Key Points
Restricting transgenic antigen expression to differentiated antigen-presenting cells protects hematopoietic progenitors from immune attack.
Restricting transgenic antigen expression to differentiated antigen-presenting cells promotes tolerogenic outcomes.
Abstract
Bone marrow (BM) or hematopoietic stem cell (HSC) transplantation is used as curative therapy for hematologic malignancies. Incorporation of gene therapy to drive tolerogenic expression of antigens is a promising strategy to overcome the limited long-term efficacy of autologous HSC transplantation for autoimmune diseases. HSC engraftment and tolerance induction is readily achieved after myeloablative or immune-depleting conditioning regardless of the cellular compartment in which antigen is expressed. It is unclear whether the efficiency of engraftment and tolerance induction is influenced by targeting antigen to specific cellular compartments. This is particularly important when using clinically feasible low-intensity conditioning aimed at preserving infectious immunity in individuals where immunologic memory exists to the autoantigen to be expressed. Here we demonstrate that, under immune-preserving conditions, confining expression of a transgenically expressed antigen to dendritic cells permits stable, long-term engraftment of genetically modified BM even when recipients are immune to the expressed antigen. In contrast, broader expression within the hematopoietic compartment leads to graft rejection and therapeutic failure because of antigen expression in HSCs. These findings are relevant to the clinical application of genetically engineered HSCs and provide evidence that careful selection of promoters for HSC-mediated gene therapy is important, particularly where tolerance is sought under immune-preserving conditions.
Introduction
Bone marrow transplantation (BMT) and, more recently, hematopoietic stem cell transplantation (HSCT) has become a routine treatment for hematologic malignancies but is also exploited for primary or acquired hematologic or immune deficiencies. For the latter, inclusion of gene therapy can be used to replace defective genes, such as in X-linked severe combined immunodeficiency (X-SCID), and adenosine deaminase (ADA) deficiency.1
BMT/HSCT has also been adapted for autoimmune disease. Early studies of allogeneic BMT achieved complete resistance to disease with full or mixed allogeneic chimerism indicating some autoimmune diseases could be cured from within the hematopoietic compartment. However, allogeneic or mismatched BMT/HSCT remains a significant procedure with substantial transplant-related mortality and is suitable only for life-threatening diseases. Alternatively, myeloablative and nonmyeloablative conditioning are highly immune-depleting, and used in conjunction with autologous HSCT (aHSCT) for recovery of hematopoiesis, can provide an “immunologic reset.“ This avoids the risks of allogeneic BMT/HSCT and subsequent GVHD but alleviates autoimmune disease experimentally2-4 and clinically (reviewed by Coleman and Steptoe5 ). In multiple sclerosis, approximately 70% of patients treated with aHSCT remain progression-free 3 years after treatment.6 In rheumatoid arthritis and systemic lupus erythematosus, aHSCT reduces disease scores7,8 and in type 1 diabetes pancreatic β-cell function is improved.9 Indeed, aHSCT may be more effective than conventional immunotherapies currently or recently trialed for type 1 diabetes.10 Despite encouraging clinical results for aHSCT, relapse rates can be high, probably because of re-emergence of pathogenic immune cell populations or their incomplete removal by the conditioning used.
One means to minimize disease re-emergence might be to increase pretransplant conditioning but current nonmyeloablative conditioning is already toxic and immune-suppressive, especially in patients with autoimmune multi-organ pathology. Incorporation of active tolerance-inducing strategies holds promise and is a potential means to overcome limitations associated with current aHSCT approaches (reviewed by Coleman and Steptoe5 ). Enforced expression of antigen is a robust approach to inducing immune-tolerance and prevents priming of T-cell and B-cell responses to expressed proteins.11,12 We and others have shown that combining gene therapy with BMT/HSCT to drive antigen expression induces antigen-specific tolerance and is a powerful means to prevent development of autoimmune disease.13,14
To progress the feasibility of aHSCT for established (or progressing) autoimmune diseases, 2 goals must be met (1) to reverse established pathogenic immunity and prevent disease relapse, and (2) to minimize toxicity and nonspecific immune-depletion. Incorporation of enforced antigen expression into aHSCT through gene therapy could limit relapses by preventing re-emergence of pathogenic T-cell specificities. Importantly, recent demonstrations that memory and effector T-cell responses can be turned-off by enforced antigen expression,15-17 could address the key therapeutic challenge for autoimmune and other diseases driven by pathogenic T-cell responses which is turning-off pre-existing immunity that underlies and perpetuates disease while limiting the need for toxic conditioning. Achieving the second goal, however, means that tolerogenic HSCT (tHSCT) must be performed under conditions where substantial immune-resistance to establishment of donor hematopoiesis is probable, regardless of whether the procedure is potentially tolerogenic or not. A similar challenge faces HSCT-mediated gene therapy in individuals sensitized to the replacement protein.
Genetically engineered antigen-specific tolerance can occur when antigen is ubiquitously expressed,18 targeted to APCs11,19 or even when expressed in nonhematopoietic tissues and cross-presented by APCs.20 Successful engraftment and tolerance induction can be achieved after myeloablative conditioning or conditioning regimes that lead to immune depletion, regardless of which cell type antigen is expressed in.13,14,21 However, it remains undefined whether engraftment or efficiency of tolerance induction is influenced by the antigen targeting approach used. In particular, as the goal is to avoid generalized immune depletion or immune suppression, this information is critically required for gene-engineered BMT/HSCT performed using immune-preserving conditions.
Here we define the influence of antigen targeting on engraftment and generation of a tolerogenic environment after transfer of gene-engineered BM and hematopoietic progenitor cells (HPCs) under immune-preserving conditions. We compared engraftment using myeloablative or immune-preserving conditions when a transgene was expressed ubiquitously, by diverse MHC class II-expressing APC or specifically by CD11chi conventional dendritic cells (DCs). We show that for effective engraftment and generation of a long-lasting tolerogenic environment, antigen must be targeted with high fidelity to fully differentiated APCs. This provides important insight into the requirements for designing effective strategies for tolerogenesis and gene therapy using genetically modified HSCs in, for example autoimmune disease or primed individuals.
Methods
Mice
C57BL/6J (CD45.2+), B6.SJLptprca (CD45.1+) and BALB/c mice were purchased from the Animal Resources Facility (Perth, Australia). B6.SJL mice were crossed with OT-I mice22 to generate CD45.1+/CD45.2+ OT-I mice, CD11c.OVA (11c.OVA) mice express OVA in CD11chi conventional DC,11 MII.OVA mice provided by Professor Francis Carbone (University of Melbourne, Australia) express OVA in MHC class II positive cells (primarily DCs and B cells)16 and K5.mOVA mice express OVA in skin keratinocytes23 and were all bred at the Biological Resources Facility (Brisbane, Australia). 11c.OVA mice were backcrossed to B6.SJLptprca mice to generate CD45.1+ 11c.OVA mice. Actin.OVA mice expressing OVA in all cell types24 were a gift from Professor Bill Heath (University of Melbourne, Australia). MII.GFP mice expressing an I-Ab-GFP fusion protein25 were a kind gift of Professor Barbara Fazekas de St Groth (Centenary Institute, Sydney, Australia) and were bred at the QIMR (Brisbane, Australia) animal facility. All animal procedures were approved by the University of Queensland Animal Ethics Committee.
Bone marrow transplantation and adoptive transfers
Mice were euthanized, femurs and tibias collected into mouse-tonicity phosphate buffered saline (MT-PBS), and BM collected by flushing with MT-PBS/2.5% FCS. Erythrocytes were lysed (NH4Cl/Tris buffer), cells washed (MT-PBS/2.5% FCS), and prepared for injection (in MT-PBS) or for treatment. Hematopoietic progenitor cells (HPCs) were prepared by high speed FACS sorting of lin−c-kit+ cells from bulk BM. Mice were irradiated (200-400 cGy in a single dose or 2 × 550 cGy 3 hours apart, 137Cs source) as stated in figure legends 3 to 4 hours before intravenous injection of BM (1 × 107) or HPCs (2 × 105). Mice subject to high dose (1100 cGy total) received neomycin supplemented water for 4 weeks after BMT. To transfer naïve OT-I T cells, single-cell suspensions of pooled mesenteric, inguinal, axillary, and brachial lymph nodes (LNs) were prepared and transferred (5 × 106) as described,11 and memory/effector OT-I cells were prepared for transfer (2 × 106) as described.15 Where indicated OT-I T cells were CFSE labeled11 before transfer.
In vivo treatments and assays
Mice were immunized with OVA/QuilA or keyhole limpet hemocyanin (KLH; 50 ug)/OVA (50ug)/QuilA (20 ug) using the procedure described.15 Sham challenge was QuilA alone in PBS. T cells or NK1.1+ cells were depleted by administration of anti-CD4 and anti-CD8 mAb (GK1.5 and 53-5.8) or anti-NK1.1 (PK136) 200 ug 3 days before experiment commencement and again at day 0 and 7. ELISpot assays (IFN-γ) were performed as previously described11 and data were expressed as OVA257-264 or KLH-stimulated minus background. Skin grafting used K5.mOVA and BALB/c donors and double-grafting was performed as described.26
Flow cytometry
Antibodies were from Biolegend, BD Pharmingen, or grown, purified, and conjugated in-house. Flow cytometric analyses were performed as previously described.11 In experiments where cells where enumerated this was typically performed using a flow-based bead counting assay as described.15 Intracellular cytokine staining (ICS) was performed as previously described.15 Tracking peripheral blood leukocyte populations was performed by flow cytometric analysis of erythrocyte-lysed whole blood as previously described.27 Long-term HSC and committed progenitor cell analyses were as described.28,29 Data were collected using a FACScanto or FACS LSR Fortessa (BD Bioscience) and analyzed using DIVA Version 5.0.1 (BD Bioscience) or FlowJo Version 6.3 (TreeStar) software. Engraftment is expressed as the proportion of donor cells within the lineage analyzed or within the total CD45+ cell population.
Transcriptional analyses
Total RNA was extracted from sorted cell preparations (as noted in figure legends) using RNeasy Mini Kit (QIAGEN). cDNA was generated with an Omniscript cDNA synthesis kit (QIAGEN). TaqMan assays were used to quantitate expression of I-Abβ (Mm00439216_m1) and OVA (Gg03366808_m1) relative to HPRT (Mm03024075_m1) using the 2-ΔΔCT method. Assays were performed on a Rotor-Gene 6000 (Corbett Life Science) in triplicate, repeated twice for RNA samples from 3 independent sorted cell populations.
Statistical analysis
Statistical analyses were performed using the Student t test for pairwise comparison of means, or 1-way ANOVA with the Newman-Keuls posttest for multiple comparisons (GraphPad Version 5.03 software). Skin graft survival was plotted using Kaplan-Meier analysis and group survival compared using log rank test (GraphPad Version 5.03 software).
Results
Bone marrow encoding APC-targeted or ubiquitously expressed antigen induces T-cell tolerance after myeloablative transplantation
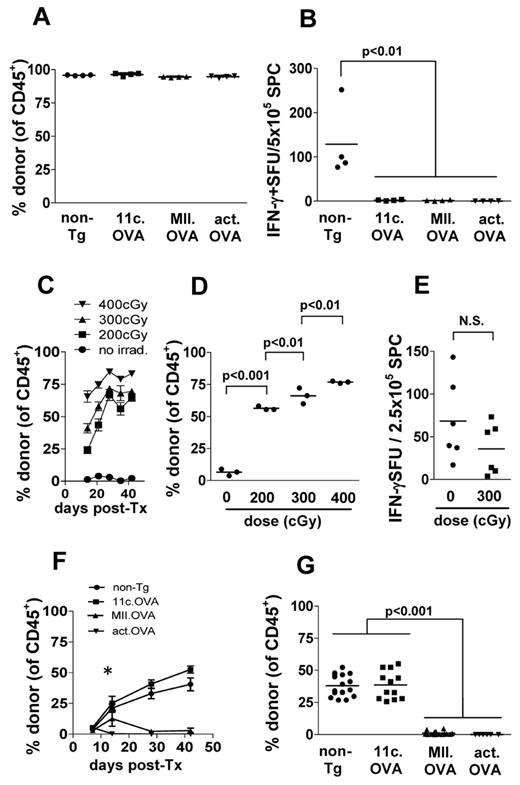
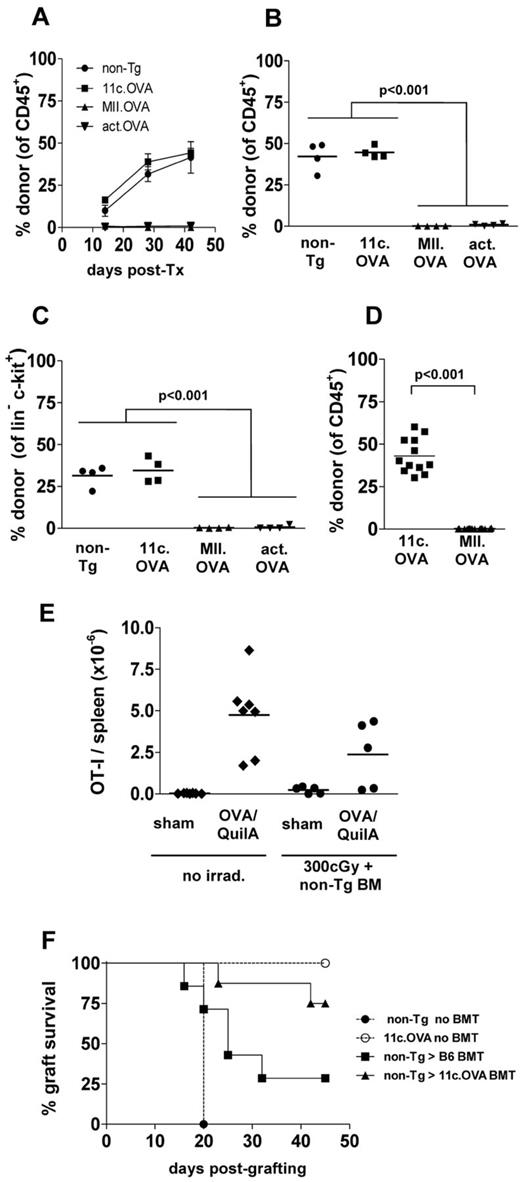
Transfer of BM encoding cognate antigen under myeloablative conditions induces antigen-specific T-cell tolerance and prevents development of autoimmune disease. To determine whether targeting antigen to APC influences tolerance, we compared BM transfer from mice expressing OVA ubiquitously (actin.OVA)24 or genetically targeted to APC by either the CD11c promoter which targets expression to conventional CD11chi DC (11c.OVA)11 or an MHC class II promoter (MII.OVA) which targets expression to a more diverse range of APC and MHC class II+ cells.16 Recipient mice were exposed to high-dose (1100 cGy) irradiation, BM transferred, and then tolerance to OVA tested 6 weeks later. Regardless of whether OVA was targeted to APC (11c.OVA, MII.OVA) or ubiquitously expressed (actin.OVA), donor-type leukocytes predominated in all recipients (Figure 1A). To test OVA-specific T-cell tolerance, BM recipients were immunized with OVA/QuilA. One week after immunization, ELISpot analysis of CD8+ T-cell responses showed that OVA-specific responsiveness was ablated in recipients of OVA-encoding BM regardless of whether antigen was targeted to APC or not (Figure 1B). From this, we conclude that ubiquitous and APC-targeted antigen expression were both permissive for induction of T-cell tolerance through BMT.
Targeting antigens with the CD11c promoter permits engraftment of antigen-encoding BM under immune-preserving conditions. (A-B) B6.SJL (CD45.1 congenic) mice were irradiated (1100 cGy) and BM (107) from C57Bl/6 (non-Tg), 11c.OVA, MII.OVA, or actin.OVA (act.OVA) mice injected intravenously. Six weeks later engraftment was assessed in peripheral blood leukocytes (PBL) by flow cytometry (A) and mice immunized with OVA/QuilA. A further week later spleens were harvested and IFN-γ production in response to OVA257-264 determined by ELISpot (B). (C-D) B6.SJL recipients were irradiated (200, 300, 400 cGy) and C57Bl/6 (non-Tg) BM (2 × 107) transferred intravenously. At the designated time-points, PBL (C) or 6 weeks after transfer spleens (D) were analyzed by flow cytometry. (E) C57BL/6 (non-Tg) mice were irradiated, rested for 2 weeks, immunized with KLH/QuilA, and then 7 days later splenocytes prepared and IFN-γ ELISpots performed. (F-G) B6.SJL recipients were irradiated (300 cGy) and C57Bl/6 (non-Tg), 11c.OVA, MII.OVA, or actin.OVA (act.OVA) BM (107) transferred intravenously. At the designated timepoints PBL (F) or 6 weeks after transfer, spleens (G) were analyzed by flow cytometry. Data are shown for individual mice pooled from 2 experiments (A-B), mean ± SD (n = 3; C), individual mice (D) from a representative experiment, mean ± SD (n = > 6 mice per group except d42, n = > 4/gp; F), or individual mice pooled from 2 (E), or 4 or more experiments (F-G). *MII.OVA @ d14 is greater than MII.OVA at d7, 28, 42, and less than 11c.OVA and non-Tg @ d14 (P < .001).
Targeting antigens with the CD11c promoter permits engraftment of antigen-encoding BM under immune-preserving conditions. (A-B) B6.SJL (CD45.1 congenic) mice were irradiated (1100 cGy) and BM (107) from C57Bl/6 (non-Tg), 11c.OVA, MII.OVA, or actin.OVA (act.OVA) mice injected intravenously. Six weeks later engraftment was assessed in peripheral blood leukocytes (PBL) by flow cytometry (A) and mice immunized with OVA/QuilA. A further week later spleens were harvested and IFN-γ production in response to OVA257-264 determined by ELISpot (B). (C-D) B6.SJL recipients were irradiated (200, 300, 400 cGy) and C57Bl/6 (non-Tg) BM (2 × 107) transferred intravenously. At the designated time-points, PBL (C) or 6 weeks after transfer spleens (D) were analyzed by flow cytometry. (E) C57BL/6 (non-Tg) mice were irradiated, rested for 2 weeks, immunized with KLH/QuilA, and then 7 days later splenocytes prepared and IFN-γ ELISpots performed. (F-G) B6.SJL recipients were irradiated (300 cGy) and C57Bl/6 (non-Tg), 11c.OVA, MII.OVA, or actin.OVA (act.OVA) BM (107) transferred intravenously. At the designated timepoints PBL (F) or 6 weeks after transfer, spleens (G) were analyzed by flow cytometry. Data are shown for individual mice pooled from 2 experiments (A-B), mean ± SD (n = 3; C), individual mice (D) from a representative experiment, mean ± SD (n = > 6 mice per group except d42, n = > 4/gp; F), or individual mice pooled from 2 (E), or 4 or more experiments (F-G). *MII.OVA @ d14 is greater than MII.OVA at d7, 28, 42, and less than 11c.OVA and non-Tg @ d14 (P < .001).
Low-dose irradiation facilitates effective engraftment of transferred BM
To develop an immune-preserving conditioning strategy for BMT we trialed nonmyeloablative low-dose irradiation. B6.SJL (CD45.1+) recipients were exposed to low-dose irradiation (200, 300, 400 cGy) and C57BL/6 (CD45.2+) BM transferred. Donor BM engrafted and gave rise to mature leukocytes in a time and dose-dependent manner with high proportions of donor-derived leukocytes present in blood from approximately 3 weeks after transfer (Figure 1C). At the end of the test period, the proportion of donor-derived leukocytes in secondary lymphoid tissue (Figure 1D) reflected that in blood indicating either site was suitable for analysis of donor-type cell development. To determine the extent to which the low-dose irradiation used might impact on the ability to prime an effector T-cell response, non-Tg mice were exposed to low-dose (300 cGy) irradiation, rested for 14 days, immunized with KLH/OVA/QuilA, and then the in vitro recall tested by ELISpot a further 7 days later. Low-dose irradiation moderately impacted on the scale of the in vitro KLH recall (Figure 1E) indicating substantial preservation of T-cell responses under the conditions used. OVA recall responses determined by OVA257-264 stimulation in ELISpot were reduced by a similar extent (185 ± 29 versus 85 ± 21 SFU/2.5 × 105 SPC, respectively, not shown).
Targeting antigen to dendritic cells permits high levels of antigen-encoding bone marrow engraftment after immune-preserving low-dose irradiation
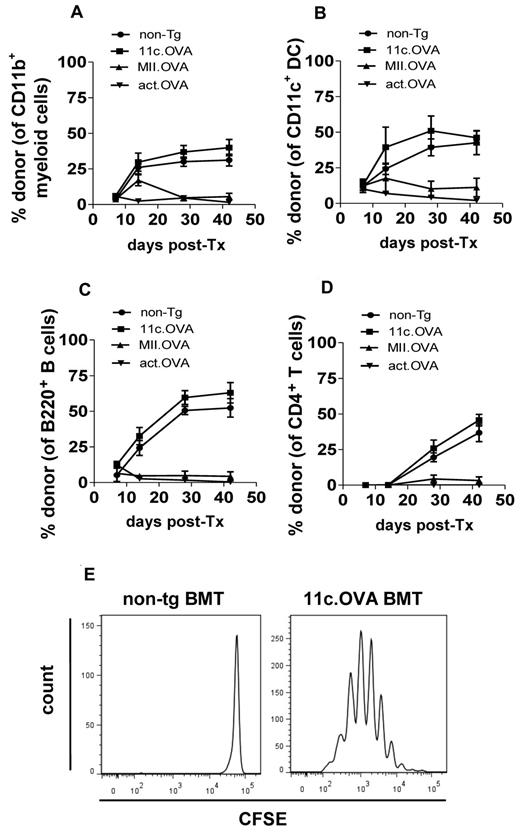
A challenge for therapeutic tolerance induction using antigen-encoding HSC with immune-preserving conditioning is the presence of pre-existing populations of naive or antigen-experienced T cells specific for the encoded antigen. This is also pertinent in gene-replacement strategies where inhibitory T-cell or antibody responses have developed. Under such conditions, ubiquitous expression of antigen may be disadvantageous and could lead to immune resistance to BM/HSC engraftment or clearance of the progeny of engrafted cells. To assess this, we compared whether engraftment differed when transgenic protein was expressed ubiquitously or targeted to differentiated APC populations. BM from non-Tg, actin.OVA, 11c.OVA, or MII.OVA donors was transferred to low-dose (300 cGy) irradiated B6.SJL mice. By 6 weeks after transfer, donor-derived cells comprised approximately 50% of leukocytes in blood and spleen of recipients of non-Tg and 11c.OVA BM (Figure 1F-G). Consistent with the proposal that widespread expression of antigen could lead to immune-mediated graft failure, in actin.OVA BM recipients almost no donor-type leukocytes were detected (Figure 1F-G). Intriguingly, in MII.OVA BM recipients, there was a small and transient accumulation of donor-type leukocytes around 2 weeks after transfer, but this rapidly waned and by 6 weeks after BM transfer, few donor-type cells were apparent either in blood or spleen (Figure 1F-G). When individual lineages were examined, donor type myeloid cells, DCs, and B cells developed rapidly in recipients of non-Tg and 11c.OVA BM, but as expected development of T cells was slower, because of the requirement for migration of progenitors to the thymus. On closer examination of lineage-specific development, it was apparent that in MII.OVA BM recipients there was transient development of donor-type DCs and myeloid cells, and to a lesser extent B cells, but these cells then waned in number. In recipients of actin.OVA BM, transferred cells appeared to be cleared rapidly without transient accumulation in blood (Figure 2A-D). This suggested that actin.OVA cells were rapidly killed on transfer, possibly through induction of anti-OVA immunity because of widespread OVA expression whereas development of anti-OVA immunity in MII.OVA BM recipients possibly required initial development of OVA-expressing donor-type DC and myeloid cells. When CFSE-labeled OT-I T cells were transferred 4 weeks after BMT extensive proliferation was apparent in 11c.OVA BM recipients indicating that the DCs that developed in these mice presented OVA-derived peptides and did not represent a subset of DCs devoid of OVA expression (Figure 2E).
OVA-presenting dendritic cells develop after transfer of 11c.OVA BM. (A-E) B6.SJL (CD45.1 congenic) mice were irradiated (300 cGy) and BM (107) from C57Bl/6 (non-Tg), 11c.OVA, MII.OVA, or actin.OVA (act.OVA) mice injected intravenously. At the designated timepoints PBL were analyzed by flow cytometry (A-D). Four weeks after BM transfer CFSE-labeled CD45.1+ OT-I cells were transferred intravenously to non-Tg and 11c.OVA BM recipients. Three days later LNs were recovered and CFSE dilution in OT-I (CD45.1+CD8+) T cells were assessed by flow cytometry. Data are mean ± SD (n = 4-10) pooled from 2 or more experiments (A-D) or representative of 2 mice analyzed in 2 separate experiments (E).
OVA-presenting dendritic cells develop after transfer of 11c.OVA BM. (A-E) B6.SJL (CD45.1 congenic) mice were irradiated (300 cGy) and BM (107) from C57Bl/6 (non-Tg), 11c.OVA, MII.OVA, or actin.OVA (act.OVA) mice injected intravenously. At the designated timepoints PBL were analyzed by flow cytometry (A-D). Four weeks after BM transfer CFSE-labeled CD45.1+ OT-I cells were transferred intravenously to non-Tg and 11c.OVA BM recipients. Three days later LNs were recovered and CFSE dilution in OT-I (CD45.1+CD8+) T cells were assessed by flow cytometry. Data are mean ± SD (n = 4-10) pooled from 2 or more experiments (A-D) or representative of 2 mice analyzed in 2 separate experiments (E).
Engraftment of BM encoding OVA under an MHCII promoter is limited by host T cells
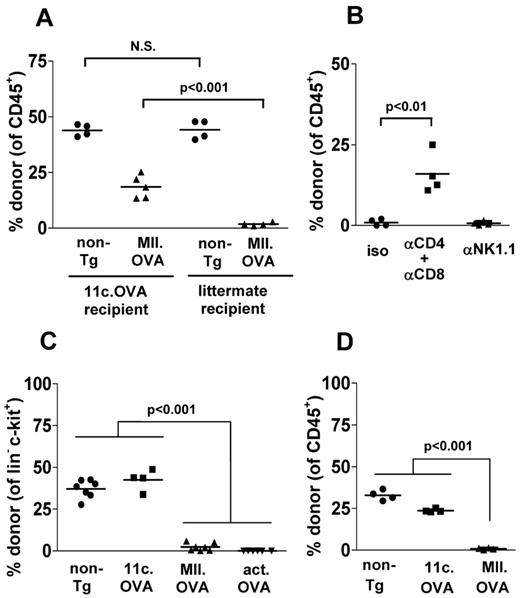
Induction of OVA-specific immunity and T cell–mediated rejection of OVA-expressing hematopoietic stem or progenitor cells is a ready explanation for engraftment failure of actin.OVA BM where OVA is expressed ubiquitously. However, failure of MII.OVA BM to engraft was unexpected as it was proposed restricting OVA expression to mature MHC class II+ APCs would protect MII.OVA hematopoietic stem and progenitor cells (HSPCs) from immune rejection. To determine whether engraftment of MII.OVA BM was limited by T cell–mediated immunity, 11c.OVA mice devoid of OVA-specific T-cell reactivity11 or OVA-ve littermate controls that are not OVA-tolerant were used as recipients. Non-Tg (CD45.2+) BM engrafted equally in low-dose irradiated (300 cGy) CD45.1+ 11c.OVA mice and OVA-ve littermate controls (Figure 3A). MII.OVA BM engraftment was substantially restored in 11c.OVA mice compared with OVA-ve littermates (Figure 3A). In non-Tg recipient mice, depletion of CD4+ and CD8+ T cells restored MII.OVA BM engraftment whereas depletion of NK cells did not (Figure 3B). Together the data indicate that induction of OVA-specific T-cell responses limits development of donor-type leukocytes in MII.OVA and actin.OVA BM recipients, possibly through rejection of HSPCs.
OVA-specific T-cell responses underlie failure of MII.OVA engraftment. (A) CD45.1+ 11c.OVA or non-transgenic littermate controls were irradiated (300 cGy) and BM (107) from non-Tg (C57Bl/6) or MII.OVA mice injected intravenously. Six weeks later donor leukocytes were enumerated in peripheral blood. (B) B6.SJL (CD45.1+) mice were administered anti-CD4 and anti-CD8, anti-NK1.1 or isotype mAbs, irradiated (300 cGy) and BM (107) from MII.OVA mice injected intravenously. An additional dose of anti-CD4 and anti-CD8 mAb was administered 3 weeks after BM transfer. Six weeks after BMT donor leukocytes were enumerated in peripheral blood. (C) B6.SJL (CD45.1+) mice were irradiated (300 cGy) and BM (107) from non-Tg (C57Bl/6), 11c.OVA, MII.OVA, and actin.OVA mice injected intravenously. Six weeks later donor HPC (lin−c-kit+) were enumerated in BM. (D) B6.SJL (CD45.1+) mice were irradiated (300 cGy) and sorted lin−c-kit+ HPCs (2 × 105) from non-Tg (C57Bl/6), 11c.OVA or MII.OVA mice injected intravenously. Six weeks later donor leukocytes were enumerated in peripheral blood. Data represent individual mice pooled from a minimum of 2 experiments.
OVA-specific T-cell responses underlie failure of MII.OVA engraftment. (A) CD45.1+ 11c.OVA or non-transgenic littermate controls were irradiated (300 cGy) and BM (107) from non-Tg (C57Bl/6) or MII.OVA mice injected intravenously. Six weeks later donor leukocytes were enumerated in peripheral blood. (B) B6.SJL (CD45.1+) mice were administered anti-CD4 and anti-CD8, anti-NK1.1 or isotype mAbs, irradiated (300 cGy) and BM (107) from MII.OVA mice injected intravenously. An additional dose of anti-CD4 and anti-CD8 mAb was administered 3 weeks after BM transfer. Six weeks after BMT donor leukocytes were enumerated in peripheral blood. (C) B6.SJL (CD45.1+) mice were irradiated (300 cGy) and BM (107) from non-Tg (C57Bl/6), 11c.OVA, MII.OVA, and actin.OVA mice injected intravenously. Six weeks later donor HPC (lin−c-kit+) were enumerated in BM. (D) B6.SJL (CD45.1+) mice were irradiated (300 cGy) and sorted lin−c-kit+ HPCs (2 × 105) from non-Tg (C57Bl/6), 11c.OVA or MII.OVA mice injected intravenously. Six weeks later donor leukocytes were enumerated in peripheral blood. Data represent individual mice pooled from a minimum of 2 experiments.
OVA-specific T-cell responses target HSPCs
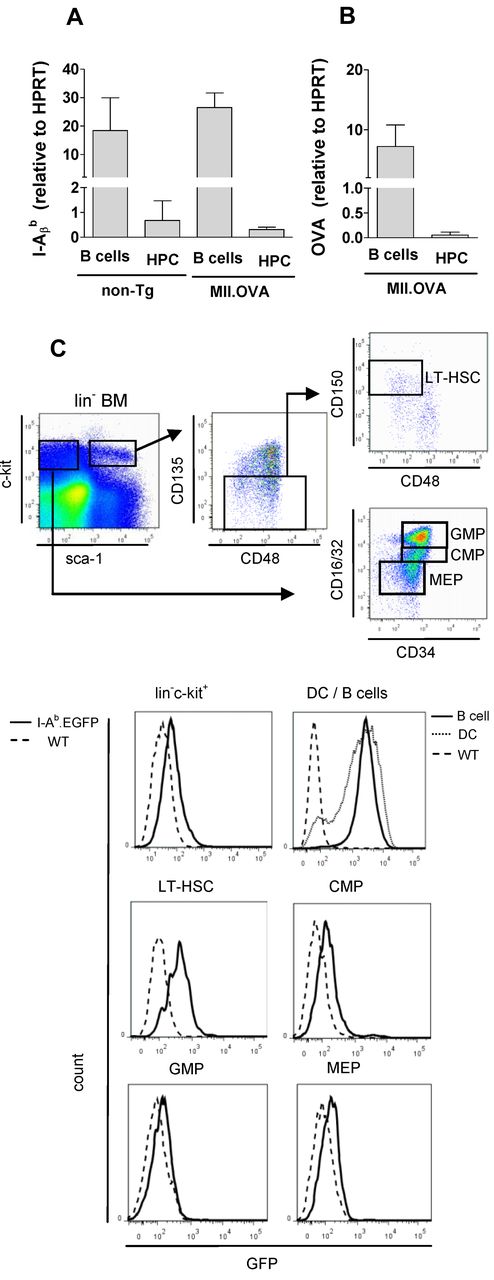
Donor-type lin-c-kit+ HSPCs were frequent in BM of non-Tg and 11c.OVA BM recipients but not in MII.OVA and actin.OVA BM recipients (Figure 3C) consistent with a failure to engraft. In actin.OVA mice, all cells, including HSPC, express OVA and can be directly targeted by, for instance, OVA-specific CTL, whereas in MII.OVA BM only MHC class II APC (DCs and B cells) would be expected to express OVA. To test whether failure of MII.OVA BM engraftment was the result of T-cell responses against mature OVA-expressing APC in transferred BM, we transferred sorted lin−c-kit+ HSPCs, devoid of differentiated APCs. lin−c-kit+ HSPCs from non-Tg and 11c.OVA effectively established donor hematopoiesis whereas those from MII.OVA donor did not (Figure 3D) suggesting that HPCs in MII.OVA BM were direct targets for immune rejection. This raised the possibility that these cells ectopically expressed OVA or unexpectedly expressed MHC class II and consequently the OVA transgene. To test this, we used qPCR to probe for MHC class II and OVA expression in HPCs and mature APCs. As expected, high levels of I-Aβb mRNA was detected in B cells from both non-Tg and MII.OVA mice and unexpectedly, but consistent with our data to date, we also detected I-Aβb mRNA in lin−c-kit+ HSPC from non-Tg and MII.OVA mice (Figure 4A). Consistent with this, mRNA encoding OVA was detected at high levels in B cells of MII.OVA mice and also, but at a reduced level, in HSPCs (Figure 4B). To validate this finding and define in detail the MHC class II+ cell population(s) within lin−c-kit+ cells we analyzed MHC class II expression using I-Ab-EGFP mice where EGFP serves as a marker of MHC class II expression. In lin−c-kit+ cells, EGFP was apparent in a low proportion of cells. Further subdivision of BM populations showed EGFP expression in a high proportion of long-term repopulating HSCs (LT-HSCs; lin−c-kithiSca1+CD135−CD48−CD150+) but at a level much lower than in B cells or DC (Figure 4C). Interestingly, MHC class II expression appeared to diminish as cells differentiated into committed progenitors, such as common myeloid progenitors (CMPs), granulocyte macrophage progenitors (GMPs), and megakaryocyte/erythroid progenitors (MEPs; Figure 4C). It was therefore clear that use of the MHC class II promoter led to detrimental off-target expression of OVA in HSPCs.
HSCs express MHC class II. (A-B) B cells (CD19+; A) and HPCs (lin−c-kit+; B) were sorted from non-Tg (C57BL/6) and MII.OVA mice and mRNA isolated. Relative levels of MHC class II (I-Aβb) and OVA mRNA were determined by TaqMan qPCR. Data are mean ± SD of 3 separate cell preps assayed twice for each sample. (C) BM was harvested from WT (C57BL/6, dotted line) and IAb.EGFP (solid line) mice and analyzed by flow cytometry to define lin−c-kit+ HPCs, long-term HSCs (LT-HSCs), common myeloid progenitors (CMPs), granulocyte/macrophage progenitors (GMPs), and megakaryocyte/erythroid progenitors (MEPs) using the gating strategy shown. B cells (CD19+, dark line) and DCs (CD11c+, gray line) were gated from spleen cell suspensions and compared with wild-type B cells (dashed line). Data are representative of 3 to 5 mice analyzed in parallel in 2 separate experiments.
HSCs express MHC class II. (A-B) B cells (CD19+; A) and HPCs (lin−c-kit+; B) were sorted from non-Tg (C57BL/6) and MII.OVA mice and mRNA isolated. Relative levels of MHC class II (I-Aβb) and OVA mRNA were determined by TaqMan qPCR. Data are mean ± SD of 3 separate cell preps assayed twice for each sample. (C) BM was harvested from WT (C57BL/6, dotted line) and IAb.EGFP (solid line) mice and analyzed by flow cytometry to define lin−c-kit+ HPCs, long-term HSCs (LT-HSCs), common myeloid progenitors (CMPs), granulocyte/macrophage progenitors (GMPs), and megakaryocyte/erythroid progenitors (MEPs) using the gating strategy shown. B cells (CD19+, dark line) and DCs (CD11c+, gray line) were gated from spleen cell suspensions and compared with wild-type B cells (dashed line). Data are representative of 3 to 5 mice analyzed in parallel in 2 separate experiments.
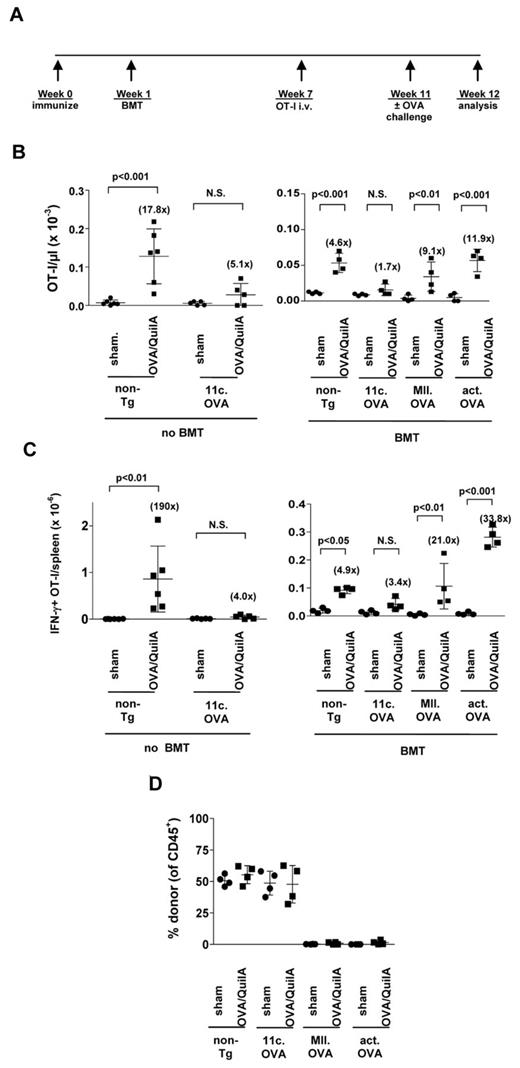
Restricting antigen expression to differentiated APCs facilitates antigen-encoding BM engraftment in primed recipients
As HPC-based therapies may be used in individuals with established transgene-specific immunity we tested whether this might impact BM engraftment. Recipient mice (CD45.1+) were immunized with OVA/QuilA, 2 weeks later mice were irradiated (300 cGy) and BM from non-Tg, 11c.OVA, MII.OVA, or actin.OVA mice injected intravenously. Donor-type leukocyte development was extensive and proceeded as expected in recipients of non-Tg and 11c.OVA BM (Figure 5A-B). Unlike unimmunized recipients (Figure 2) there appeared to be no transient development of donor-type cells in recipients of MII.OVA or actin.OVA BM (Figure 5A) consistent with rapid clearance of transferred cells. Analysis of BM showed that failure to develop peripheral donor-type leukocyte populations in recipients of MII.OVA and actin.OVA BM again coincided with the absence of donor-type lin−c-kit+ HSPCs (Figure 5C). Given that primed hosts could possibly bear detrimental antibody specificities that could limit engraftment, we determined whether cellular immunity alone is sufficient to prevent engraftment of HSPCs. OVA-specific OT-I TCR transgenic memory T cells15 were transferred to CD45.1+ mice and 7 days later recipients were irradiated (300 cGy) and BM from 11c.OVA and MII.OVA donors transferred intravenously. As observed for immunized BM recipients, BM from MII.OVA but not 11c.OVA donors failed to establish donor hematopoiesis (Figure 5D). To determine the extent to which memory T-cell responses might be impacted by the transplant procedure, memory OT-I T cells were transferred and mice irradiated and injected with non-Tg BM or left untreated. Under these conditions recall responses of the transferred OT-I T cells permit determination of the effects of irradiation directly on pre-existing memory T cells regardless of the extent of endogenous T-cell regeneration in the irradiated mice. Recipients of non-Tg BM showed approximately a 50% reduction in the number of OT-I T cells present in spleen after OVA challenge (Figure 5E) indicating that, although reduced, a substantial residual recall response was present.
Restricting antigen expression to CD11c+ DCs permits BM engraftment and tolerance induction in primed recipients. (A-C) B6.SJL (CD45.1+) recipients were immunized (OVA/QuilA) and 2 weeks later irradiated (300 cGy) and BM (107) from C57Bl/6, 11c.OVA, MII.OVA or actin.OVA (act.OVA) mice injected intravenously. At the depicted times (A) or 6 weeks later (B-C) donor leukocytes were enumerated in blood (A) or spleen (B) and lin−c-kit+ HPC enumerated in BM (C). (D-E) Memory OT-I T cells were transferred to B6.SJL mice and 1 week later mice were irradiated (300 cGy) and BM (1 × 107) from non-Tg, 11c.OVA or MII.OVA mice injected intravenously. Six weeks later donor leukocytes were enumerated in spleen using flow cytometry (D) or mice were sham or OVA-challenged and OT-I cells (CD45.1+CD8+Va2+) in spleen enumerated (E). (F) C57Bl/6 mice were irradiated (300 cGy) and BM (107) from C57Bl/6 or 11c.OVA mice injected intravenously. Four weeks later mice were double-grafted with skin from K14.mOVA mice and BALB/c allogeneic controls and graft survival monitored. Data represent mean ± SD from n = 4/group (A), individual mice pooled from a minimum of 2 experiments (B-E), or data pooled from 2 experiments (F).
Restricting antigen expression to CD11c+ DCs permits BM engraftment and tolerance induction in primed recipients. (A-C) B6.SJL (CD45.1+) recipients were immunized (OVA/QuilA) and 2 weeks later irradiated (300 cGy) and BM (107) from C57Bl/6, 11c.OVA, MII.OVA or actin.OVA (act.OVA) mice injected intravenously. At the depicted times (A) or 6 weeks later (B-C) donor leukocytes were enumerated in blood (A) or spleen (B) and lin−c-kit+ HPC enumerated in BM (C). (D-E) Memory OT-I T cells were transferred to B6.SJL mice and 1 week later mice were irradiated (300 cGy) and BM (1 × 107) from non-Tg, 11c.OVA or MII.OVA mice injected intravenously. Six weeks later donor leukocytes were enumerated in spleen using flow cytometry (D) or mice were sham or OVA-challenged and OT-I cells (CD45.1+CD8+Va2+) in spleen enumerated (E). (F) C57Bl/6 mice were irradiated (300 cGy) and BM (107) from C57Bl/6 or 11c.OVA mice injected intravenously. Four weeks later mice were double-grafted with skin from K14.mOVA mice and BALB/c allogeneic controls and graft survival monitored. Data represent mean ± SD from n = 4/group (A), individual mice pooled from a minimum of 2 experiments (B-E), or data pooled from 2 experiments (F).
Transfer of 11c.OVA BM under immune-preserving conditions leads to effective tolerance in recipients
One goal of tolerogenic therapies, and possibly a useful addition to gene-replacement therapies, is to provide an ongoing state of permanent tolerance. An advantage of genetically engineered HSCs in this respect is that long-term engraftment would lead to continued generation of tolerogenic APCs throughout the recipient's lifespan. To first test whether facilitating engraftment by restricting antigen expression to differentiated APCs led to antigen-specific tolerance in recipients, we performed skin grafting experiments that tested both the specificity of tolerance and whether immunity sufficient to reject whole tissue was preserved by the experimental conditions used. Compared with unirradiated controls, rejection of OVA-expressing skin was slowed and the extent of reject reduced in recipients of non-Tg BM (Figure 5F) indicating some blunting of the effector response by the BM transplant procedure but the majority of grafts were rejected by 45 days after grafting. In contrast, most OVA-expressing skin grafts were accepted in 11c.OVA BM recipients (Figure 5F). Cotransplanted BALB/c allogeneic grafts were rejected with equivalent tempo (by day 11 after grafting, not shown) in all groups suggesting that irradiation may have the greatest effect retention of T-cell responsiveness where the antigen-specific precursor frequency is low.
Effective engraftment, facilitated by antigen targeting, is required for long-term tolerance in primed recipients
Skin grafting indicated robust antigen-specific tolerance in 11c.OVA BM recipients quite proximal to BMT, and possibly a failure to reinstate an OVA-specific repertoire in recipients of 11c.OVA BM. We next determined specifically whether tolerogenic antigen presentation persisted in 11c.OVA BM recipients. Naive CD8+ OT-I T cells are effectively tolerized on adoptive transfer to 11c.OVA or MII.OVA mice. In this system when tolerance is induced, some OT-I cells are deleted, but residual tolerant OT-I T cells are rendered incapable of expanding or producing IFN-γ in response to antigen challenge.11 Conversely, in the absence of tolerance OT-I T cells retain antigen responsiveness. Therefore, we chose to use this transfer setting as an assay to probe the tolerogenicity of donor BM-derived APCs at an extended time after transplantation. One advantage of this assay is its independence of the presence or absence of an endogenous OVA-specific repertoire. To model BMT in a pre-immune setting, mice were immunized, irradiated (300 cGy), and non-Tg, 11c.OVA, MII.OVA or actin.OVA BM transferred. Six weeks later a population of naive OVA-specific CD8+ T cells (CD45.1+/CD45.2+ OT-I) was transferred (Figure 6A). After 4 weeks to allow “tolerance induction” in the adoptively transferred naive OT-I T cells, mice were sham or OVA-challenged to test tolerance induction. In untransplanted non-Tg control mice treated in parallel, OT-I T cells expanded substantially and IFN-γ production was induced in response to OVA challenge (Figure 6B-C left panels) whereas no significant expansion or IFN-γ production was observed in equivalently treated 11c.OVA controls (Figure 6B-C left panels). Although responsiveness was reduced somewhat compared with untransplanted controls, in MII.OVA and actin.OVA BM recipients, OT-I T cells expanded and the number of IFN-γ-producing OT-I T cells increased significantly in response to OVA challenge to a similar extent as seen in non-Tg BMT controls indicating that a tolerogenic environment was not generated (Figure 6B-C right panels). In distinct contrast, OT-I did not expand significantly nor was the number of IFN-γ-producing OT-I T cells significantly increased by OVA challenge of 11c.OVA BM recipients (Figure 6B-C right panels) indicating effective tolerance induction. Inactivation of the transferred naive OT-I T cells was associated with effective engraftment of OVA-encoding 11c.OVA BM (Figure 6D) despite similar potential tolerogenicity (Figure 1). A further indication that OVA-specific T cells were silenced was that no significant change in the proportion of donor-derived leukocytes was observed in OVA/QuilA challenged 11c.OVA BM recipients (Figure 6D).
11c.OVA BM establishes a long-term tolerogenic environment in primed recipients. (A) Experimental plan. (B-D) B6.SJL (CD45.1+) recipients were immunized (OVA/QuilA), 2 weeks later irradiated (300 cGy) and BM (107) from C57Bl/6; non-Tg, 11c.OVA, MII.OVA, or actin.OVA (act.OVA) mice injected intravenously. Six weeks later CD45.1+CD45.2+ OT-I LN cells (5 × 106) were transferred intravenously. Four weeks later mice were sham (PBS/QuilA) or OVA (OVA/QuilA) challenged. One week after challenge the number of OT-I T cells (CD45.1+CD45.2+CD8+) were enumerated in peripheral blood (B) and the number of IFN-γ producing OT-I T cells in spleen (C) was determined by flow cytometry. Donor chimerism was determined in peripheral blood 1 week after challenge (D). Data represent individual mice pooled from a minimum of 2 experiments. Bars show mean ± SD.
11c.OVA BM establishes a long-term tolerogenic environment in primed recipients. (A) Experimental plan. (B-D) B6.SJL (CD45.1+) recipients were immunized (OVA/QuilA), 2 weeks later irradiated (300 cGy) and BM (107) from C57Bl/6; non-Tg, 11c.OVA, MII.OVA, or actin.OVA (act.OVA) mice injected intravenously. Six weeks later CD45.1+CD45.2+ OT-I LN cells (5 × 106) were transferred intravenously. Four weeks later mice were sham (PBS/QuilA) or OVA (OVA/QuilA) challenged. One week after challenge the number of OT-I T cells (CD45.1+CD45.2+CD8+) were enumerated in peripheral blood (B) and the number of IFN-γ producing OT-I T cells in spleen (C) was determined by flow cytometry. Donor chimerism was determined in peripheral blood 1 week after challenge (D). Data represent individual mice pooled from a minimum of 2 experiments. Bars show mean ± SD.
Discussion
Autologous HSCT for autoimmune disease shows great promise but toxicity, procedure-associated immune suppression, and a general trend for disease relapse limits application and effectiveness. Addition of gene therapy to aHSCT to induce antigen-specific tolerance is a potential means to overcome limitations of current aHSCT approaches.5 We show that, when considering addition of gene therapy to aHSCT, targeting expression of a therapeutic protein to fully differentiated cells may be beneficial. In fact, to achieve immunologic tolerance under immune-preserving conditions, we show targeting expression to differentiated APCs with a high degree of fidelity as a strict requirement.
It is well-defined from studies using transgenesis,11,30 antibody-antigen conjugates,31 or other means that presentation of antigen by steady-state APCs is a powerful inducer of T-cell tolerance. An additional path to immune tolerance is transfer of transgenic or virally engineered BM or HSPCs encoding an antigen of interest to achieve de novo antigen expression and this can prevent autoimmune disease development.13,14 However, therapeutic goals for established or progressive autoimmune disease extend beyond this and 2 key goals exist: to reverse pre-existing pathogenic immunity and prevent disease relapse, while limiting non-specific immune-depletion. Using gene therapy to incorporate antigen expression into aHSCT to attain these outcomes would increase the feasibility and broaden the potential disease applications of aHSCT for non-life threatening diseases but also requires application under immune-preserving conditions. Studies of tolerogenic HSCT (tHSCT) for established autoimmune disease are limited, but to date most studies of tHSCT have used myeloablative or other intense conditioning to facilitate donor cell engraftment and eliminate mature immune cells, principally naive or primed T cells. Even so-called “nonmyeloablative” approaches to tHSCT in primed recipients typically use immune-depleting agents such as anti–T-cell antibodies to achieve immune ablation.14,32,33 A potential difficulty of limiting immune ablation is that recipient T cells can exert considerable immune-resistance to cells expressing endogenous, transgenic or virally engineered neoantigens leading to cellular rejection, and in the case of BMT or HSCT, graft failure.34-36 This could be particularly problematic in an autoimmune disease setting where recipients have expanded populations of memory and effector cells specific for the proteins to be encoded by transferred BM/HSPCs.37 Targeting therapeutic gene expression to fully differentiated APCs and avoiding expression in stem or progenitor cells is an effective route around the problem of immune-resistance to engraftment of gene-modified BM. Our data indicate the immunogenicity of transferred BM increases as gene expression becomes more widespread.
Incorporating enforced antigen expression through gene therapy is a potentially powerful means to prevent disease relapse after aHSCT. Here, establishing effective engraftment of gene-engineered BM in primed recipients, either by immune ablation (Figure 1) or by targeting gene expression to differentiated APC (Figures 1 and 5) was crucial to establishing a tolerogenic environment in recipients and one which is presumably capable of silencing and/or preventing re-emergence of pathogenic T-cell specificities in autoimmune disease. Indeed, expression of antigen targeted to DC continuously inactivates cognate antigen-specific T cells that escape thymic negative selection.30 Recent demonstrations that memory and effector T-cell responses can be turned-off by enforced antigen expression,15-17 provide tantalizing hints that tHSCT may be a path to reversing pre-existing pathogenic T-cell responses that underlie and perpetuate autoimmune and other T cell–mediated diseases (reviewed by Coleman and Steptoe5 ). Conceivably, this could provide an antigen-specific immune reset without the need for non-specific immune depletion used to remove pre-existing T-cell populations.
Previous studies have shown that transfer of BM or HSPC encoding a transgene under either a ubiquitously expressing or an MHC class II (HLA-DR) promoter can lead to clearance of cognate antigen-expressing tumors.38,39 The results of the present study provide insights into how transfer of gene-engineered BM has led to either immunogenic or tolerogenic outcomes, when on the surface, tolerogenesis might be considered the logical outcome. Although any BMT is potentially tolerogenic, without establishment of long-term hematopoiesis priming and establishment or expansion of memory occurs. Prolonged antigen presentation, that occurs as the result of successful establishment of hematopoiesis, promotes tolerance.15 From this it can be predicted that as observed here and hinted at in other studies,38 failed tHSCT will probably generate long-lived memory directed at encoded proteins even if expressed by tolerogenic APCs. This provides a strong rationale for any potential clinical application, that effective establishment of ongoing hematopoiesis and transgene expression after tHSCT is assured and is a warning to the potential consequences if this fails. However, on the other hand, once facilitated by high fidelity targeting to differentiated APCs, our data indicate engraftment is resistant to subsequent immune challenge.
In transgenic mice, T-cell tolerance occurs regardless of whether antigen is expressed solely by APCs or not.11,18-20 Whether the proximal tolerance-inducing cells under the latter circumstance are professional APCs (DCs, B cells, macrophages) or nonhematopoietic cells is unclear. Given that APCs are powerful inducers of tolerance it is apparent that for tHSCT as long as antigen is expressed in APC progeny of engrafting cells, tolerance should ensue. For tHSCT, no systematic comparisons of the relative benefit of different promoters or gene-expression targeting have been performed. In transgenic mice antigen expressed primarily in B cells and DCs, from an MHC class II promoter, leads to rapid tolerance induction in effector and memory CD8+ T cells without development of detrimental effector function that is present when tolerance is induced by antigen expressed from a DC-specific promoter.16 The present study confirms that under myeloablative conditions promoter choice or targeting of expression is of little consequence for generation of tolerance after tHSCT. This is consistent with previous studies in autoimmune disease models where, to our knowledge, no requirement for APC-restricted antigen expression has yet been found. Similarly, our data imply immune-depleting conditioning may be critical to the success of clinical gene replacement therapy where ubiquitously expressing promoter elements have been used to drive therapeutic gene expression. Although anticipating that an MHC class II promoter may have advantages over a DC-specific promoter for tHSCT for the reasons discussed in this paper, unanticipated stage-specific expression of MHC class II in HSPC was a detrimental factor when immune-preserving conditioning was used and indicates that gene expression must be targeted by the promoter used with high fidelity only to differentiated APCs. In human HPCs, MHC class II is expressed heterogeneously by CD34+CD38+ and CD34+CD38− HPCs and may define lineage differentiation potential. Our finding of MHC class II expression restricted, among HSPC, almost entirely to LT-HSC was unexpected but is supported by several studies.40-42 The data reiterate the necessity of careful investigation of candidate promoters for successful application of gene therapy, particularly with reference to the specific intended application. Although these studies have used BM and HSPCs from transgenic mice, the findings are equally pertinent to the use of virally engineered cells that might be considered for clinical application.
For gene therapy, both clinically and experimentally, promoters that drive gene expression with a ubiquitous distribution have typically been used. Initially, endogenous viral long terminal repeat (LTR) promoters and enhancers were used but the high level expression along with potential oncogenesis is considered detrimental, prompting attempts to use less-strong, but ubiquitously expressing promoters, such as those for phosphoglycerate kinase (PGK) or elongation factor 1a (EF1α).43 However, it is thought that in some circumstances, for example, expression of β-globin genes in thalassemia that cell-lineage specific promoters, in this case endogenous β-globin promoters may be advantageous to achieve the high levels and development-stage specific expression required.44 Studies in experimental models of leukocyte adhesion deficiency type 145,46 and Artemis immunodeficiency47 show that the promoter selection can determine outcomes in ways that are not readily predicted. Again, this highlights the importance of careful, tailored, promoter selection for gene therapy based, preferably, on experimental studies. Viral vectors using APC-specific promoter/antigen constructs have been successfully used to achieve transgene expression in B cells and DCs in mouse19,21 and human39 systems. These studies validate the potential of APC-targeting promoters and our findings indicate that levels of expression from APC-directed promoters are sufficient for tolerance induction. Although direct comparisons of transgene expression levels in conventional transgenesis and viral-vector mediated gene-transfer are rare, both systems can lead to high levels of gene expression and promoter choice may be more critical than the means of gene transfer.45-49 Importantly, studies using virally transduced BM for induction of tolerance have replicated the outcomes of transgenic BM studies14,21,50 but testing in viral gene-transfer settings will provide important validation of the findings reported here.
In summary, we demonstrate that targeting antigen expression with high-fidelity to mature APCs, such as DCs, enables long-term engraftment and generation of a tolerogenic environment in recipients that are naive or primed to the protein encoded by gene-engineered HSC. Our results provide a strong rationale for using carefully selected promoters to restrict antigen expression to differentiated cells when HSCs that encode potentially immunogenic protein(s) are transferred under immune-preserving conditions. Targeting does not alter the inherent tolerogenicity of tHSCT but is required to permit the engraftment on which the fulfillment of tolerogenic potential is dependent. As an example, in the case of autoimmune disease, targeting expression of autoantigen(s) to DCs may be the best approach for tolerance, engraftment, and safety.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Professors Francis Carbone and William Heath, University of Melbourne; Professor Leonard Harrison, Walter and Eliza Hall Institute; and Dr Barbara Fazekas de St Groth, Centenary Institute, for providing mice. The authors thank Dr Antje Blumenthal and Ryan Galea for assistance with qPCR. The authors also thank staff of the Biologic Resources Facility and Mater Medical Research Institute for help with animal procedures.
This work was supported by project grant APP1013066 from the National Health and Medical Research Council (NHMRC) of Australia. M.L.C. was supported by a UQ Postgraduate Research Award. R.J.S. was supported by an NHMRC Career Development Award and ARC Future Fellowship. J.W.W. supported by a fellowship from Perpetual Trustees. R.T. was the recipient of an Australian Research Council Future Fellowship and support from Arthritis Queensland. G.R.H. is supported by an NHMRC Australia Fellowship. S.W.L. is supported by the Leukemia Foundation and NHMRC.
Authorship
Contribution: M.A.C. and R.J.S. designed and performed experiments and wrote the paper; S.W.L., J.A.B., and J.W.W. designed and performed experiments and analyses; C.M.D performed experiments; and G.R.H and R.T. designed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: R. J. Steptoe, UQ Diamantina Institute, The University of Queensland, Princess Alexandra Hospital, Level 4 R Wing Building 1, Woolloongabba, 4102 Queensland, Australia; e-mail: r.steptoe@uq.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal