was compared with the null model (trivial model with intercepts only) using the likelihood ratio test. These statistical analysis were performed with SAS Version 9.2 and R Version 2.13.231 using R packages pcsl32 and lmtest.33
The level of statistical significance was set to 5%.
Results
Prevalence of FVIII-binding antibodies in healthy individuals and in different cohorts of hemophilia A patients
We investigated plasma samples from 600 healthy individuals, 77 subjects with hemophilia A and no history of FVIII inhibitors, 20 subjects with hemophilia A and FVIII inhibitors, 23 subjects with hemophilia A after successful ITI, and 9 subjects with acquired hemophilia A for the presence of FVIII-binding antibodies (IgM, IgA, IgG1, IgG2, IgG3, IgG4). We found the prevalence of FVIII-binding antibodies to be 19% (116 of 600) in healthy individuals, 34% (26 of 77) in patients with hemophilia A and no history of FVIII inhibitors, 100% (20 of 20) in patients with hemophilia A and FVIII inhibitors, 39% (9 of 23) in patients with hemophilia A after successful ITI, and 100% (9 of 9) in patients with acquired hemophilia A (Table 1). When we differentiated the results by antibody titers ≥ 1:80 and antibody titers < 1:80, it became apparent that most of the FVIII-binding antibodies that we found in healthy individuals and patients without FVIII inhibitors were antibodies with titers < 1:80 (Table 1). Nevertheless, 2% (14 of 600) of healthy individuals, 5% (4 of 77) of patients without FVIII inhibitors and 4% (1 of 23) of patients after successful ITI had antibodies with titers ≥ 1:80 that we could confirm for specificity by competition assays. As expected, all hemophilia A patients with inhibitors and all acquired hemophilia A patients had FVIII-binding antibodies with titers ≥ 1:80 in at least 1 IgG subclass.
Estimated prevalence of FVIII-binding antibodies in healthy individuals and in different patient cohorts
| Patient type . | Sample size . | Prevalence of positive individuals, % (95% CI) . | Prevalence of antibodies with titers ≥ 1:80, % (95% CI) . | Prevalence of antibodies with titers < 1:80, % (95% CI) . |
|---|---|---|---|---|
| Healthy | 600 | 19 (16-22) | 2 (1-4) | 17 (14-20) |
| Severe hemophilia A without inhibitor (HA-no/INH) | 77 | 34 (24-45) | 5 (2-13) | 31 (22-42) |
| Severe hemophilia A after successful ITI (HA-ITI) | 23 | 39 (22-59) | 4 (1-21) | 35 (19-55) |
| Severe hemophilia A with inhibitor (HA-INH) | 20 | 100 (84-100) | 100 (84-100) | 0 (0-16) |
| Acquired hemophilia A (Acqu-HA) | 9 | 100 (70-100) | 100 (70-100) | 0 (0-30) |
| Patient type . | Sample size . | Prevalence of positive individuals, % (95% CI) . | Prevalence of antibodies with titers ≥ 1:80, % (95% CI) . | Prevalence of antibodies with titers < 1:80, % (95% CI) . |
|---|---|---|---|---|
| Healthy | 600 | 19 (16-22) | 2 (1-4) | 17 (14-20) |
| Severe hemophilia A without inhibitor (HA-no/INH) | 77 | 34 (24-45) | 5 (2-13) | 31 (22-42) |
| Severe hemophilia A after successful ITI (HA-ITI) | 23 | 39 (22-59) | 4 (1-21) | 35 (19-55) |
| Severe hemophilia A with inhibitor (HA-INH) | 20 | 100 (84-100) | 100 (84-100) | 0 (0-16) |
| Acquired hemophilia A (Acqu-HA) | 9 | 100 (70-100) | 100 (70-100) | 0 (0-30) |
Estimated prevalence of FVIII-binding antibodies in healthy individuals, in patients with hemophilia A without inhibitors, and in patients with hemophilia A after successful immune tolerance induction therapy (ITI). Some samples contained more than one population of antibodies of different Ig isotypes and IgG subclasses which were individually assessed for antibody titers ≥ 1:80 and antibody titers < 1:80, respectively. Therefore, the prevalence of antibody titers ≥ 1:80 and antibody titers < 1:80 does not necessarily add up to the total prevalence of positive individuals.
FVIII indicates factor VIII; and CI, confidence interval.
Specificity of FVIII-binding antibodies found in healthy individuals and in patients with hemophilia A without detectable inhibitors
The question arose of whether antibodies with titers ≥ 1:80 found in healthy individuals and in patients without detectable FVIII inhibitors were directed against nonfunctional domains of FVIII, for example, against the B-domain, which could explain the lack of neutralizing capacity. To approach this question, we tested the reactivity of all plasma samples with antibody titers ≥ 1:80 for their reactivity against BDD FVIII. Our results demonstrate that 18 of 19 samples analyzed bound to full-length FVIII but not to BDD FVIII. Only 1 plasma sample, obtained from a healthy individual, contained antibodies that bound to both full-length FVIII and BDD FVIII (Table 2).
Titers and specificities of FVIII-binding antibodies and results of FVIII inhibitor assays
| Study cohort/identification code . | Antibody titer against FVIII . | Binding to BDD FVIII . | Competition by FVIII . | FVIII inhibitors, (BU/mL) . |
|---|---|---|---|---|
| Healthy | ||||
| Healthy 67 | 1:320 | No | Yes | < 0.6 |
| Healthy 549 | 1:160 | No | Yes | < 0.6 |
| Healthy 226 | 1:80 | No | Yes | < 0.6 |
| Healthy 262 | 1:80 | No | Yes | < 0.6 |
| Healthy 510 | 1:80 | Yes | Yes | < 0.6 |
| Healthy 304 | 1:80 | No | Yes | < 0.6 |
| Healthy 480 | 1:80 | No | Yes | < 0.6* |
| Healthy 79 | 1:320 | No | Yes | < 1.0* |
| Healthy 217 | 1:80 | No | Yes | < 0.6 |
| Healthy 512 | 1:160 | No | Yes | < 1.0* |
| Healthy 20 | 1:80 | No | Yes | < 0.6 |
| Healthy 398 | 1:80 | No | Yes | < 0.6 |
| Healthy 62 | 1:80 | No | Yes | < 0.6 |
| Healthy 282 | 1:80 | No | Yes | < 0.6 |
| HA-noINH | ||||
| HA-noINH 7 | 1:320 | No | Yes | < 0.6 |
| HA-noINH 13 | 1:80 | No | Yes | < 0.6 |
| HA-noINH 52 | 1:80 | No | Yes | < 0.6 |
| HA-noINH 77 | 1:80 | No | Yes | < 0.6 |
| HA-ITI | ||||
| HA-ITI 5 | 1:80 | No | Yes | < 0.6 |
| Study cohort/identification code . | Antibody titer against FVIII . | Binding to BDD FVIII . | Competition by FVIII . | FVIII inhibitors, (BU/mL) . |
|---|---|---|---|---|
| Healthy | ||||
| Healthy 67 | 1:320 | No | Yes | < 0.6 |
| Healthy 549 | 1:160 | No | Yes | < 0.6 |
| Healthy 226 | 1:80 | No | Yes | < 0.6 |
| Healthy 262 | 1:80 | No | Yes | < 0.6 |
| Healthy 510 | 1:80 | Yes | Yes | < 0.6 |
| Healthy 304 | 1:80 | No | Yes | < 0.6 |
| Healthy 480 | 1:80 | No | Yes | < 0.6* |
| Healthy 79 | 1:320 | No | Yes | < 1.0* |
| Healthy 217 | 1:80 | No | Yes | < 0.6 |
| Healthy 512 | 1:160 | No | Yes | < 1.0* |
| Healthy 20 | 1:80 | No | Yes | < 0.6 |
| Healthy 398 | 1:80 | No | Yes | < 0.6 |
| Healthy 62 | 1:80 | No | Yes | < 0.6 |
| Healthy 282 | 1:80 | No | Yes | < 0.6 |
| HA-noINH | ||||
| HA-noINH 7 | 1:320 | No | Yes | < 0.6 |
| HA-noINH 13 | 1:80 | No | Yes | < 0.6 |
| HA-noINH 52 | 1:80 | No | Yes | < 0.6 |
| HA-noINH 77 | 1:80 | No | Yes | < 0.6 |
| HA-ITI | ||||
| HA-ITI 5 | 1:80 | No | Yes | < 0.6 |
Titers and specificities of FVIII-binding antibodies and results of FVIII inhibitor assays found in healthy individuals and in hemophilia A patients without FVIII inhibitors. All cases with titers ≥ 1:80 are included.
FVIII indicates factor VIII; HA-noINH, hemophilia A patients without inhibitors; and A-ITI, hemophilia A patients after successful immune tolerance induction therapy (ITI).
Samples were tested in-house with a commercially available kit.
Isotype and IgG subclass distribution of FVIII-binding antibodies found in healthy individuals and in patients with hemophilia A
As several previous reports indicated that the production of certain IgG subclasses might be associated with the involvement of different CD4+ T helper cell subtypes in the immune response against protein antigens,8,34,35 the analysis of Ig isotypes and IgG subclasses of anti-FVIII antibodies could contribute to a better understanding of the regulation of unwanted immune responses against FVIII products. Our results confirm previous reports which showed that the antibody response in patients with FVIII inhibitors is not restricted isotypically and involves all IgG subclasses.18 IgG1 and IgG4 antibodies were most prominent in both hemophilia A patients with inhibitors and in patients with acquired hemophilia A. IgG2 and IgG3 were less frequently detected in these cohorts. IgM and IgA antibodies were rarely seen (Table 3 and Figure 1). The isotype and IgG subclass distribution of FVIII-binding antibodies in healthy individuals and in patients without FVIII inhibitors was different. IgG4 was completely absent in these cohorts (Table 3 and Figure 1). Instead, IgG1, IgG3, and IgA were prominent in healthy individuals; IgG1 and IgG3 were dominant in patients without a history of FVIII inhibitors and IgG1 was dominant in patients after successful ITI.
Estimated prevalence of Ig isotypes and IgG subclasses of FVIII-binding antibodies found in healthy individuals and in different cohorts of hemophilia A patients
| Study cohort . | Sample size . | IgG1, %* . | IgG2, %* . | IgG3, %* . | IgG4, %* . | IgA, %* . | IgM, %* . |
|---|---|---|---|---|---|---|---|
| Healthy | 600 | 6 | 1 | 6 | 0 | 6 | 1 |
| Hemophilia A without inhibitor (HA-noINH) | 77 | 19 | 1 | 13 | 0 | 4 | 3 |
| Hemophilia A after successful ITI (HA-ITI) | 23 | 30 | 9 | 4 | 0 | 0 | 0 |
| Hemophilia A with inhibitor (HA-INH) | 20 | 95 | 35 | 25 | 95 | 10 | 5 |
| Acquired hemophilia A (Acqu-HA) | 9 | 100 | 67 | 22 | 100 | 11 | 11 |
| Study cohort . | Sample size . | IgG1, %* . | IgG2, %* . | IgG3, %* . | IgG4, %* . | IgA, %* . | IgM, %* . |
|---|---|---|---|---|---|---|---|
| Healthy | 600 | 6 | 1 | 6 | 0 | 6 | 1 |
| Hemophilia A without inhibitor (HA-noINH) | 77 | 19 | 1 | 13 | 0 | 4 | 3 |
| Hemophilia A after successful ITI (HA-ITI) | 23 | 30 | 9 | 4 | 0 | 0 | 0 |
| Hemophilia A with inhibitor (HA-INH) | 20 | 95 | 35 | 25 | 95 | 10 | 5 |
| Acquired hemophilia A (Acqu-HA) | 9 | 100 | 67 | 22 | 100 | 11 | 11 |
FVIII indicates factor VIII; and ITI, immune tolerance induction therapy.
Many samples contained different populations of antibodies that were individually assessed for prevalence. Therefore, the sum of the prevalence of the individual isotypes and subclasses may be > 100%.
Titers of FVIII-binding antibodies assessed for individual Ig isotypes and IgG subclasses. The detected titers of Ig isotypes and IgG subclasses of FVIII-binding antibodies for (A) healthy individuals, (B) hemophilia A patients without inhibitors (HA-noINH), (C) hemophilia A patients with inhibitors (HA-INH), (D) acquired hemophilia A (Acqu-HA) patients, and (E) hemophilia A patients after successful ITI (HA-ITI) are shown. Plasma samples were diluted at least 1:20. Samples that did not give a positive signal at this minimum dilution were considered as negative (not detectable [ND]). The dotted line at a titer of 1:80 indicates the minimum titer required for proof of specificity. Titers of < 1:80 were too low to be confirmed for specificity.
Titers of FVIII-binding antibodies assessed for individual Ig isotypes and IgG subclasses. The detected titers of Ig isotypes and IgG subclasses of FVIII-binding antibodies for (A) healthy individuals, (B) hemophilia A patients without inhibitors (HA-noINH), (C) hemophilia A patients with inhibitors (HA-INH), (D) acquired hemophilia A (Acqu-HA) patients, and (E) hemophilia A patients after successful ITI (HA-ITI) are shown. Plasma samples were diluted at least 1:20. Samples that did not give a positive signal at this minimum dilution were considered as negative (not detectable [ND]). The dotted line at a titer of 1:80 indicates the minimum titer required for proof of specificity. Titers of < 1:80 were too low to be confirmed for specificity.
Next, we were interested to trace the presence of multiple isotypes and IgG subclasses of anti-FVIII antibodies in the same plasma sample. The simultaneous development of different isotypes or IgG subclasses of anti-FVIII antibodies would confirm the polyclonal nature of the antibody response and might also indicate the involvement of different CD4+ T helper cell subtypes in the regulation of the immune response against FVIII. Our results clearly demonstrate that almost all samples obtained from hemophilia A patients with inhibitors, and all samples obtained from patients with acquired hemophilia A contained multiple isotypes and IgG subclasses of anti-FVIII antibodies (Table 4). In contrast, only a fraction of healthy individuals and patients without FVIII inhibitors were found to have detectable anti-FVIII antibodies of multiple isotypes and IgG subclasses (Table 4).
Subjects with multiple Ig Isotypes and/or IgG subclasses of FVIII-binding antibodies
| No. (%) of positive samples that contain multiple Ig isotypes/IgG subclasses, identification code . | Titer of FVIII-binding antibodies . | |||||
|---|---|---|---|---|---|---|
| IgG1 . | IgG2 . | IgG3 . | IgG4 . | IgA . | IgM . | |
| Healthy: 7 of 116 positives (6%), sample size: n = 600 | ||||||
| Healthy 12 | neg | neg | 1:80 | neg | neg | 1:20 |
| Healthy 54 | neg | neg | 1:20 | neg | neg | 1:40 |
| Healthy 93 | 1:20 | neg | neg | neg | 1:20 | neg |
| Healthy 111 | 1:20 | neg | neg | neg | 1:40 | neg |
| Healthy 297 | 1:40 | 1:40 | ||||
| Healthy 311 | 1:40 | 1:40 | ||||
| Healthy 595 | 1:20 | 1:40 | ||||
| Hemophilia A without inhibitor: 5 of 26 positives (19%), sample size: n = 77 | ||||||
| HA-noINH 13 | 1:80 | 1:40 | ||||
| HA-noINH 28 | 1:20 | 1:20 | ||||
| HA-noINH 55 | 1:40 | 1:40 | ||||
| HA-noINH 63 | 1:40 | 1:20 | ||||
| HA-noINH 77 | 1:80 | 1:20 | ||||
| Hemophilia A with inhibitor: 19 of 20 positives (95%), sample size: n = 20 | ||||||
| HA-INH 1 | 1:80 | 1:320 | 1:20 | |||
| HA-INH 2 | 1:80 | 1:640 | ||||
| HA-INH 3 | 1:80 | 1:640 | ||||
| HA-INH 4 | 1:320 | 1:160 | 1:80 | 1:1280 | ||
| HA-INH 5 | 1:40 | 1:1280 | ||||
| HA-INH 6 | 1:160 | 1:1280 | ||||
| HA-INH 7 | 1:640 | 1:20 | 1:5120 | |||
| HA-INH 8 | 1:2560 | 1:320 | 1:40 | 1:10 240 | ||
| HA-INH 9 | 1:1280 | 1:5120 | 1:40 | |||
| HA-INH 10 | 1:160 | 1:40 | 1:80 | 1:10 240 | ||
| HA-INH 11 | 1:5120 | 1:640 | 1:163840 | 1:20 | ||
| HA-INH 12 | 1:80 | 1:20 | 1:1280 | |||
| HA-INH 14 | 1:80 | 1:40 | ||||
| HA-INH 15 | 1:20 | 1:160 | ||||
| HA-INH 16 | 1:80 | 1:40 | 1:320 | |||
| HA-INH 17 | 1:80 | 1:20 | 1:160 | 1:320 | ||
| HA-INH 18 | 1:80 | 1:320 | ||||
| HA-INH 19 | 1:160 | 1:320 | ||||
| HA-INH 20 | 1:80 | 1:40 | 1:640 | |||
| Acquired hemophilia A: 9 of 9 positives (100%), sample size: n = 9 | ||||||
| Acqu-HA1 | 1:5120 | 1:40 | 1:20 480 | 1:20 | ||
| Acqu-HA 2 | 1:1280 | 1:320 | ||||
| Acqu-HA 3 | 1:2560 | 1:80 | 1:640 | |||
| Acqu-HA 4 | 1:320 | 1:1280 | ||||
| Acqu-HA 5 | 1:10 240 | 1:320 | 1:80 | 1:2560 | ||
| Acqu-HA 6 | 1:40 960 | 1:160 | 1:320 | 1:2560 | ||
| Acqu-HA 7 | 1:5120 | 1:20 | 1:5120 | 1:40 | ||
| Acqu-HA 8 | 1:2560 | 1:5120 | ||||
| Acqu-HA 9 | 1:1280 | 1:80 | 1:20 480 | |||
| Hemophilia A after successful ITI: 1 of 9 positives (11%), sample size: n = 23 | ||||||
| HA-ITI 11 | 1:40 | 1:40 | ||||
| No. (%) of positive samples that contain multiple Ig isotypes/IgG subclasses, identification code . | Titer of FVIII-binding antibodies . | |||||
|---|---|---|---|---|---|---|
| IgG1 . | IgG2 . | IgG3 . | IgG4 . | IgA . | IgM . | |
| Healthy: 7 of 116 positives (6%), sample size: n = 600 | ||||||
| Healthy 12 | neg | neg | 1:80 | neg | neg | 1:20 |
| Healthy 54 | neg | neg | 1:20 | neg | neg | 1:40 |
| Healthy 93 | 1:20 | neg | neg | neg | 1:20 | neg |
| Healthy 111 | 1:20 | neg | neg | neg | 1:40 | neg |
| Healthy 297 | 1:40 | 1:40 | ||||
| Healthy 311 | 1:40 | 1:40 | ||||
| Healthy 595 | 1:20 | 1:40 | ||||
| Hemophilia A without inhibitor: 5 of 26 positives (19%), sample size: n = 77 | ||||||
| HA-noINH 13 | 1:80 | 1:40 | ||||
| HA-noINH 28 | 1:20 | 1:20 | ||||
| HA-noINH 55 | 1:40 | 1:40 | ||||
| HA-noINH 63 | 1:40 | 1:20 | ||||
| HA-noINH 77 | 1:80 | 1:20 | ||||
| Hemophilia A with inhibitor: 19 of 20 positives (95%), sample size: n = 20 | ||||||
| HA-INH 1 | 1:80 | 1:320 | 1:20 | |||
| HA-INH 2 | 1:80 | 1:640 | ||||
| HA-INH 3 | 1:80 | 1:640 | ||||
| HA-INH 4 | 1:320 | 1:160 | 1:80 | 1:1280 | ||
| HA-INH 5 | 1:40 | 1:1280 | ||||
| HA-INH 6 | 1:160 | 1:1280 | ||||
| HA-INH 7 | 1:640 | 1:20 | 1:5120 | |||
| HA-INH 8 | 1:2560 | 1:320 | 1:40 | 1:10 240 | ||
| HA-INH 9 | 1:1280 | 1:5120 | 1:40 | |||
| HA-INH 10 | 1:160 | 1:40 | 1:80 | 1:10 240 | ||
| HA-INH 11 | 1:5120 | 1:640 | 1:163840 | 1:20 | ||
| HA-INH 12 | 1:80 | 1:20 | 1:1280 | |||
| HA-INH 14 | 1:80 | 1:40 | ||||
| HA-INH 15 | 1:20 | 1:160 | ||||
| HA-INH 16 | 1:80 | 1:40 | 1:320 | |||
| HA-INH 17 | 1:80 | 1:20 | 1:160 | 1:320 | ||
| HA-INH 18 | 1:80 | 1:320 | ||||
| HA-INH 19 | 1:160 | 1:320 | ||||
| HA-INH 20 | 1:80 | 1:40 | 1:640 | |||
| Acquired hemophilia A: 9 of 9 positives (100%), sample size: n = 9 | ||||||
| Acqu-HA1 | 1:5120 | 1:40 | 1:20 480 | 1:20 | ||
| Acqu-HA 2 | 1:1280 | 1:320 | ||||
| Acqu-HA 3 | 1:2560 | 1:80 | 1:640 | |||
| Acqu-HA 4 | 1:320 | 1:1280 | ||||
| Acqu-HA 5 | 1:10 240 | 1:320 | 1:80 | 1:2560 | ||
| Acqu-HA 6 | 1:40 960 | 1:160 | 1:320 | 1:2560 | ||
| Acqu-HA 7 | 1:5120 | 1:20 | 1:5120 | 1:40 | ||
| Acqu-HA 8 | 1:2560 | 1:5120 | ||||
| Acqu-HA 9 | 1:1280 | 1:80 | 1:20 480 | |||
| Hemophilia A after successful ITI: 1 of 9 positives (11%), sample size: n = 23 | ||||||
| HA-ITI 11 | 1:40 | 1:40 | ||||
FVIII indicates factor VIII; ITI, immune tolerance induction therapy; and neg, titer < 1:20.
Correlation between Bethesda titer and titer of FVIII-binding antibodies in patients with FVIII inhibitors
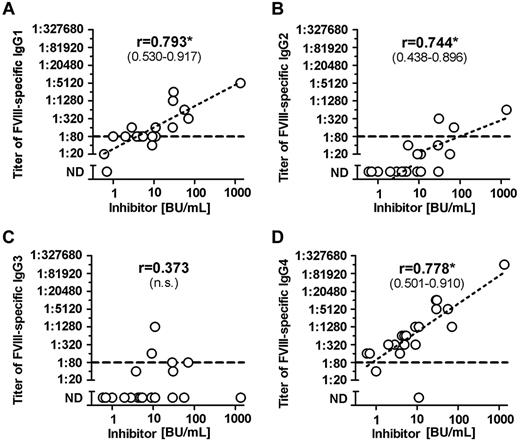
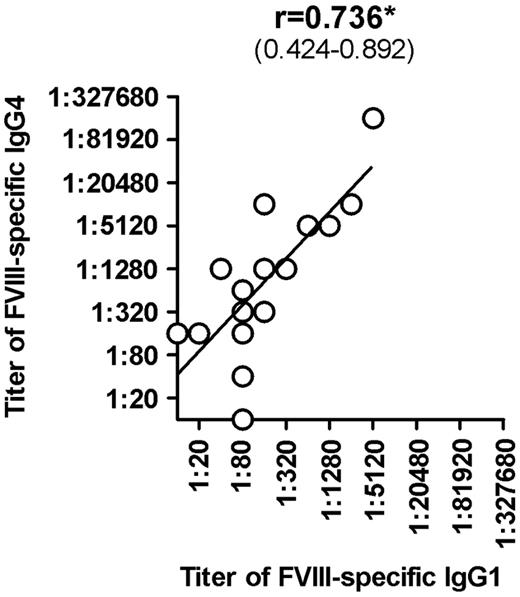
Once we completed the comprehensive analysis of FVIII-binding antibodies in different cohorts of hemophilia A patients and in healthy individuals, we were interested to determine whether titers of FVIII-binding antibodies would correlate with Bethesda titers of neutralizing antibodies. Furthermore, we wanted to know which of the IgG subclasses that contributed to FVIII-binding antibodies in patients with FVIII inhibitors would give the best correlation to Bethesda titers of FVIII inhibitors. Our results indicate that IgG1 and IgG4 antibodies provide the best correlation between titers of FVIII-binding antibodies and Bethesda titer of FVIII inhibitors (Figure 2). Furthermore, we found a significant correlation between titers of IgG1 and IgG4 subclasses of FVIII-binding antibodies in patients with FVIII inhibitors (Figure 3). We also observed a statistically significant correlation with Bethesda titers for IgG2 FVIII-binding antibodies. No statistically significant correlation was observed for IgG3 antibodies (Figure 2). We did not perform a correlation analysis for IgM or IgA antibodies because of the low number of positive samples with IgM and IgA FVIII-binding antibodies found in hemophilia A patients with inhibitors. Overall, these results support the idea that multiple IgG subclasses contribute to FVIII inhibitors.
Correlation between titers of FVIII-binding antibodies and inhibitor titers in hemophilia A patients with inhibitors (HA-INH). The correlation between titers of FVIII-binding antibodies and inhibitor titers in hemophilia A patients with inhibitors was determined for the IgG subclasses (A) IgG1, (B) IgG2, (C) IgG3, (D) IgG4 using the 2-tailed nonparametric correlation according to Spearman, and calculated using GraphPad Prism 5 Software. The correlation coefficient (r) is given for each IgG subclass; 95% confidence intervals are indicated in parentheses when the correlation is significant (*P ≤ .05). The correlation between titers of IgA and IgM FVIII-binding antibodies and inhibitor titers was not done because of the low number of FVIII-binding IgA and IgM antibodies detected in hemophilia A patients with inhibitors. The dotted line at a titer of 1:80 indicates the minimum titer required for proof of specificity. Titers of < 1:80 were too low to be confirmed for specificity. n.s. indicates not significant; and ND, not detectable.
Correlation between titers of FVIII-binding antibodies and inhibitor titers in hemophilia A patients with inhibitors (HA-INH). The correlation between titers of FVIII-binding antibodies and inhibitor titers in hemophilia A patients with inhibitors was determined for the IgG subclasses (A) IgG1, (B) IgG2, (C) IgG3, (D) IgG4 using the 2-tailed nonparametric correlation according to Spearman, and calculated using GraphPad Prism 5 Software. The correlation coefficient (r) is given for each IgG subclass; 95% confidence intervals are indicated in parentheses when the correlation is significant (*P ≤ .05). The correlation between titers of IgA and IgM FVIII-binding antibodies and inhibitor titers was not done because of the low number of FVIII-binding IgA and IgM antibodies detected in hemophilia A patients with inhibitors. The dotted line at a titer of 1:80 indicates the minimum titer required for proof of specificity. Titers of < 1:80 were too low to be confirmed for specificity. n.s. indicates not significant; and ND, not detectable.
Correlation between titers of FVIII-specific IgG4 and titers of FVIII-specific IgG1 in hemophilia A patients with inhibitors (HA-INH). The correlation between titers of FVIII-specific IgG4 and titers of FVIII-specific IgG1 in hemophilia A patients with inhibitors was determined using the 2-tailed nonparametric correlation according to Spearman, and calculated using GraphPad Prism 5 software. The correlation coefficient (r) and the 95% confidence intervals (in parentheses) are indicated. The correlation is significant (*P ≤ .05).
Correlation between titers of FVIII-specific IgG4 and titers of FVIII-specific IgG1 in hemophilia A patients with inhibitors (HA-INH). The correlation between titers of FVIII-specific IgG4 and titers of FVIII-specific IgG1 in hemophilia A patients with inhibitors was determined using the 2-tailed nonparametric correlation according to Spearman, and calculated using GraphPad Prism 5 software. The correlation coefficient (r) and the 95% confidence intervals (in parentheses) are indicated. The correlation is significant (*P ≤ .05).
Correlation analysis of plasma donor covariates (age, sex, and geography) with the prevalence of FVIII-binding antibodies in healthy individuals
FVIII-binding antibodies in healthy individuals represent self-reactive antibodies that might be associated with immune reactions against a self-protein. Previous reports indicated that the prevalence of self-reactive antibodies in healthy individuals increases with age.36 Therefore, we asked whether the prevalence of FVIII-binding antibodies in healthy individuals correlates with the age of the blood donors (Figure 4A-D). In addition, we were interested to study the potential impact of sex and geography on the prevalence of these antibodies.
Ig Isotypes and IgG subclasses of FVIII-binding antibodies in healthy individuals in relationship to age and sex. Plasma samples obtained from healthy individuals were analyzed for FVIII-binding antibodies of the Ig isotypes and IgG subclasses (A) IgG1, (B) IgG2, (C) IgG3, (D) IgG4, (E) IgA, and (F) IgM. Healthy individuals were equally distributed by age between 18 and 66 years for both men (▴; n = 300) and women (▿; n = 300); see also supplemental Figure 2. Plasma samples were diluted at least 1:20. Samples that did not give a positive signal at this minimum dilution were considered as ative (not detectable [ND]). The dotted line at a titer of 1:80 indicates the minimum titer required for proof of specificity. Titers of < 1:80 were too low to be confirmed for specificity.
Ig Isotypes and IgG subclasses of FVIII-binding antibodies in healthy individuals in relationship to age and sex. Plasma samples obtained from healthy individuals were analyzed for FVIII-binding antibodies of the Ig isotypes and IgG subclasses (A) IgG1, (B) IgG2, (C) IgG3, (D) IgG4, (E) IgA, and (F) IgM. Healthy individuals were equally distributed by age between 18 and 66 years for both men (▴; n = 300) and women (▿; n = 300); see also supplemental Figure 2. Plasma samples were diluted at least 1:20. Samples that did not give a positive signal at this minimum dilution were considered as ative (not detectable [ND]). The dotted line at a titer of 1:80 indicates the minimum titer required for proof of specificity. Titers of < 1:80 were too low to be confirmed for specificity.
A zero-inflated Poisson regression model was used to model the most prominent FVIII-binding antibodies in healthy individuals (IgG1, IgG3, and IgA, see Table 5) using subject-specific covariates (age, sex, and geography). Regarding FVIII-binding IgG1 and IgG3 antibodies, the statistical model provided no statistical evidence that covariates age, sex, and geography of the blood donor contributed to their development. On the other hand, for FVIII-binding IgA antibodies, there was a statistically significant contribution of age and geography of the blood donor to the development of titers. The fitted statistical model states that controlling for geography of the blood donor, the odds of having an IgA titer ≥ 1:20 increased by a factor of 1.395 (95% CI, 1.051-1.852) for each 10-year increase in age. In addition, holding age at a fixed value, the odds of having a titer ≥ 1:20 over the odds of having a titer ≥ 1:20 in Austria was 1.00 (95% CI, 0.190-5.29) in Elkhart, 1.35 (95% CI, 0.282-6.47) in Lakeland, 1.80 (95% CI, 0.400-8.11) in Ammon, 3.55 (95% CI, 0.881-14.29) in Fargo, and 5.57 (95% CI, 1.44-21.5) in Laredo.
Statistical modeling of the relation of IgG1, IgG3, and IgA FVIII-binding antibodies in healthy individuals
| FVIII-binding antibody . | Model . | Log-likelihood . | Residual degrees of freedom . | χ2 statistic . | P . |
|---|---|---|---|---|---|
| IgG1* | Null model§ | −172.3 | 598 | 7.9 | .8938 |
| Full specified model¶ | −168.4 | 584 | |||
| IgG3† | Null model§ | −183.4 | 598 | 21.9 | .0803 |
| Full specified model¶ | −172.5 | 584 | |||
| IgA‡ | Null model§ | −177.0 | 598 | 31.0 | .0055 |
| Full specified model¶ | −161.5 | 584 |
| FVIII-binding antibody . | Model . | Log-likelihood . | Residual degrees of freedom . | χ2 statistic . | P . |
|---|---|---|---|---|---|
| IgG1* | Null model§ | −172.3 | 598 | 7.9 | .8938 |
| Full specified model¶ | −168.4 | 584 | |||
| IgG3† | Null model§ | −183.4 | 598 | 21.9 | .0803 |
| Full specified model¶ | −172.5 | 584 | |||
| IgA‡ | Null model§ | −177.0 | 598 | 31.0 | .0055 |
| Full specified model¶ | −161.5 | 584 |
Statistical modeling of the relation of IgG1, IgG3, and IgA FVIII-binding antibodies in healthy individuals with covariates age, sex, and location of the plasma center using a zero-inflated Poisson regression model.
The comparison of the full specified model with the null model using the likelihood ratio test resulted in a P value of .8938 indicating that age, sex, and location of the plasma center taken together as predictor variables (not just individually) did not result in a statistically significant improvement in the model fit.
The comparison of the full specified model with the null model using the likelihood ratio test resulted in a P value of .0803 indicating that age, sex, and location of the plasma center taken together as predictor variables (not just individually) did not result in a statistically significant improvement in the model fit.
The comparison of the full specified model with the null model using the likelihood ratio test resulted in a P value of .0055 indicating that age, sex, and location of the plasma center taken together as predictor variables (not just individually) resulted in a statistically significant improvement in the model fit compared to the trivial model. The full specified model was further refined based on clinical input and the akaike information criterion (AIC) criteria resulting in a final zero-inflated Poisson regression model consisting of the intercept only in the titer ≥ 1:20 component and with covariates age and plasma center in the titer < 1:20 component which resulted also in a statistically significant improvement in the model fit compared to the null model (P = .0051).
Null model: zero-inflated Poisson regression model without covariates (ie, trivial model with intercepts only).
Full specified model: zero-inflated Poisson regression model with covariates age, sex, and location of the plasma center in the titer ≥ 1:20 component as well as in the titer < 1:20 component.
Discussion
The development of antibodies against FVIII in patients with hemophilia A has been shown to consist of both neutralizing (FVIII inhibitors) and nonneutralizing antibodies.18 Furthermore, the presence of nonneutralizing FVIII-binding antibodies has been reported not only in patients but also in healthy individuals.12,20 Yet, the actual prevalence of total FVIII-binding antibodies in healthy individuals and in hemophilia A patients with and without FVIII inhibitors remains unclear.
Our study introduces a comprehensive analysis of the prevalence of FVIII-binding antibodies found in healthy individuals and in different cohorts of patients with hemophilia A. Importantly, we conducted a thorough investigation not only of the neutralizing antibodies but of the total FVIII-binding antibody response, including a breakdown of Ig isotypes and IgG subclasses. To ensure the high quality of our dataset, the assays used in our study were established in accordance with the most recent regulatory guidelines.24,25
Investigations of self-reactive antibodies against FVIII have presented conflicting results. The 2 largest studies found a prevalence ranging from 3% (4 of 150)12 to 17% (85 of 500).19 Our results reveal an overall prevalence of FVIII-binding antibodies in healthy individuals of 19% (116 of 600). We discovered though that these antibodies were mostly of titers < 1:80 which were to low to confirm their specificity for FVIII. Therefore, we cannot exclude that these low-titer antibodies represent multireactive antibodies. Only 2% (14 of 600) of our cohort of healthy individuals had antibodies with titers ≥ 1:80 which we could confirm for specificity to full-length FVIII. Thirteen of 14 of these plasma samples with titers ≥ 1:80 bound to full-length FVIII but not to B-domain–deleted FVIII, providing a hint that these antibodies might be directed against an epitope in the B-domain. An alternative explanation could be that the lack of the B-domain causes structural alterations in the FVIII protein resulting in a loss of conformational epitopes that are recognized by antibodies that bind to full-length FVIII but not to B-domain–deleted FVIII. One of 14 of the samples contained antibodies that bound to both full-length and B-domain–deleted FVIII. We hypothesize that these antibodies are of low affinity because they do not seem to negatively impact hemostasis. Recent reports describe a role of low-affinity self-reactive antibodies in the maintenance of immune homeostasis.16,17 If this would be the case for FVIII-binding antibodies found in healthy individuals, these antibodies might be involved in the maintenance of peripheral immune tolerance against FVIII.
Interestingly, we observed a statistically significant contribution of age and geography to the development of FVIII-binding IgA antibodies in healthy individuals. While IgA was described to be induced by TGFβ-producing CD4+ T cells, T cell–independent mechanisms for the induction of low-affinity IgA antibodies have also been reported.37 Signals delivered by serum monomeric IgA are essential in controlling the immune system by preventing the development of autoimmunity and inflammation.38 Increases of autoantibodies with age have been previously interpreted to be mainly because of either environmental factors such as viral infections and resulting cross-reactivity, or as ongoing changes in immune regulatory mechanisms, such as a decline in regulatory T-cell function.36 We hypothesize that the correlation of IgA to age and geography could thus be because of both environmental and genetic factors and that these antibodies are in fact of low affinity and possibly involved in immune regulation.
In our cohort of hemophilia A patients without inhibitors, the prevalence of FVIII-binding antibodies was 34% (26 of 77). Nevertheless, only 5% (4 of 77) of these patients had FVIII-binding antibodies with titers ≥ 1:80. Inhibitor patients who had undergone successful immune tolerance therapy were found to have a prevalence of FVIII-binding antibodies of 39% (9 of 23) with only 4% (1 of 23) of them having antibody titers ≥ 1:80. As expected, 100% of plasma samples tested from hemophilia A patients with inhibitors (20 of 20) and from acquired hemophilia A patients (9 of 9) contained detectable FVIII-binding antibodies. All of them had titers ≥ 1:80 which were confirmed for specificity to FVIII. For comparison, 3 recent studies using bead-based assays reported a prevalence of 50% (23 of 46), 33% (13 of 39), and 18% (28 of 210) for FVIII-binding antibodies in hemophilia A patients without inhibitors.12,13,39 Another study which used an ELISA-based approach found a prevalence of 12% (6 of 49).40 In contrast to our study which only included patients with severe hemophilia A (FVIII activity < 1%), the studies presented by Krudysz-Amblo et al, Lebreton et al, and Zakarija et al included patients with all severities of hemophilia A.12,13,39 We believe that discrepancies in results for the prevalence of FVIII-binding antibodies between the various studies might be because of differences in the patient populations investigated and different assay formats. The study by Lebreton et al presented findings indicating that FVIII-binding antibodies found in patients without inhibitors are directed against light chain, heavy chain, or B-domain, with a clear dominance of antibodies directed against the heavy chain.13 On the other hand, results presented by Vincent et al found antibody specificities directed against only the B-domain.40 Our own data presented in this study seem to confirm the results reported by Vincent et al, although we did identify 1 of 600 healthy individuals with FVIII-binding antibody titers ≥ 1:80 that recognized both full-length FVIII and BDD FVIII.
The antibody response against FVIII in patients with hemophilia A and FVIII inhibitors was demonstrated to be a polyclonal IgG response that is not restricted isotypically. IgG1 and IgG4 were reported to be the major components of anti-FVIII antibodies.3,11,18 Similar to previous findings, IgG1 and IgG4 were the dominant subclasses of antibodies against FVIII found in our cohorts of patients with FVIII inhibitors. Both subclasses correlated well in titer with the titers of neutralizing antibodies. Samples tested from acquired hemophilia A patients possessed a similar antibody pattern as patients with congenital hemophilia A and FVIII inhibitors. Strikingly, IgG4 was only found in patients with FVIII inhibitors but not in healthy individuals, in patients without FVIII inhibitors, or in patients after successful ITI. For comparison, the most recent study investigating IgG subclass distribution in hemophilia A patients undergoing ITI by van Helden et al found detectable FVIII-binding IgG4 antibodies in 16 of 20 patients.11 The 4 patients negative for IgG4 had low Bethesda titers (< 2.0 BU/mL). The sensitivity of the assays used by van Helden et al was in the range of 20 ng/mL. For comparison, the sensitivity of our assay for FVIII-binding IgG4 antibodies was 0.8 ng/mL which is approximately 25-fold more sensitive than the assay used by van Helden et al. Thus, minor differences in the prevalence of FVIII-binding IgG4 antibodies between our study and the study by van Helden et al might be because of differences in the sensitivity of the assays used. Our findings highlight the significance of IgG4 in the neutralizing immune responses against FVIII, and raise the question of which immunologic pathways are responsible for the differentiation of FVIII-specific B cells into IgG4-producing plasma cells. Furthermore, it provokes an investigation into understanding how these pathways are triggered selectively in patients with neutralizing antibodies against FVIII. The class switch to IgG4 has been described to depend on a type 2 helper CD4+ T-cell response.41 Moreover, growing evidence links IgG4 to IL-10–producing regulatory CD4+ T cells, as IL-10 was shown to be required to drive the differentiation of IgG4-switched B cells to IgG4-secreting plasma cells.42 IgG4 has long been considered as a nonpathologic antibody subclass with anti-inflammatory properties.41 It is functionally different from other IgG subclasses because of its poor ability to activate complement and Fc-receptor–expressing effector cells.43 van der Neut Kolfschoten et al reported that IgG4 antibodies can exchange their Fab arms by swapping a heavy chain and attached light chain (half-molecule) with a heavy-light chain pair from another molecule, which would result in functional monovalency of IgG4 molecules and prevent cross-linking of identical antigens.44 Recently, a new class of IgG4-related systemic fibroinflammatory diseases was described. One of the immunologic characteristics of these diseases is the activation of regulatory T (Treg) cells,45,46 which is indicated by a higher expression level of the forkhead box P3 (FOXP3) mRNA in tissues as well as infiltrates of CD4+CD25+ Treg cells at affected sites and increased numbers of CD4+CD25high Treg cells in the blood.45,46 These findings further support the association of IgG4 with the activation of Treg cells. Still, it remains to be seen whether the dominant induction of IgG4 FVIII-binding antibodies found in patients with hemophilia A and FVIII inhibitors is indeed linked to the activation of Treg cells.
In summary, our data confirm that FVIII-binding antibodies are found not only in hemophilia A patients with FVIII inhibitors but also in patients without FVIII inhibitors and in healthy individuals. The distribution of Ig isotypes and IgG subclasses of FVIII-binding antibodies differs between patients with FVIII inhibitors on one hand and patients without inhibitors and healthy subjects on the other hand. Total absence of detectable FVIII-binding IgG4 in all study populations except patients with FVIII inhibitors is the most striking finding of this study. These differences could be indicative of different immune regulatory pathways that drive antibody responses against FVIII in the different study cohorts. For a better understanding on how the immune system decides whether or not do develop FVIII inhibitors, future studies should focus on the longitudinal analysis of FVIII-binding antibodies in early phases of FVIII replacement therapy, which are likely to be most important for the regulation of FVIII-specific immune responses in patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Eva Altinger, Margit Danzler, Neriman Duman, and Maria Hirschler for technical assistance. The authors also thank Alfred Weber for critical discussion.
This work was supported by Baxter BioScience.
Authorship
Contribution: S.F.J.W. and C.J.H. designed research, established validated assay formats, performed experiments, analyzed and interpreted data, and wrote the manuscript; F.M.H. and P.A. established validated assay formats, and analyzed and interpreted data; M.J.W. performed statistical modeling of antibody titers; J.O., C.M., J.W., and A.T. provided patient samples and interpreted data; H.P.S. and F.S. interpreted data; and B.M.R. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: S.F.J.W., C.J.H., F.M.H., P.A., M.J.W., H.P.S., F.S., and B.M.R. are employees of Baxter Bio-Science. The remaining authors declare no competing financial interests.
Correspondence: Birgit M. Reipert, Baxter Innovation GmbH, Industriestrasse 72, A-1220 Vienna, Austria; e-mail: birgit_reipert@baxter.com.
References
Author notes
S.F.J.W. and C.J.H. contributed equally to the studies described.

![Figure 1. Titers of FVIII-binding antibodies assessed for individual Ig isotypes and IgG subclasses. The detected titers of Ig isotypes and IgG subclasses of FVIII-binding antibodies for (A) healthy individuals, (B) hemophilia A patients without inhibitors (HA-noINH), (C) hemophilia A patients with inhibitors (HA-INH), (D) acquired hemophilia A (Acqu-HA) patients, and (E) hemophilia A patients after successful ITI (HA-ITI) are shown. Plasma samples were diluted at least 1:20. Samples that did not give a positive signal at this minimum dilution were considered as negative (not detectable [ND]). The dotted line at a titer of 1:80 indicates the minimum titer required for proof of specificity. Titers of < 1:80 were too low to be confirmed for specificity.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/6/10.1182_blood-2012-07-444877/4/m_zh89991301900001.jpeg?Expires=1763481393&Signature=ebcvOvZmR~xuiVjT3I0UurAgZlrga7cHrjI~L9lvkmXDOUAli3UGfcq~uSjSCmDHwohtjN8PKpkZ2gVB4xZhKniWSPPjJL5kMqjp4l7OoJy8OZCf7uBY32FKxdEP09Go--RrwGBK0pPZmdijBqUvLQLN08eq7WFsU8-sntMqp3G5MjXNLgL8~O5JGIYC5y7wdnDE1cBm2PJYNouJKjm-8SxA~e8TwNOCbpAaiu5frhx7PkuKc-mvuAevyV~08ThCILOd-TYMIO~PXMXIzCS6twGJXE410PPt6DNXpDaV9FWn0ijDgOch8qUFpSn92AtCs-VPsqv2vuC2Oxhp15-xLg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Ig Isotypes and IgG subclasses of FVIII-binding antibodies in healthy individuals in relationship to age and sex. Plasma samples obtained from healthy individuals were analyzed for FVIII-binding antibodies of the Ig isotypes and IgG subclasses (A) IgG1, (B) IgG2, (C) IgG3, (D) IgG4, (E) IgA, and (F) IgM. Healthy individuals were equally distributed by age between 18 and 66 years for both men (▴; n = 300) and women (▿; n = 300); see also supplemental Figure 2. Plasma samples were diluted at least 1:20. Samples that did not give a positive signal at this minimum dilution were considered as ative (not detectable [ND]). The dotted line at a titer of 1:80 indicates the minimum titer required for proof of specificity. Titers of < 1:80 were too low to be confirmed for specificity.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/6/10.1182_blood-2012-07-444877/4/m_zh89991301900004.jpeg?Expires=1763481393&Signature=h3XXngj2B3ujEEJB9MzpN5E7zC7zztCM~5vDzu3qqUlOQ~22XBcyXS9P81UKxS6QfGZHMuzCJkYJeWNhnJi2k1asT7NE-9QCLnvUwJAEEn~pWWVlzzE6ViATwnWZTLnRysAjyh5KwS93oi-NXGGb~Sq4~7Y5ECH1YE71pgz78NuUeN3zfBv9j~XnzImjYnDj3RqRXjn1WBdr9FDgwb7bPwStExDoFqqQsX1OaU6iOmXEAUng1TC-3CAbC89t~MNTjyVs79G023QC5QZUisofzgomYOO2EtK0rDpmr3E71UVKS3RZo6meUldV6uqVsscF08jN8g3wF7c1h6ZbtcZx~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)