In this issue of Blood, Haso and collegues report on the generation of new anti-CD22 chimeric antigen receptors (CARs) and the importance of careful epitope selection when designing CARs against leukemia.1
The treatment of chemotherapy-resistant acute lymphoblastic leukemia (ALL) is a major challenge in adult and pediatric oncology. Besides new therapies to inhibit pathways responsible for leukemia proliferation, immunotherapeutic strategies involving monoclonal antibodies directed against antigens expressed on the leukemic cells or cell therapy employing T lymphocytes or natural killer (NK) cells to eradicate malignant cells before or after allogeneic hematopoietic stem cell transplantation (HSCT) are increasingly used in clinical trials. An example of how powerfully the immune system can attack and completely eliminate leukemic blasts when directly targeted against antigens expressed on the target cells is the novel class of anti-CD3/anti-CD19 bi-specific single-chain antibody constructs (BiTEs), which recruits T lymphocytes through the CD3 antigen of the T-cell receptor (TCR) complex for redirected lysis of CD19-positive malignant cells. In clinical studies, impressive numbers of minimal residual disease (MRD)–negative remissions have been observed in adult and pediatric patients with chemorefractory disease before or after allogeneic HSCT.2-4
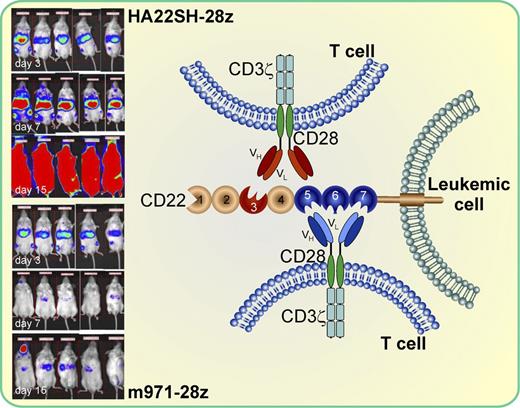
The siglec CD22 consists of 7 extracellular IgG-like domains and is expressed on B-cell leukemias and lymphomas. CAR T cells using different binding sites via the incorporation of single-chain variable fragments VH and VL derived from different anti-CD22 antibodies (m971 and HA22SH) and co-transduced with 2 co-stimulatory molecules (CD28 and CD3ζ) differ in their in vitro and in vivo antileukemic activity against CD22-positive target cells. CAR cells incorporating a more membrane-proximal epitope on CD22 (m971-28z) had a significantly higher in vitro activity and were more effective in reducing the leukemic burden of mice in a xenograft model and prolonging their survival compared with the CARs expressing the more distal binding site (HA22SH-28z). The red color of the mice in the insert is a measure for their leukemic burden at day 3 prior to injection of m971 or HA22SH CAR T cells and at days 7 and 15 after injection. Parts of the figure were adapted from Figure 6A in the article by Haso et al that begins on page 1165.
The siglec CD22 consists of 7 extracellular IgG-like domains and is expressed on B-cell leukemias and lymphomas. CAR T cells using different binding sites via the incorporation of single-chain variable fragments VH and VL derived from different anti-CD22 antibodies (m971 and HA22SH) and co-transduced with 2 co-stimulatory molecules (CD28 and CD3ζ) differ in their in vitro and in vivo antileukemic activity against CD22-positive target cells. CAR cells incorporating a more membrane-proximal epitope on CD22 (m971-28z) had a significantly higher in vitro activity and were more effective in reducing the leukemic burden of mice in a xenograft model and prolonging their survival compared with the CARs expressing the more distal binding site (HA22SH-28z). The red color of the mice in the insert is a measure for their leukemic burden at day 3 prior to injection of m971 or HA22SH CAR T cells and at days 7 and 15 after injection. Parts of the figure were adapted from Figure 6A in the article by Haso et al that begins on page 1165.
Another way to harness the powerful antileukemic activity of the immune system is the genetic engineering of T lymphocytes that express CARs.5 First-generation CARs incorporated a single-chain variable fragment (scFv) derived from a monoclonal antibody and the signaling motif from the CD3ζ chain.6 Further improvements were achieved through the addition of 1 (second-generation CAR) or 2 (third-generation CAR) costimulatory activating motifs, including CD28, 4-1BB (CD137), and/or CD134 (OX-40), all of which led to increased cytotoxicity and enhanced proliferation.7 The efficacy of such engineered T lymphocytes has already been demonstrated in patients with progressive B-cell malignancies8 and in a patient with chronic lymphoid leukemia, who was treated with a low dose of autologous T cells redirected against the leukemic cells through the expression of a chimeric receptor with specificity for the B-cell antigen CD19 coupled with co-stimulatory CD137 and the CD3ζ signaling domain. After infusion, a tremendous expansion of these engineered T cells and a complete remission was observed.9 More recently, impressive results have been reported in 10 patients (9 adults with refractory CLL and 1 child with refractory ALL) and 4 of the 9 patients including the child with ALL achieved complete remission.10 Moreover, a significant expansion of the autologous infused T cells and persistence for at least up to 2 years was observed. Besides the CD19 antigen, CD22 is another member of the B-cell antigen family with similar distribution as CD19. CD22 belongs to the sialic acid binding Ig-like lectin (Siglec) family and consists of 7 extracellular IgG-like domains. CD22 is expressed on precursor B-ALL and has already been used as a target structure using toxins conjugated to anti-CD22 monoclonal antibodies (mAbs).
Here, Haso et al have generated 10 different gene constructs to generate CAR T cells targeting the CD22 antigen.1 They used 3 scFv (high-affinity scFv HA22, standard-affinity scFv BL22, and fully human scFv derived from mAb m971) directed against the CD22 antigen and fused them to various TCR signaling domains with or without an IgG heavy chain constant domain (CH2CH3). In contrast to the binding sites targeted by mAbs HA22 and BL22, which recognize an epitope located more membrane-distal in domain 3 of the 7 extracellular IgG-like domains, m971 binds membrane-proximal within IgG domains 5 through 7. They then compared the in vitro activity against fresh leukemic blasts as well as cell lines. They could show that the cytotoxic activity of the CAR T cells co-expressing the m971 scFv and the CD28 and CD3ζ costimulatory molecules (second-generation CARs) were superior to the HA22 or BL22 CARs using the same co-stimulatory molecules. In addition, neither increasing the distance between the T-cell surface and the CD22 epitope with CH2CH3 nor the generation of third-generation CARs through incorporating 2 co-stimulatory domains significantly increased their activity. Even increasing the binding affinity of the CARs using high-affinity BL22-derived scFv did not result in an increase of the lytic activity compared with the CARs expressing the standard-affinity scFv derived from BL22, demonstrating that the affinity is not a driving factor for CAR activity. Cytokine production of the different CARs was similar to their cytotoxic activity and second-generation m971 and HA22 CARs were more active in IFN-γ, TNF-α, and IL-2 production.
In a next step, the authors then compared the in vivo activity of the 2 second-generation CARs expressing m971 and HA22 with the co-stimulatory molecules CD28 and CD3ζ in a xenograft model. After injection of NALM6 leukemic blasts, the 2 best second-generation CAR-transduced T cells were injected into the mice. Compared with the mock-treated mice, the CD22-CAR treatment was associated with a longer survival and the mice treated with the m971 expressing T cells had the longest survival and the lowest tumor burden (see figure). These data convincingly demonstrate that the binding site of the CAR on the CD22 antigen can be very important in the generation of new and more effective CARs and may be even more important than other parameters, such as additional co-stimulatory molecules or the distance between the T-cell surface and the targets. It can be anticipated that the CAR technology will open a new chapter in medicine for the treatment of refractory malignant diseases and that the observations by Haso et al will play an important role in the future development of other CARs directed against suitable antigens expressed on malignant cells.
Conflict-of-interest disclosure: The author declares no competing financial interest. ■