Key Points
Mab-based immunotherapy prevents Treg expansion and limits immunosuppressive activity.
Abstract
Regulatory T cells (Tregs) down-regulate immunity and are associated with chronic viral infections, suggesting that their inhibition might be used to treat life-threatening diseases. Using the FrCasE mouse retroviral model, we have recently shown that short mAb-based immunotherapies can induce life-long protective immunity. This finding has a potentially important therapeutical impact because mAbs are increasingly used to treat severe viral infections. We now report that poor anti-FrCasE immunity in infected mice is due to Treg expansion in secondary lymphoid organs because depletion of Tregs restored humoral and cytotoxic T lymphocyte (CTL) antiviral responses. Kinetic analyses show that Treg expansion is not a consequence of chronicity, but rather is associated with viral spread. Moreover, Treg adoptive transfers indicate that production of the immunosuppressive cytokine IL-10 is essential for preventing a protective immune response. Finally, treatment of infected mice with a virus-neutralizing IgG2a shortly after infection prevents Treg expansion and limits immunosuppressive activity. This effect is rapid, necessary for the development of protective immunity, and depends on mAb effector functions. Therefore, manipulating Tregs may be necessary to confer robust antiviral immunity in the context of mAb-based therapy. This concept likely applies to cancer treatment because vaccine-like effects of mAbs have also been observed in certain cancer immunotherapies.
Introduction
Regulatory T cells (Tregs) are a subset of CD4+ T lymphocytes down-regulating the immune system.1 They are the key modulators of the establishment and/or maintenance of viral chronicity and constitute a barrier to efficient vaccination and immunotherapy strategies.2 The implication of Tregs in chronic viral infection was first described in mice infected with the Friend virus complex (FV)3 and was then extended to other persistent viruses, including HIV,4 HBV,5,6 HCV,5,6 EBV,7 LCMV,8 HSV,9 and HPVs.10 It is increasingly clear that controlling this immunosuppressive cell subset would have widespread clinical applications to fight life-threatening viral diseases.11
mAbs constitute the largest class of biotherapeutics.12 Their main current applications are in cancer and inflammatory diseases,12 but they also have an enormous potential to treat both acute and chronic severe viral infections. Although the first antiviral mAb to be commercialized (in 1998) was an anti-RSV to treat an infant respiratory disease,13 many of the current antiviral mAbs have been developed more recently.12 Illustrating this trend, humanized mAbs targeting diverse viruses (Ebola, WNV, CMV, human and avian influenza, SARS, Hanta, and Nipah) responsible for acute diseases have recently shown promising results in preclinical animal settings. Some of them are now undergoing clinical trials.14 Many chronic infections have also been targeted. Several human anti-HIV mAbs have been tested in nonhuman primates (see Hessell et al15 and Ng et al16 ) and in clinical trials, and broadly neutralizing human anti-HIV mAbs have been isolated recently.17 Several human anti-HCV mAbs have also been developed recently,18 some of which are currently being tested in the clinical setting.19
The direct effects of mAbs, such as inhibition of soluble effectors or of cell membrane signaling receptors, virus neutralization, and NK-dependent (antibody-dependent cell-mediated cytotoxicity) or complement-dependent cytolysis on their targets are, in general, well studied.12 In addition, we have shown recently that short neutralizing mAb-based immunotherapies may exert immunomodulatory effects translating into life-long immune protection (see end of “Introduction”),20-24 which has potentially important consequences for treating life-threatening viral diseases in humans. The recent demonstration that passive neutralizing mAbs could not only control viremia in young macaques infected with a hybrid simian HIV strain, but also enhance B-cell responses in a perinatal setting16 further supports this concept. Similar vaccine-like effects were also documented recently in 2 cancer models immunotherapy treated with mAbs directed to tumor cell membrane proteins.25,26 This emphasizes that the immunomodulatory actions of mAbs may find applications in a wide array of pathologies with both a direct therapeutical benefit for patients and an indirect one for society: mAb-based therapies are expensive irrespective of the disease concerned, and the capacity to induce long-lasting immune protection by short treatments would considerably reduce their costs. Elucidating the cellular and molecular mechanisms underlying the reinforcement of endogenous immunity of mAb-treated patients will be a major step toward improved immunotherapies
To test the concept that short immunotherapies with antiviral mAbs can trigger long-lasting protective immunity, we resorted to the FrCasE mouse simple retrovirus model because of its multiple advantages: (1) the manifestations of pathologic effects are rapid with a 100% incidence and a clear symptomology (fatal neurodegeneration within 2 months if infection occurs before day 5-6 after birth or fatal erythroleukemia within 4-5 months if infection occurs on day 8; see Gros et al20 and Michaud et al24 ); (2) it is one of the rare chronic viral infection systems for which neutralizing mAbs of the same species are available, permitting investigations in immunocompetent organisms; (3) mice offer immunologic tools that are not available in other species; and (4) infection by FrCasE is reminiscent of perinatal infection by HIV, which affects 2.5 million children living with HIV infection worldwide (2009 estimate by the World Health Organization22,24 ). When pups infected perinatally with FrCasE are treated for a few days with a neutralizing IgG2a/κ mAb (667) recognizing the receptor-binding domain of FrCasE Env glycoprotein within 2 days after infection, infected/treated mice survive > 1 year with no pathologic symptoms.20,24 Healthy survival depends on a strong protective endogenous immunity because breaking it entails leukemic death.21 This immunity contrasts with the weak response developing in infected/nontreated mice and is of the Th1 type with strong (IgG2a) humoral20,22,24 and CTL21,24 arms and a strong memory component,21,24 permitting mice to resist a viral challenge long after primary infection.20 Induction of such an immunity does not result from the simple reduction of viremia during immunotherapy affording the immune system more time to react. Instead, it is because of genuine immunomodulatory actions of the mAb that depend on its ability to interact with receptors for IgGs (FcγRs),23,24 in particular on dendritic cells (DCs) that are at the heart of any adaptive immune response. Specifically, interactions between DC FcγRs and immune complexes (ICs) comprising the therapeutic antibody bound to Env on FrCasE-infected cells increases uptake of the latter cells by DCs, permits more efficient activation of DCs, and is followed by stronger anti-FrCasE CD8+ T-cell responses.24 Cellular IC/FcγRs interactions are, however, not limited to DCs but also concern NKs that are responsible for antibody-dependent cell-mediated cytotoxicity of infected cells during immunotherapy.24 In contrast, complement-dependent cytolysis has no detectable role in infected cell lysis.24
Because chronic viral infections are associated with Treg responses dampening antiviral immune responses, we wondered whether the antiviral vaccine-like effects induced by mAb-based immunotherapies could involve an inhibition of Tregs. In the present study, we characterize the Treg response induced by FrCasE and establish its role in the poor immunity mounted by infected mice. We also report that 667 mAb treatment inhibits the development of Tregs and that this control is necessary for the mounting of protective immunity in infected, 667 mAb–treated mice. This finding may have important consequences for the design of successful antiviral and anticancer immunotherapies based on the use of mAbs.
Methods
Virus stocks
Production of 667 and 672 mAbs and of the 667 F(ab′)2 fragment
The neutralizing mouse mAb 667 (IgG2a/κ) was purified and assayed as described previously.21 The mouse mAb 672 (IgM/κ) was prepared from hybridoma cell-culture supernatant and purified using the IgM purification kit from Pierce.23 The 667 F(ab′)2 fragment was prepared by proteolysis of 667 with pepsin. The Fc fragment was removed by protein A chromatography and the F(ab′)2 fragment was collected in the unbound fraction and quality controlled as described in Nasser et al.23
Viral infection, immunotherapy, and follow-up
We used inbred 129/Sv/Ev mice (H-2Db haplotype). Three days after birth, infections were induced intraperitoneally with 1-5 focus-forming units (FFU; low-inoculum infections) as described previously.23 Under these conditions, mice develop leukemia with kinetics similar to those of mice infected with 5 × 104 FFU (high-inoculum infection) on day 8 after birth (see Michaud et al24 ). Treatments with the 667 and 672 mAbs or the 667 F(ab′)2 fragment consisted of repeated IP administrations (15 μg of antibody each time) starting 1-2 hours after FrCasE inoculation (which is sufficient for establishment of infection), as described previously.20-24 Mice were examined daily for clinical signs of neurodegeneration or erythroleukemia.20-24 Plasma anti-FrCasE serum Igs were assayed by ELISA as described previously.20,27 For challenge experiments, mice were injected intravenously with a mixture containing 25 μL of FrCasE suspension (5 × 105 FFU/mL) and 1 × 106 infected fresh splenocytes.
Flow cytometry analyses
Flow cytometry was conducted using the FACSCanto II device (BD Biosciences). The data were processed using FlowJo Version 8.8.7 software (TreeStar). FrCasE-infected cells were assayed using an anti-Env mAb labeled with FITC (see Nasser et al23 and Michaud et al24 ). Staining of cell-surface markers (CD4, CD8, CD25, CTLA4, GITR, Helios, and CD39) and intracellular proteins (granzyme B [GzmB], FoxP3, IFN-γ, IL10, and TGF-β) were performed using BD Biosciences, BD Pharmingen, eBiosciences, or BioLegend antibodies that were FITC-, APC-Alexa Fluor–, PE-, or PE-Cy7–conjugated GzmB, FoxP3, IFN-γ, IL-10, and TGF-β using the Cytofix/Cytoperm intracellular staining kit (BD Biosciences).
In vivo depletion of CD4+CD25+ T cells
The anti-CD25 IL-2Rα mAb produced (PC61 hybridoma; BioXcell) was used for in vivo depletion of CD25+ T cells, as described previously.28 Infected/nontreated mice received 4 injections (25 μg each) of this mAb on days 2, 4, 7, and 9 after challenge. Depletion of Tregs was monitored by flow cytometry of CD25highFoxP3+CD4+ T cells.
Purification and adoptive transfer of Tregs
CD4+CD25+ T cells were isolated from splenocytes of donor mice using the MACS CD4+CD25+ regulatory T-cell magnetic isolation kit (Miltenyi Biotec). Cell purity (> 95%) was verified by flow cytometry using FoxP3 staining. For adoptive transfer, 3 × 105 purified cells in 0.1 mL of PBS were tail-vein injected into recipient mice.
In vitro T-cell activation
A total of 5 × 105 splenocytes were incubated at 37°C for 5 hours in 96-well round-bottom plates in 200 μL of RPMI culture medium containing 5 μg/mL of brefeldin A (Sigma-Aldrich). T-cell activation was assessed in IFN-γ flow cytometry assays using phorbol 12-myristate 13-acetate (50 ng/mL) + ionomycin (500 ng/mL)–stimulated splenocytes as positive controls and untreated cells as negative controls.
In vitro Treg activation
A total of 5 × 105 Tregs purified as described in “In vitro T-cell activation” were incubated at 37°C for 72 hours in 96-well round-bottom plates in 200 μL of RPMI medium containing 1mM Na-pyruvate (100mM; GIBCO), 1× nonessential amino acids (MEM NEAA, 100×; GIBCO), 50μM β-mercaptoethanol (50mM; GIBCO), 20 ng/mL of recombinant mouse IL-2 (Sigma-Aldrich), and 0.5 μg/mL of anti-CD28 antibody (BD Biosciences). For TCR-stimulation experiments, wells were precoated at 37°C for 2 hours with an anti-CD3 mAb (BD Biosciences) using a 1 μg/mL solution in bicarbonate buffer, pH 8.8. Next, 5 μg/mL of brefeldin A (Sigma-Aldrich) was added 5 hours before labeling for flow cytometry analysis.
Treatment of mice with the anti–IL-10 mAb
Mice were injected intraperitoneally once a day using 0.5 mg of the anti–IL-10 JES5-2A5 mAb (BioXcell) from day 5 (day of Treg grafting) to day 10 after viral challenge (day of sacrifice).
In vivo cytotoxicity assays
The experiments were conducted as described previously.21,24 Spleens were recovered from 10-day-old FrCasE-infected or noninfected pups. Splenocytes from noninfected mice were labeled with the vital dye CFSE (Molecular Probes) at a concentration of 0.5μM (CFSElow cells) and splenocytes from infected mice were labeled in a 5μM CFSE solution (CFSEhigh cells). The 2 cell populations were mixed in a 1:1 ratio before IV administration to recipient mice. The ratio of CFSElow/CFSEhigh cells in the spleens was assayed by flow cytometry 24 hours later and the CTL activity against infected splenocytes was calculated from the ratio of CFSEhigh/CFSElow cells, as described previously.21,24
Flow cytometry assay of CD8+ T cells specific for FrCasE-infected cells
Splenocytes were labeled with both an APC-conjugated anti-CD8+ T-cell antibody and a PE-conjugated MHC class I H-2Db tetramer (Beckman Coulter) displaying the immunodominant Friend virus GagL epitope (Db-GagL tetramers) as described previously.24
Statistics
Data are presented as means ± SD. Statistical significance was established using the Student t test. P < .05 was considered significant.
Ethics statement
All procedures for animal handling and experiments were approved by the local animal facility ComEth Institutional Review Board under the supervision of the French Languedoc-Roussillon Region Ethics Committee (CEEA-LR) on animal experimentation.
Results
Treg response induced on FrCasE infection
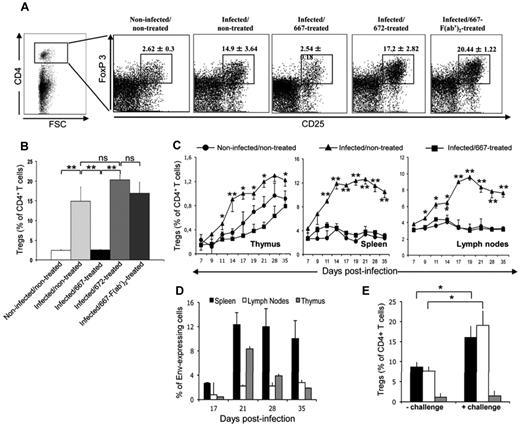
We first investigated whether FrCasE induced a Treg response associating with chronicity after neonatal infection. We compared Treg abundances in the spleens of control (noninfected/nontreated) and perinatally infected (infected/nontreated) mice at 8 weeks of age using classic flow cytometry parameters for Tregs: positivity for both CD4 and the FoxP3 transcription factor and expression of high levels of CD25 (IL-2 receptor α chain). CD4+CD25highFoxP3+ Tregs were much more abundant in adult infected/nontreated mice (14.9% ± 3.64% of total CD4+ cells; Figure 1A-B) than in adult noninfected/nontreated mice (2.4% ± 0.3% of total CD4+ cells; Figure 1A-B), showing induction of a Treg response after infection by FrCasE.
Treg responses in immunotherapy-treated and nontreated infected mice. (A) Flow cytometry analyses of Treg responses in mice 8 weeks of age. Mice were infected neonatally with FrCasE. Infected mice were immunotherapy treated shortly after infection using repeated administration of 667, 667-F(ab′)2, or 672 for 5 days (see “Methods”) and compared with control noninfected/nontreated and infected/nontreated animals. Treg levels were assayed by flow cytometry 8 weeks later on the basis of CD4, CD25high, and FoxP3 positivity and were expressed as a percentage of total CD4+ T cells. (B) Statistical analysis of Treg responses in mice 8 weeks of age. Three experiments comprising 3 mice each were conducted as described in panel A. The data are presented as means ± SEM of Tregs expressed as a percentage of total CD4+ T cells. The statistical significance between groups was established using the Student t test. **P < .01. (C) Treg development in the thymi, spleens, and lymph nodes of noninfected/nontreated, infected/nontreated, and infected/667-treated mice. The different groups of mice were infected and treated as in panel A. Tregs were assayed as in panels A and B in total CD4+ cells recovered from each organ. Data are presented as means ± SEM of 3 independent experiments conducted with 2 mice per time point. (D) Infection kinetics in the thymi, spleens, and lymph nodes of infected/nontreated mice. Mice were infected and cells were then prepared at various time points, from the thymi, spleens, and lymph nodes for flow cytometry quantification of Env expression at their surface, as described in “Methods.” Values are the means ± SEM of 3 independent experiments conducted with 3 mice per condition. (E) Effect of FrCasE viral challenge on Treg levels in infected/nontreated mice. Mice were infected and subjected to viral challenge 8 weeks later, as described in “Methods,” and Treg levels were assayed after another 8 days, as in panels A and B. Values are the means ± SEM of 3 experiments conducted with 3 mice each. The statistical significance between groups was established using the Student t test. *P < .05.
Treg responses in immunotherapy-treated and nontreated infected mice. (A) Flow cytometry analyses of Treg responses in mice 8 weeks of age. Mice were infected neonatally with FrCasE. Infected mice were immunotherapy treated shortly after infection using repeated administration of 667, 667-F(ab′)2, or 672 for 5 days (see “Methods”) and compared with control noninfected/nontreated and infected/nontreated animals. Treg levels were assayed by flow cytometry 8 weeks later on the basis of CD4, CD25high, and FoxP3 positivity and were expressed as a percentage of total CD4+ T cells. (B) Statistical analysis of Treg responses in mice 8 weeks of age. Three experiments comprising 3 mice each were conducted as described in panel A. The data are presented as means ± SEM of Tregs expressed as a percentage of total CD4+ T cells. The statistical significance between groups was established using the Student t test. **P < .01. (C) Treg development in the thymi, spleens, and lymph nodes of noninfected/nontreated, infected/nontreated, and infected/667-treated mice. The different groups of mice were infected and treated as in panel A. Tregs were assayed as in panels A and B in total CD4+ cells recovered from each organ. Data are presented as means ± SEM of 3 independent experiments conducted with 2 mice per time point. (D) Infection kinetics in the thymi, spleens, and lymph nodes of infected/nontreated mice. Mice were infected and cells were then prepared at various time points, from the thymi, spleens, and lymph nodes for flow cytometry quantification of Env expression at their surface, as described in “Methods.” Values are the means ± SEM of 3 independent experiments conducted with 3 mice per condition. (E) Effect of FrCasE viral challenge on Treg levels in infected/nontreated mice. Mice were infected and subjected to viral challenge 8 weeks later, as described in “Methods,” and Treg levels were assayed after another 8 days, as in panels A and B. Values are the means ± SEM of 3 experiments conducted with 3 mice each. The statistical significance between groups was established using the Student t test. *P < .05.
The Treg response induced by FrCasE is inhibited by the 667-mAb immunotherapy
We also addressed whether the induction of the Tregs in infected/nontreated mice could be inhibited on 667 mAb treatment. This was tested in the spleens of 8-week-old mice that were treated with 667 mAb shortly after infection (infected/667-treated mice). Treg levels at adult age were dramatically reduced in infected/667-treated mice compared with infected/nontreated animals (Figure 1A-B). Infected mice were then treated with either the 672 mAb (infected/672-treated) or the 667 F(ab′)2 (infected/667-F(ab′)2–treated). The 672 mAb is a neutralizing IgM/κ recognizing an epitope contained within that of the 667 mAb.23 It displays no binding activity for FcγRs, but shows high binding activity for complement, whereas the 667 F(ab′)2 is devoid of any effector function. None of these treatments inhibited the induction of Tregs (17.2% ± 2.82% and 20.44% ± 1.22% at week 8, respectively; Figure 1A-B). Therefore, the vaccine-like effect of the 667 immunotherapy23,24 associated with an inhibition of Treg expansion by FrCasE that depended on the presence of the Fc part of the administered mAb. The effector function involved was most probably binding to FcγR, as shown in former work23 and in the present study by the fact that neither the 667 F(ab′)2 nor the 672 mAb had an effect despite the high complement-binding activity of 672.
Inhibition of Treg expansion by 667 is a rapid event
We also investigated when the inhibition of Treg expansion by 667 immunotherapy occurred. First, we compared Treg appearance kinetics in noninfected/nontreated and infected/nontreated mice from day 7-35 after infection in central (thymus) and peripheral lymphoid organs (lymph nodes and spleen), where murine leukemia viruses propagate (Figure 1C). In control noninfected/nontreated mice, the fraction of Tregs among total CD4+ T cells steadily increased in the thymus throughout the analysis period and remained nearly constant in spleen and lymph nodes (Figure 1C), which is consistent with central immune system development. In the thymus of infected/nontreated mice, Tregs levels increased moderately (50%) and represented a small fraction (1.2%) of total CD4+ cells (Figure 1C). In contrast, they dramatically increased in spleen and lymph nodes (3- and 4-fold, respectively), where they represented a large fraction of CD4+ T cells (12% and 10%, respectively; Figure 1C). In all 3 organs, differences from noninfected/nontreated mice appeared rapidly, as early as day 9 after infection. Interestingly, Treg expansion was associated with viral spread in infected/nontreated mice, as shown here by flow cytometry of retroviral Env-expressing cells in thymi, spleens, and lymph nodes (Figure 1D) and as described previously23 by assay of plasma viremia. Specifically, Treg levels reached a maximum by days 14-19, depending on the organ, remained relatively stable for the period of analysis, and were correlated with high levels of cell infection in the 3 organs. Finally, an increase in Tregs was observed in the spleens and lymph nodes, but not in the thymi, when infected/nontreated mice were virally challenged on week 8 after infection (Figure 1E), raising the possibility of antigen-dependent induction of Treg proliferation in secondary lymphoid organs. Strikingly, no detectable induction of Tregs was seen in the thymi, spleens, or lymph nodes of infected/667-treated animals from day 7-35 after infection (Figure 1C). This was in contrast to infected/672-treated– and infected/667-F(ab′)2–treated mice behaving as infected/nontreated ones (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We conclude that inhibition of Treg expansion by 667 immunotherapy was a rapid, if not an immediate, event.
Treg response in FrCasE-infected mice is correlated with poor CTL activation
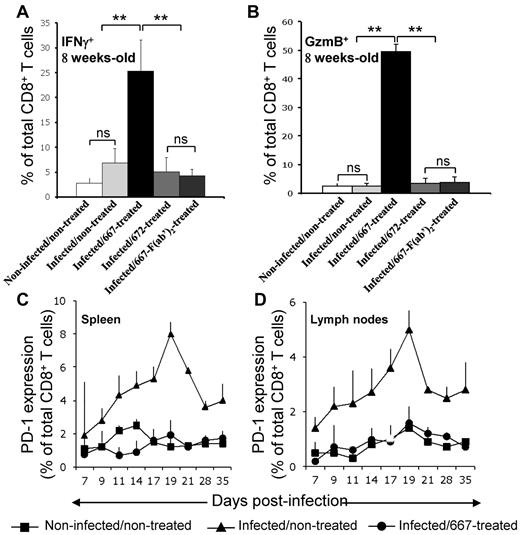
We then investigated whether Tregs in infected/nontreated mice contributed to infection chronicity and if a lack of Treg expansion in infected/667-treated animals was necessary for the generation of protective immunity. Having shown previously that the number of CTLs specific for FrCasE-infected cells was much lower in infected/nontreated than in infected/667-treated mice,21,24 we first assessed whether the levels of Tregs and the activation state of CTLs could be inversely correlated between these 2 groups of animals. The number of CTLs specific to infected cells being too low in infected/nontreated mice to conduct a rigorous analysis, we compared total splenic CD8+ T cells positive for the functional markers IFN-γ and GzmB in different groups of mice 8 weeks after infection. CD8+IFNγ+ and CD8+GzmB+ cell numbers were very low in noninfected/nontreated and infected/nontreated mice compared with infected/667-treated mice (Figure 2A-B). Consistent with a role for 667 effector functions in protective immunity induction, results for infected/672-treated and infected/667-F(ab′)2–treated mice were similar to those of infected/nontreated animals. Therefore, increased levels of Tregs not only associated with lower levels of CTLs directed at FrCasE-infected cells, but also with poorer CTL activation. We next investigated whether these features could be associated with increased levels of PD-1 because this marker is most often a negative regulator expressed at the surface of CD8+ T lymphocytes, where it promotes apoptosis and exhaustion.29 This was achieved at different times points after infection in spleens and lymph nodes (Figure 2C-D) because these organs showed the highest levels of induced Tregs in infected/nontreated mice (Figure 1C). Basal levels of PD-1+CD8+ T cells remained comparable in noninfected/nontreated and infected/667-treated animals for the entire duration of the experiment. In contrast, PD-1 was strongly induced in infected/nontreated mice with accumulation kinetics comparable to those of Tregs up to day 19 (at which time Treg numbers have already reached their plateau). Infected/672-treated and infected/667-F(ab′)2–treated mice behaved like infected/nontreated mice (supplemental Figure 2). Therefore, PD-1 expression levels are inversely correlated with the number of CD8+ T cells expressing IFNγ and GzmB in the various groups of animals analyzed, raising the possibility that Tregs exerted their effects on anti-FrCasE CTLs, at least in part via the induction of PD-1 in infected/nontreated mice (see “Discussion”).
CTL responses in infected/nontreated mice and infected/immunotherapy–treated animals. (A) Expression of IFNγ+ in CTLs. Mice were infected neonatally and immunotherapy treated as indicated and IFNγ was assayed by flow cytometry in CD8+ T cells in 8-week-old mice. Three mice per group were used in 3 independent experiments. Data are presented as means ± SEM. The statistical significance between groups was established using the Student t test. **P < .01. (B) Expression of GzmB (Gzm) in CTLs. Experiments were conducted as in panel A with an anti-Gzm antibody. Kinetic expression of PD-1 at the surface of CD8+ T cells in spleens (C) and lymph nodes (D). Mice were neonatally infected, 667 immunotherapy treated or not, and PD-1 was assayed by flow cytometry at the surface of CD8+ T cells at various time points. Values are the means ± SEM of 3 independent experiments conducted with 2 mice per time point. Data corresponding to 667-F(ab′)2– and 672-treated mice are presented in supplemental Figure 2.
CTL responses in infected/nontreated mice and infected/immunotherapy–treated animals. (A) Expression of IFNγ+ in CTLs. Mice were infected neonatally and immunotherapy treated as indicated and IFNγ was assayed by flow cytometry in CD8+ T cells in 8-week-old mice. Three mice per group were used in 3 independent experiments. Data are presented as means ± SEM. The statistical significance between groups was established using the Student t test. **P < .01. (B) Expression of GzmB (Gzm) in CTLs. Experiments were conducted as in panel A with an anti-Gzm antibody. Kinetic expression of PD-1 at the surface of CD8+ T cells in spleens (C) and lymph nodes (D). Mice were neonatally infected, 667 immunotherapy treated or not, and PD-1 was assayed by flow cytometry at the surface of CD8+ T cells at various time points. Values are the means ± SEM of 3 independent experiments conducted with 2 mice per time point. Data corresponding to 667-F(ab′)2– and 672-treated mice are presented in supplemental Figure 2.
Tregs repress both cellular and humoral anti-FrCasE immune responses in infected/nontreated mice
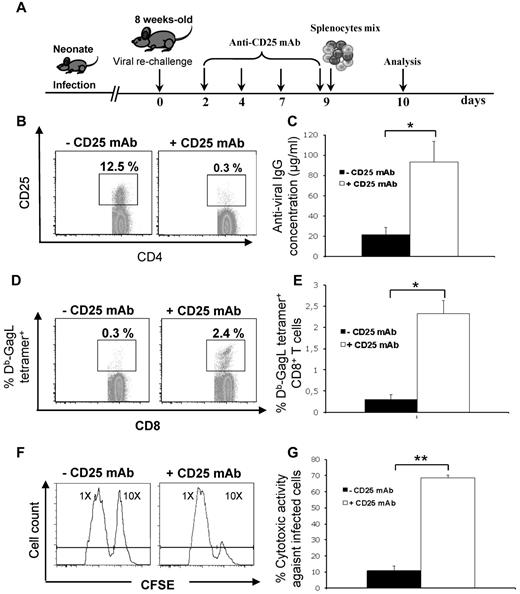
We also assessed the functional role of Tregs in the poor anti-FrCasE humoral and CTL responses in infected/nontreated mice. Mice were infected perinatally and rechallenged at 8 weeks of age before analysis of both types of responses (Figure 3A) in the presence or absence (Figure 3B) of Tregs, which were depleted via repeated IP administration of an anti-CD25 mAb (96% efficiency), as described previously.28 The anti-FrCasE humoral response was 5-fold more elevated in Treg-depleted mice compared with control animals (Figure 3C), was predominantly of the IgG2a isotype, and was as neutralizing as that found in infected/667-treated mice of the same age (not shown). Treg depletion restored humoral immunity in both infected/672-treated and infected/667-F(ab′)2–treated mice with endogenous IgG levels comparable to those observed in infected/667-treated mice (not shown). Anti-FrCasE CTL immunity being dependent on the immunodominant GagL epitope derived from the retroviral Gag protein precursor, we used a fluorescent Db-GagL tetramer to monitor it by flow cytometry.21,24 Treg depletion induced an 8-fold increase in the percentages of Db-GagL tetramer+CD8+ splenic cells (Figure 3D-E). Moreover, an in vivo cell-killing assay (see “Methods” and legend to Figure 3) showed that CTL activity against FrCasE-infected cells was 7-fold higher in Treg-depleted mice (Figure 3F-G). Consistent with the role of the 667 FcγR-binding effector function in the induction of protective immunity,23,24 infected/667-F(ab′)2–treated and infected/672-treated mice behaved similarly to infected/nontreated animals concerning both humoral and CTL responses (not shown). Therefore, Tregs induced by FrCasE in infected/nontreated mice inhibited the mounting of efficient antiviral humoral and cellular responses.
Restoration of the anti-FrCasE immune response in infected/nontreated mice on Treg depletion. (A) Experimental design. Mice were infected perinatally. At 8 weeks of age, they were rechallenged to facilitate immune response analysis, which was followed by Treg depletion by repeated injections of an anti-CD25 mAb. Anti-FrCasE IgGs and Db-GagL+CD8+ T cells were assayed 10 days after challenge. To assay the in vivo anti-FrCasE CTL activity, a mixture of splenocytes loaded with the GagL peptide or a control peptide was administered 9 days after challenge and death of GagL-loaded cells was assayed on the following day, as described in “Methods.” (B) Depletion of Tregs. Mice were treated as described in panel A. CD25highCD4+ T cells were assayed by flow cytometry analysis for FoxP3 positivity at the moment of immune response analysis. (C) Anti-FrCasE Ig concentrations. Antiviral IgG concentrations were assayed by ELISA in the sera of mice 10 days after rechallenge, as described in panel A. Three independent experiments with 5 mice per group were conducted. Data are presented as means ± SEM. The statistical significance between groups was established using the Student t test. *P < .05. (D-E) Flow cytometry assay of Db-GagL tetramer+CD8+ T cells in Treg-depleted and Treg-nondepleted infected/nontreated mice. Analyses were carried out 10 days after challenge as described in panel A. (D) Representative flow cytometry analysis of CD8+ splenocytes of an individual mouse. (E) Means ± SEM of 3 independent experiments with 5 mice per group. The statistical significance between groups was established using the Student t test. *P < .05. (F-G) In vivo assay of anti-FrCasE–infected cell CTL responses. FrCasE-infected splenocytes were labeled with the CFSE vital dye at a 10× concentration and splenocytes recovered from naive mice were labeled with CFSE at a 1× concentration. Both types of splenocytes were mixed in an approximately 1:1 ratio and the mixture was administered to Treg-depleted and Treg-nondepleted infected/nontreated mice on day 9 after challenge. Cell death was monitored on the following day by CFSE flow cytometry assay of splenocytes recovered from killed mice. (F) Individual mouse analysis. Left peaks correspond to noninfected splenocytes and right peaks to infected splenocytes. The decrease of the right peak in the presence of the anti-CD25 mAb is indicative of CTL activity against FrCasE-infected cells. (G) Means ± SEM of 3 independent experiments with 5 mice per group. The statistical significance between groups was established using the Student t test. **P < .01.
Restoration of the anti-FrCasE immune response in infected/nontreated mice on Treg depletion. (A) Experimental design. Mice were infected perinatally. At 8 weeks of age, they were rechallenged to facilitate immune response analysis, which was followed by Treg depletion by repeated injections of an anti-CD25 mAb. Anti-FrCasE IgGs and Db-GagL+CD8+ T cells were assayed 10 days after challenge. To assay the in vivo anti-FrCasE CTL activity, a mixture of splenocytes loaded with the GagL peptide or a control peptide was administered 9 days after challenge and death of GagL-loaded cells was assayed on the following day, as described in “Methods.” (B) Depletion of Tregs. Mice were treated as described in panel A. CD25highCD4+ T cells were assayed by flow cytometry analysis for FoxP3 positivity at the moment of immune response analysis. (C) Anti-FrCasE Ig concentrations. Antiviral IgG concentrations were assayed by ELISA in the sera of mice 10 days after rechallenge, as described in panel A. Three independent experiments with 5 mice per group were conducted. Data are presented as means ± SEM. The statistical significance between groups was established using the Student t test. *P < .05. (D-E) Flow cytometry assay of Db-GagL tetramer+CD8+ T cells in Treg-depleted and Treg-nondepleted infected/nontreated mice. Analyses were carried out 10 days after challenge as described in panel A. (D) Representative flow cytometry analysis of CD8+ splenocytes of an individual mouse. (E) Means ± SEM of 3 independent experiments with 5 mice per group. The statistical significance between groups was established using the Student t test. *P < .05. (F-G) In vivo assay of anti-FrCasE–infected cell CTL responses. FrCasE-infected splenocytes were labeled with the CFSE vital dye at a 10× concentration and splenocytes recovered from naive mice were labeled with CFSE at a 1× concentration. Both types of splenocytes were mixed in an approximately 1:1 ratio and the mixture was administered to Treg-depleted and Treg-nondepleted infected/nontreated mice on day 9 after challenge. Cell death was monitored on the following day by CFSE flow cytometry assay of splenocytes recovered from killed mice. (F) Individual mouse analysis. Left peaks correspond to noninfected splenocytes and right peaks to infected splenocytes. The decrease of the right peak in the presence of the anti-CD25 mAb is indicative of CTL activity against FrCasE-infected cells. (G) Means ± SEM of 3 independent experiments with 5 mice per group. The statistical significance between groups was established using the Student t test. **P < .01.
The absence of induced Tregs is necessary for protective immunity induced by 667 immunotherapy
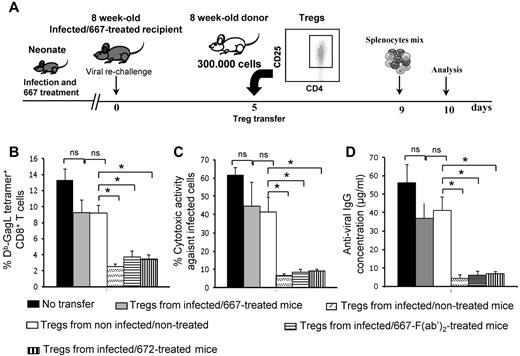
We then addressed whether the absence of induced Tregs in infected/667-treated mice was necessary for the induction of protective immunity by investigating whether Tregs from infected/nontreated mice could disrupt their anti-FrCasE immunity (see Figure 4A for the experimental scheme). Highly enriched Treg populations from 8-week-old infected/nontreated (78% ± 5% of CD4+CD25+FoxP3+ cells) and noninfected/nontreated mice (84% ± 3% of CD4+CD25+FoxP3+ cells) were prepared and administered to infected/667-treated mice of the same age, which were virally challenged 5 days earlier to optimize the analysis of humoral and CTL immune responses. In contrast to Tregs originating from noninfected/nontreated animals, which had only a moderate effect, those from infected/nontreated mice reduced: (1) the number of CTLs specific for FrCasE-infected cells (Figure 4B), (2) CTL activity against FrCasE-infected cells (Figure 4C), and (3) humoral immunity against FrCasE (Figure 4D). CTLs from infected/667-treated mice receiving Tregs from infected/nontreated animals also had reduced levels of IFNγ and GzmB (not shown). This was reminiscent of what happened in infected/nontreated mice, in which high Treg levels were associated not only with reduced levels of CTLs directed at infected cells, but also with a reduction in their activation state (Figure 2C-D). Underlining functional differences between Tregs from infected/nontreated and infected/667-treated mice, infected/667-treated mice grafted with Tregs purified from other infected/667-treated animals of the same age (82% ± 6% of CD4+CD25+FoxP3+ cells) behaved like those grafted with control Tregs from noninfected/nontreated mice (Figure 4B-D). Moreover, Tregs prepared from 8-week-old, infected/667-F(ab′)2–treated (79% ± 4% of CD4+CD25+FoxP3+ cells) or infected/672-treated mice (86% ± 5% of CD4+CD25+FoxP3+ cells) induced a strong and rapid inhibition of endogenous anti-FrCasE immunity developing in infected/667-treated mice (Figure 4B-D). Therefore, because none of the differences observed in these experiments was attributable to differences in Treg enrichment, our data indicate that Tregs from infected/nontreated mice and infected/667-treated animals show different functional properties: whereas those induced in infected/nontreated mice by FrCasE exerted strong suppressive activity on anti-FrCasE immunity, those present in infected/667-treated animals were tolerant to this immunity.
Disruption of anti-FrCasE immune response in infected/667-treated mice on transfer of Tregs from infected/nontreated mice. (A) Experimental design. Mice were infected neonatally and immunotherapy treated with 667. They were virally challenged at 8 weeks of age and subjected to adoptive transfer with purified Tregs (3 × 105 cells) from various origins before analysis of humoral and CTL immune responses, conducted as in Figure 3. Tregs were purified from 8-week-old noninfected/nontreated, infected/nontreated, infected/667-treated, infected/667-F(ab′)2–treated, or infected/672-treated mice. (B) Assay of GagL-specific CD8+ T cells. (C) In vivo assay of anti-FrCasE–infected cell CTL responses. (D) Assay of anti-FrCasE IgGs. For panels B, C and D, 3 independent experiments with 3 mice per group were conducted. Data are presented as means ± SEM. The statistical significance between groups was established using the Student t test. *P < .05.
Disruption of anti-FrCasE immune response in infected/667-treated mice on transfer of Tregs from infected/nontreated mice. (A) Experimental design. Mice were infected neonatally and immunotherapy treated with 667. They were virally challenged at 8 weeks of age and subjected to adoptive transfer with purified Tregs (3 × 105 cells) from various origins before analysis of humoral and CTL immune responses, conducted as in Figure 3. Tregs were purified from 8-week-old noninfected/nontreated, infected/nontreated, infected/667-treated, infected/667-F(ab′)2–treated, or infected/672-treated mice. (B) Assay of GagL-specific CD8+ T cells. (C) In vivo assay of anti-FrCasE–infected cell CTL responses. (D) Assay of anti-FrCasE IgGs. For panels B, C and D, 3 independent experiments with 3 mice per group were conducted. Data are presented as means ± SEM. The statistical significance between groups was established using the Student t test. *P < .05.
Tregs from infected/nontreated and infected/667-treated mice show phenotypic differences
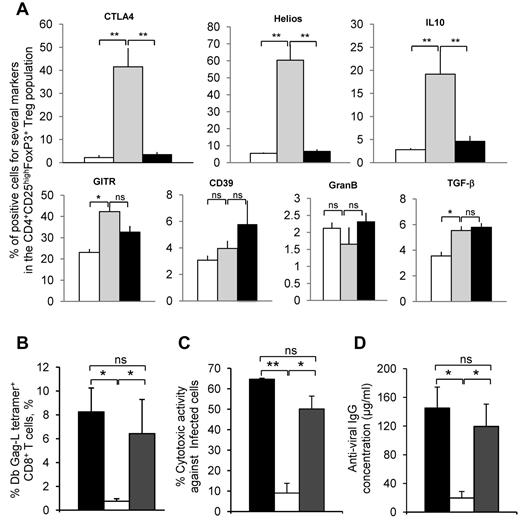
We then phenotyped CD4+CD25+FoxP3+ cells in further detail using well-described Treg phenotypical and functional markers (CTLA4, Helios, GITR, CD39, and GzmB) and the intracellular expression of the immunosuppressive cytokines TGF-β and IL-10 to identify functional differences between Tregs from infected/nontreated and infected/667-treated mice. For this, Tregs from 8-week-old noninfected/nontreated, infected/nontreated, or infected/667-treated mice purified from spleens were analyzed with flow cytometry after TCR stimulation. No major differences were observed for GITR, CD39, GzmB, or TGF-β (Figure 5A). In contrast, higher levels of CTLA4, Helios, and intracellular IL-10, 3 markers often associated with Treg activation and immunosuppressive activity,29-31 were clearly detected in Tregs from infected/nontreated mice, whereas the other 2 groups of mice showed comparable amounts (Figure 5A).
Phenotyping of Tregs from infected/nontreated and infected/667-treated mice and the role of IL-10 produced by Tregs from infected/nontreated mice. (A) Phenotyping of Tregs. Tregs were prepared from 8-week-old noninfected/nontreated, infected/nontreated, or infected/667-treated mice and subjected to TCR stimulation, as described in “Methods,” before flow cytometry analyses of the indicated proteins. Two independent experiments with 3 mice per group were conducted. Data are presented as means ± SEM. The statistical significance between groups was established using the Student t test. *P < .05; **P < .01. (B-D) Role of IL-10 in Tregs from infected/nontreated mice. Tregs were purified from 8-week-old infected/nontreated mice and grafted to infected/667-treated mice of the same age. These mice were virally challenged at the same time, as described in “Methods.” Half of the animals were subjected to IP administration of a neutralizing anti–mouse IL-10 mAb from day 5-10 after viral challenge/Treg grafting and the other half were left untreated. Immune response analyses were carried out on day 10 after grafting as in Figure 4, and the experiments were conducted as described in “Methods.” (B) Assay of GagL-specific CD8+ T cells. (C) In vivo assay of anti-FrCasE–infected cell CTL responses. (D) Assay of anti-FrCasE IgGs. Two independent experiments with 4 mice per group were conducted. Data are presented as means ± SEM. The statistical significance between groups was established using the Student t test. *P < .05; **P < .01.
Phenotyping of Tregs from infected/nontreated and infected/667-treated mice and the role of IL-10 produced by Tregs from infected/nontreated mice. (A) Phenotyping of Tregs. Tregs were prepared from 8-week-old noninfected/nontreated, infected/nontreated, or infected/667-treated mice and subjected to TCR stimulation, as described in “Methods,” before flow cytometry analyses of the indicated proteins. Two independent experiments with 3 mice per group were conducted. Data are presented as means ± SEM. The statistical significance between groups was established using the Student t test. *P < .05; **P < .01. (B-D) Role of IL-10 in Tregs from infected/nontreated mice. Tregs were purified from 8-week-old infected/nontreated mice and grafted to infected/667-treated mice of the same age. These mice were virally challenged at the same time, as described in “Methods.” Half of the animals were subjected to IP administration of a neutralizing anti–mouse IL-10 mAb from day 5-10 after viral challenge/Treg grafting and the other half were left untreated. Immune response analyses were carried out on day 10 after grafting as in Figure 4, and the experiments were conducted as described in “Methods.” (B) Assay of GagL-specific CD8+ T cells. (C) In vivo assay of anti-FrCasE–infected cell CTL responses. (D) Assay of anti-FrCasE IgGs. Two independent experiments with 4 mice per group were conducted. Data are presented as means ± SEM. The statistical significance between groups was established using the Student t test. *P < .05; **P < .01.
IL-10 is key immunosuppressor effector of Tregs in infected/nontreated mice
These data prompted us to investigate whether IL-10 produced by Tregs could play a key role in the poor immunity mounted by infected/nontreated animals. Tregs were purified from infected/nontreated mice and then grafted to 8-week-old infected/667-treated mice that were virally challenged at the same time (Figure 4A). Half of the animals were subjected to IP administration of a neutralizing anti–mouse IL-10 mAb from day 5 to day 10 after viral challenge/Treg grafting, at which time anti–IL-10–treated and nontreated mice were killed for analysis of immune response. Our data showed that: (1) the number of CTLs specific for FrCasE-infected cells (Figure 5B), (2) CTL activity against FrCasE-infected cells (Figure 5C), and (3) humoral immunity against FrCasE (Figure 5D) were maintained only in the presence of the anti–IL-10 mAb. This suggested that IL-10 was a key immunosuppressive effector of Tregs in infected/nontreated mice.
Discussion
Using the FrCasE model system, we showed previuosly that short antiviral mAb-based immunotherapies generate vaccine-like effects characterized by strong and long-lasting antiviral humoral and cytotoxic T-cell responses.20-24 This has potentially important therapeutical consequences for the treatment of patients infected with life-threatening chronic viruses. In the present study, we report that the induction of such a long-lasting, antiviral protective immunity relies critically on inhibition of the Treg response that is otherwise induced on infection and permits chronicity establishment and pathologic manifestations. Our findings confirm that manipulating Treg populations represents a necessary condition to confer robust and sustained antiviral immunity via mAb-based immunotherapy.
It is usually unclear when and how Tregs are generated in chronic infections and even whether they are the cause or the consequence of chronicity, especially in young infected organisms (with the exception of one report on immune response modulation by Tregs in HIV-exposed infants32 ). Our kinetic analyses in infected/nontreated mice in the present study indicate that Treg expansion is rapid, occurs during acute infection, and precedes resorption of this phase and establishment of FrCasE chronicity. This situation is reminiscent of that of FV33,34 (the infection model closest to FrCasE) and HSV neonatal infection, in which Tregs suppress infant antiviral CTL responses.35 Also reminiscent of FV, in which induction of Tregs is compartmentalized to sites of inflammation,36 FrCasE-induced Tregs principally expand in the lymph nodes and spleen, the main sites where viral replication occurs. Moreover, Tregs induced by FrCasE not only reduce the number and cytolytic activity of CTLs, but also alter their phenotype. Therefore, decreased expressions of IFNγ and GzmB were observed on CTLs at adult age, as was also described previously in the FV model,37 as well as increased levels of PD-1 at the end of the neonatal period. Although the latter observation is consistent with observations by others that PD-1 is expressed on exhausted CTLs in diverse chronic viral infection,38-40 it must be noted that an intact PD-1 pathway has also been found to enhance immunity during acute viral infection.41 This indicates that its role in infected/nontreated mice still requires further exploration, making it all the more important that PD-1 effects must be considered in combination with other markers of Tregs.29,42
Beyond the fact that they highlight similarities and differences between FV and the FrCasE retroviral systems, our present data rule out that Treg expansion is the consequence of infection chronicity leading to CTL exhaustion and loss of immune memory via continuous antigen stimulation, a possibility that has been raised elsewhere.43 Rather, several lines of evidence strongly suggest that Tregs induced during acute infection contribute to the maintenance of viral chronicity at later times through inhibition of the antiviral immune response. First, Tregs from infected/nontreated mice were more efficient at depressing the immune response of infected/667-treated animals in adoptive-transfer experiments than Tregs from noninfected/nontreated or infected/667 treated mice. Second, Tregs from infected/nontreated mice showed higher levels of Helios and CTLA-4, Treg markers for which high expression is usually associated with activation and suppressive potential.29,31 Third, we show that IL-10 produced by Tregs from infected/nontreated mice is a key factor necessary for these cells to exert an immunosuppressive action on grafting in infected/667-treated mice. Finally, depletion of Tregs in infected/nontreated mice led to the restoration of a strong anti-FrCasE response with both humoral and CTL arms. Similarly, results obtained in the FV model using transgenic mice (DEREG mice, which could not be used in our study because they were generated in the C57BL/6J genetic background that is resistant to FrCasE) in which Tregs could be selectively ablated showed that transient Treg depletion permitted exhausted CD8+ T cells to regain antiviral functions.44 Interestingly, transient depletion of Tregs via administration of an anti-CD25 mAb to FIV-infected cats was also shown to improve the antiviral CTL response but had no effect on the antiviral humoral response.28 Along with our present results, these data indicate that, despite possible species/system differences, the in vivo manipulation of Tregs may be used to strengthen immunotherapeutical approaches aiming at containing viral propagation and counteracting disease progression.20,22,24
The most striking observation of the present study was that the mAb-based immunotherapy rapidly inhibited the induction of Tregs normally associated with FrCasE infection. This inhibitory effect was sustained over time and was necessary for the emergence of protective humoral and CTL antiviral immunities, as shown by adoptive transfer of Tregs from infected/nontreated mice, which abrogated anti-FrCasE immunity in infected/667-treated animals. To our knowledge, this is the first study reporting such a Treg-inhibiting effect for an mAb-based immunotherapy not directly targeting this cell in the context of an antiviral or an anticancer treatment. Several nonexclusive mechanisms may explain the absence of induced Tregs in the presence of the 667 mAb. Because Treg expansion in infected/nontreated mice is a consequence of viral infection via still-to-be-elucidated mechanisms, a first possibility to consider is that limiting the amount of the agent (viral load) causing their induction by a neutralizing mAb may prevent Treg response induction. Supporting this idea, the prevalence of Tregs in lymphoid tissues has been shown to correlate with viral load45 and disease progression in HIV-infected patients.4 Although not excluding such a contribution, we do not feel that this passive mechanism is sufficient to explain the inhibition of Treg expansion. Because such an inhibitory effect is dependent on the effector function of 667, as demonstrated by the presence of an induced Treg response in infected/672-treated and infected/667-F(ab′)2–treated mice, an active mechanism(s) involving the formation of ICs between the neutralizing mAb and its cognate antigen appears more likely. We have previously reported a crucial role for the FcγR-binding function of 667 and ruled out a significant contribution for its complement-binding function in the induction of protective antiviral immunity.23,24 Moreover, we have also described previously a critical role for the interaction between DCs and ICs involving infected cells in this effect.24 It is therefore possible that enhanced activation of DCs by cellular ICs may not only favor the activation of antiviral CTLs, but also may tip the balance of CD4+ Th/Treg cells toward Treg differentiation. Also underlining a possible role for DCs in counteracting the appearance of induced Tregs, Balkow et al46 reported that, in the FV model, DC precursors are infected and do not fully mature into DCs, which is crucial for expansion of FoxP3+ Tregs. Should this also occur in FrCasE-infected/nontreated mice, it is likely that limitation of viral spread on the administration of 667 would also help to preserve DC function to generate an efficient antiviral response.
In conclusion, Treg-mediated dysfunction of immune effector cells is a matter of concern in the case of life-threatening chronic viral infections47 and in vivo manipulation of Tregs represents a new therapeutical challenge to strengthen antiviral immunity.48,49 In this context, the results of the present study suggest that, because of their immunomodulatory actions, mAb-based immunotherapies directed to viral antigens may constitute invaluable tools with which to combat the deleterious effect of Tregs in chronic viral infection. Because Tregs can suppress anticancer immune responses50 and because mAb immunotherapies targeting tumor cell-surface molecules may enhance anticancer immune responses,25,26 our findings may also have applications in the treatment of cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs E. Kremer, I. Robbins, and V. Dardahlon for critical reading of the manuscript.
This work was supported by the Centre National de la Recherche Scientifique and the Program Blanc of the Agence Nationale pour la Recherche. N.R. was supported by fellowships from the French Ministry of Higher Education and Research and from the Association pour la Recherche contre le Cancer. M.P's laboratory is an Equipe Labellisée of the French Ligue Nationale contre le Cancer.
Authorship
Contribution: R.N. and L.G. designed and performed the experiments; M. Pelegrin contributed to data interpretation and edited the manuscript; M. Plays performed the follow-up on the animals and some in vivo experimental procedures; and L.G. and M. Piechaczyk conceived the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for L.G. is Institut de Recherche en Cancérologie de Montpellier Université Montpellier 1, Montpellier, France.
Correspondence: Marc Piechaczyk, Equipe Labellisée par la Ligue contre le Cancer, Institut de Génétique Moléculaire de Montpellier, CNRS, 1919 route de Mende, 34293, Montpellier Cédex, France; e-mail: marc.piechaczyk@igmm.cnrs.fr; or Laurent Gros; e-mail: laurent.gros@inserm.fr.
References
Author notes
L.G. and M.P. are co–senior authors.