Key Points
Antimicrobial CD8+ MAIT cells are activated, exhausted, and progressively and persistently depleted during chronic HIV-1 infection.
This decline in MAIT cell level and function may seriously impair the ability to mount immune responses to bacterial and fungal pathogens.
Abstract
Mucosal-associated invariant T (MAIT) cells are an evolutionarily conserved antimicrobial MR1-restricted T-cell subset. MAIT cells are CD161+, express a Vα7.2 TCR, are primarily CD8+ and numerous in blood and mucosal tissues. However, their role in HIV-1 infection is unknown. In this study, we found levels of MAIT cells to be severely reduced in circulation in patients with chronic HIV-1 infection. Residual MAIT cells were highly activated and functionally exhausted. Their decline was associated with time since diagnosis, activation levels, and the concomitant expansion of a subset of functionally impaired CD161− Vα7.2+ T cells. Such cells were generated in vitro by exposure of MAIT cells to Escherichia coli. Notably, whereas the function of residual MAIT cells was at least partly restored by effective antiretroviral therapy, levels of MAIT cells in peripheral blood were not restored. Interestingly, MAIT cells in rectal mucosa were relatively preserved, although some of the changes seen in blood were recapitulated in the mucosa. These findings are consistent with a model in which the MAIT-cell compartment, possibly as a result of persistent exposure to microbial material, is engaged, activated, exhausted, and progressively and persistently depleted during chronic HIV-1 infection.
Introduction
HIV-1 infection is associated with a range of pathologic changes to the immune system, including systemic immune activation, CD4 T-cell loss and CD8 T-cell expansion. The state of broad and persistent immune activation develops early during infection,1,2 contributes to the rapid aging of the immune system seen during chronic progressive HIV-1 disease, and persists despite effective long-term virologic suppression by combination antiretroviral therapy (cART; reviewed in by Deeks,3 Appay et al,4 and Desai and Landay5 ). These pathologic processes lead to the progressive destruction of lymphoid organs and loss of CD4 helper T cells.6,7 Already during primary infection, HIV-1 depletes intestinal CD4 T cells and disrupts the structure and function of the intestinal immune system.8-13 One consequence of this is increased permeability of the intestinal epithelium with translocation of microbial products into the local tissue, the portal circulation, the liver and eventually into systemic circulation.14 This process may continue despite effective long-term cART.15,16 Disruption of immune homeostasis and barrier function at the mucosa is a considerable challenge for the host immune system because the microbial proteins, carbohydrates, and lipids form a range of antigens that will engage innate as well as adaptive immune mechanisms (reviewed by Brenchley and Douek17 ). Despite considerable advances in the treatment and management of HIV-1 disease, certain infections still present a significant clinical challenge particularly among HIV-infected individuals who are diagnosed at advanced stages, those who lack access to antiretroviral therapy, and those who cannot maintain adherence to therapy and clinical care.18-20 This includes an increased risk of developing bacterial pneumonia even in HIV-1–infected patients with relatively normal CD4 counts,21 indicating that impaired CD4 T-cell independent control of certain infections still exists even in the context of treated HIV-1 disease.
Mucosal-associated invariant T (MAIT) cells are a relatively recently discovered subset of unconventional, innate-like T cells that are highly abundant in mucosal tissues, liver, and peripheral blood.22-25 Human MAIT cells express an invariant T-cell receptor (TCR) carrying the Vα7.2 α-chain segment, a restricted Vβ repertoire (Vβ2 or Vβ13), and recognize antigens in complex with the evolutionarily conserved MHC-Ib–related protein (MR1).24,25 In addition to the Vα7.2 TCR segment, MAIT cells are defined by the expression of CD161, the IL-18 receptor (IL-18R), and the transcription factor ZBTB16, also known as PLZF.26-28 The majority of MAIT cells are CD8+, expressing either CD8αα or CD8αβ, with minor CD4+ or CD8/4 double-negative populations.26-29
MAIT cells recognize and respond to diverse bacterial and fungal-derived products presented by MR1 molecules expressed on antigen presenting cells (APCs), and they do this independently of the TLR pathways.27 A recent study indicated that the complementarity determining region (CDR) 1α, CDR2α, and CDR3α of the Vα7.2 TCR are crucial for MAIT TCR recognition of a variety of enteric bacteria irrespective of the Vβ usage.30 Furthermore, a very recent study indicated that MR1-presented antigens are composed of microbial vitamin metabolites.31 After antigen recognition, MAIT cells rapidly respond by secretion of both proinflammatory and tissue-protective cytokines, such as IFNγ, TNF, IL-17, and IL-22, in an innate-like manner.22,23,27,28 MAIT cells contribute to protection against certain mycobacterial and enterobacterial infections in mice, and may play a significant role in Mycobacterium tuberculosis infection in humans.27,32-34
The role of MAIT cells in HIV-1 infection is currently unknown. In this study, we examined the levels and characteristics of MAIT cells in circulation as well as in rectal mucosa in patients with chronic HIV-1 infection. Our findings support a model whereby the MAIT-cell compartment, possibly as a result of persistent exposure to microbial material, is engaged, activated, exhausted, and progressively and persistently depleted during chronic HIV-1 infection. These findings are interpreted and discussed in the context of mechanisms of HIV immunopathogenesis and consequences for control of microbial infections in HIV-1–infected patients.
Methods
Participants
HIV-1–infected patients were from the Karolinska University Hospital Huddinge Infectious Diseases Outpatient Clinic (Stockholm, Sweden), and from the Study of the Consequences of the Protease Inhibitor Era (SCOPE), San Francisco General Hospital (SFGH), or were referred by collaborating clinicians at either the University of California, Davis (UC Davis) or the University of California, San Francisco (UCSF). Patients had no history of AIDS-defining illness in the 12 months before recruitment. Healthy HIV-uninfected individuals were recruited at the Blood Transfusion Clinic at the Karolinska University Hospital Huddinge and at the SFGH. Written informed consent was obtained from all individuals in accordance with study protocols conforming to the provisions of the Declaration of Helsinki and approved by the Regional Ethics Review Board in Stockholm and the Institutional Review Board, School of Medicine, UC Davis, and the Committee on Human Subjects Research, UCSF.
Peripheral blood and rectal biopsy tissue processing
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood by Ficoll-Hypaque density gradient centrifugation (Pfizer-Pharmacia or Axis-Shield), and either rested overnight in complete medium, or cryopreserved in liquid nitrogen. Rectal biopsy tissue was obtained at 10 to 20 cm from the anal verge by flexible sigmoidoscopy.35-37 Briefly, 20 to 25 tissue pieces (∼ 3 mm diameter) were collected during each procedure and placed in complete RPMI 1640 supplemented with 15% fetal calf serum (R15 medium), and immediately transported to UC Davis for processing and analysis. Rectal mononuclear cells (RMCs) were isolated from biopsy specimens after 3 washes with R15 medium and then underwent 3 rounds of digestion in 0.5 mg/mL collagenase type II (Sigma-Aldrich) at 37°C with agitation. Each digestion was followed by disruption of the tissue by passing through a syringe with a 16-gauge blunt end needle, followed by a 70-μm cell strainer. RMCs were then washed in R15 to remove collagenase and allowed to rest overnight (37°C, 5% CO2) in R15 containing 0.5 mg/mL piperacillin-tazobactam (Zosyn; Wyeth Pharmaceuticals).
Antibodies
Anti-CD3 FITC, anti-CD3 and anti-CD69 Alexa Fluor 700, anti-CD3 and anti-CD4 Pacific Blue, anti-CD161 PECy5, anti-CD38 and anti-TNF PECy7, anti-CD27 and anti-HLA-DR APC-H7, anti-CD127 Alexa Fluor 647, and anti-IFNγ APCs were from BD Bioscience. Anti-CD4 ECD, and IOTest Beta Mark Kit for TCR Vβ analyses were from Beckman Coulter. Anti-Vα7.2 FITC and PE (clone 3C10), anti-CD8α Brilliant Violet 570, anti-CD57 Pacific Blue, and anti-Ki67 and anti–IL-17A Brilliant Violet 421 were from BioLegend. Anti–TIM-3 Alexa Fluor 488, anti–IL-18R PE, and anti-PLZF APC (clone 6318100) were from R&D Systems. Anti-CD4 Qdot 705, anti-CD8 Qdot 565, and live/dead aqua fixable cell stain were from Invitrogen. Anti–Vα7.2-biotin (a kind gift from Dr Olivier Lantz, Institut Curie, Paris, France), was visualized with streptavidin Qdot 585 (Invitrogen). Anti–MR1 mAb (clone 26.5) was kindly provided by Dr Ted Hansen (School of Medicine, Washington University, St Louis, MO).
In vitro infection and cell activation
Escherichia coli strain D21 was cultured overnight at 37°C in Luria broth and counted with the standard plate counting method. Bacteria were washed once in PBS and fixed in 1% paraformaldehyde for 5 minutes and then washed extensively before feeding to the PBMC culture at a multiplicity of infection (MOI) of 6 in the presence of 1.25 μg/mL anti-CD28 mAb (BD Bioscience). Cells were cultured in round-bottom 96-well plates at 106 cells/well and stimulated overnight, and monensin (Golgi Stop, BD Bioscience) was added for the last 6 hours of incubation. In selected experiments, monocytes were purified from fresh buffy coats using RosetteSep monocyte enrichment cocktail (StemCell Technologies), and MAIT cells were purified from freshly isolated PBMCs using anti-Vα7.2 PE antibody followed by anti–PE-microbeads (Miltenyi Biotec) and positive selection using MACS Cell Separation (Miltenyi Biotec). The purity of both enriched monocytes and Vα7.2+ T cells were > 80%. In selected experiments, MAIT cells were purified from MACS-enriched Vα7.2+ T cells by FACS-sorting (FACSAria, BD Bioscience). Monocytes were then fed PFA-fixed E coli at indicated MOIs for 3 hours, followed by the addition of Vα7.2+ T cells or MAIT cells at a 2:1 Vα7.2+/MAIT cell:monocyte ratio. Cells were then cultured for 1 to 3 days in complete medium.
Flow cytometry
Cell-surface staining was performed using directly conjugated or biotinylated antibodies in PBS containing 2% FCS and 2mM EDTA (FACS buffer) for 20 minutes on ice. For the latter, cells were washed once in cold FACS buffer followed by 20 minutes incubation with streptavidin Qdot 585 on ice. Cells were then washed once in FACS buffer and fixed in Cytofix/Cytoperm (BD Bioscience). Intracellular staining was performed after surface staining, fixation, and 1 wash in Perm/Wash buffer (BD Bioscience) using relevant intracellular mAbs in Perm/Wash on ice for 30 minutes. Cells were washed once in Perm/Wash before data acquisition. Samples were acquired on an LSRFortessa flow cytometer (BD Bioscience) equipped with 405, 488, 561, and 639 nm lasers. Single-stained polystyrene beads (BD Bioscience) were used for compensation purposes. Software-based compensation was performed in FlowJo Version 9 software (Tree Star).
Statistical analyses
Significant differences in independent samples were assessed using Fisher exact test for categorical variables, and t test or Mann-Whitney test for continuous variables as appropriate. Paired t test or Wilcoxon signed rank test was used to determine significance between paired samples. The Kruskal-Wallis test followed by Mann-Whitney test was used to detect differences across multiple samples. Rectal MAIT-cell frequencies were predicted from peripheral blood MAIT-cell frequency using simple linear regression on log10-transformed data to meet the normal distribution assumption. Correlations were evaluated using Spearman rank correlation. Statistical analyses were performed using Prism Version 5 software (GraphPad) and P values < .05 were considered significant.
Results
MAIT cells are persistently depleted in chronic HIV-1 infection
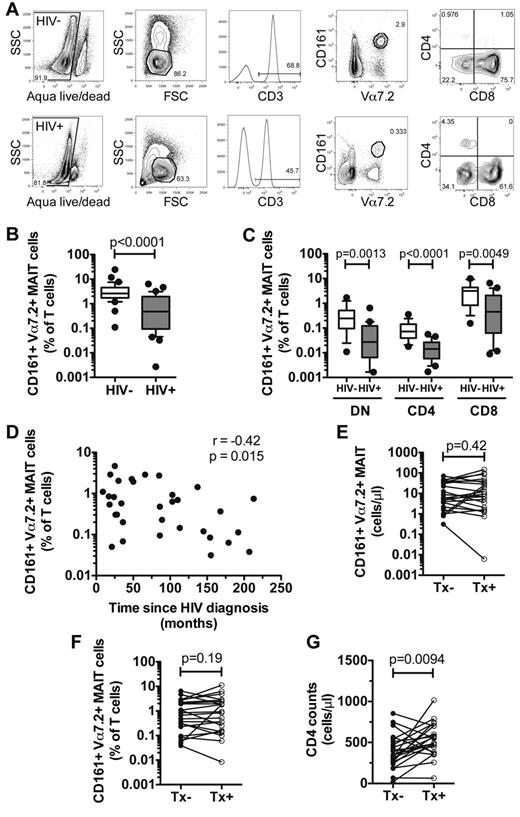
We initially determined the frequency of Vα7.2+ CD161+ MAIT cells in peripheral blood of 34 uninfected control subjects and 33 patients with chronic untreated HIV-1 infection (Figure 1A; Table 1). Levels of MAIT cells in HIV-1 patients were sharply lower compared with those of the uninfected controls (HIV+ = 1.09% ± 0.26%, HIV− = 3.92% ± 0.75%, P < .0001; Figure 1B). As previously observed,26-29 the majority of MAIT cells in blood consisted of CD8+ and CD4− CD8− (DN) subsets. We observed the presence of a CD4+ subset in all donors tested, albeit at a much smaller proportion. All MAIT-cell subsets were significantly lower in the HIV-1–infected patients (DN MAIT P = .0013; CD4 MAIT P < .0001; CD8 MAIT P = .0049; Figure 1C). In agreement with this, we did not observe significant redistribution of DN, CD4, and CD8 subsets within the total peripheral blood MAIT-cell population (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The loss of MAIT cells was associated with the time since HIV-1 diagnosis, which is a surrogate marker of time since infection (r = −0.42, P = .015; Figure 1D).
MAIT cells are depleted in the peripheral blood of chronically HIV-infected patients. (A) Identification of peripheral blood MAIT cells in uninfected and HIV-infected individuals. The frequency of total MAIT cells (B), and DN, CD4, and CD8 subsets (C) was determined in 34 HIV-1–uninfected individuals and 33 patients with chronic untreated HIV-infection. (D) The relationship between MAIT-cell frequency and time since HIV diagnosis was assessed using Spearman rank correlation. The numbers (E) and frequency (F) of MAIT cells, and CD4 T-cell counts (G) were compared in 22 HIV-infected patients before and after commencing cART. Box and whisker plots show median, IQR and the 10th to 90th percentile. Tx indicates therapy.
MAIT cells are depleted in the peripheral blood of chronically HIV-infected patients. (A) Identification of peripheral blood MAIT cells in uninfected and HIV-infected individuals. The frequency of total MAIT cells (B), and DN, CD4, and CD8 subsets (C) was determined in 34 HIV-1–uninfected individuals and 33 patients with chronic untreated HIV-infection. (D) The relationship between MAIT-cell frequency and time since HIV diagnosis was assessed using Spearman rank correlation. The numbers (E) and frequency (F) of MAIT cells, and CD4 T-cell counts (G) were compared in 22 HIV-infected patients before and after commencing cART. Box and whisker plots show median, IQR and the 10th to 90th percentile. Tx indicates therapy.
Details of HIV-1 patients and healthy controls
| . | Stockholm cohort . | San Francisco cohort . | ||||
|---|---|---|---|---|---|---|
| HIV− . | HIV+ . | P . | HIV− . | HIV+ . | P . | |
| n | 34 | 33 | 9 | 11 | ||
| Age, y‡ | 37.5 (30-51.3) | 39 (34.5-47) | .94* | 46 (45-50.2) | 47 (28-52) | .56* |
| Sex, n (%) | 16 M (47.1%) | 21 M (63.6%) | .51* | 9 M (100%) | 9 M (81.8%) | .48* |
| CD4 counts, cells/μL | ND | 446 ± 54 | ND | 494 ± 58 | .21† | |
| Viral loads, copies/mL | NA | 78 739 ± 31 580 | NA | 137 000 ± 100 005 | .46† | |
| Time since HIV diagnosis, mo | NA | 85 ± 11 | NA | 110 ± 30 | .34† | |
| . | Stockholm cohort . | San Francisco cohort . | ||||
|---|---|---|---|---|---|---|
| HIV− . | HIV+ . | P . | HIV− . | HIV+ . | P . | |
| n | 34 | 33 | 9 | 11 | ||
| Age, y‡ | 37.5 (30-51.3) | 39 (34.5-47) | .94* | 46 (45-50.2) | 47 (28-52) | .56* |
| Sex, n (%) | 16 M (47.1%) | 21 M (63.6%) | .51* | 9 M (100%) | 9 M (81.8%) | .48* |
| CD4 counts, cells/μL | ND | 446 ± 54 | ND | 494 ± 58 | .21† | |
| Viral loads, copies/mL | NA | 78 739 ± 31 580 | NA | 137 000 ± 100 005 | .46† | |
| Time since HIV diagnosis, mo | NA | 85 ± 11 | NA | 110 ± 30 | .34† | |
ND indicates not done; NA, not applicable; and M, male.
Comparisons were done on uninfected versus HIV-infected patients.
Comparisons were done on HIV-infected patients recruited in Stockholm versus San Francisco.
Median (IQR); mean ± SD shown for all parameters unless otherwise specified.
We next examined whether long-term virologic control by cART could restore the frequency and numbers of peripheral blood MAIT cells in 22 patients with chronic HIV-1 infection. We measured MAIT cells just before cART initiation and again after a median of 52 (inter-quartile range [IQR]: 39-70) months on treatment and found no significant increase in numbers (P = .19; Figure 1E), or frequency (P = .42; Figure 1F), despite significant increase in CD4 counts (P = .0094; Figure 1G). Similarly, no significant change was observed in any of the MAIT-cell subsets after initiation of cART (supplemental Figure 1B-C, respectively), except for DN MAIT numbers that were lower after long-term cART (P = .0031, supplemental Figure 1C). There was no correlation between percentage and number of MAIT cells and CD4 counts or viral loads (supplemental Figure 1D-E). We also investigated the numbers and proportion of MAIT cells in 2 elite controller patients in the cohort who were not receiving cART. These elite controller patients had been HIV-1–seropositive for a mean of 177 (153-207) months and had a mean CD4 count of 1149 cells/μL (533-1764 cells/μL) with undetectable plasma viral loads (< 20 copies/mL), and displayed MAIT-cell levels similar to those of uninfected controls (mean = 2.17%, 1.08%-3.25%). Taken together, these data indicate that peripheral blood MAIT cells are persistently depleted in chronic HIV-1 infection. Their loss is associated with the duration since HIV diagnosis and they do not recover after long-term cART.
MAIT-cell responses to E coli are impaired in chronic HIV-1 infection and partly recover during cART
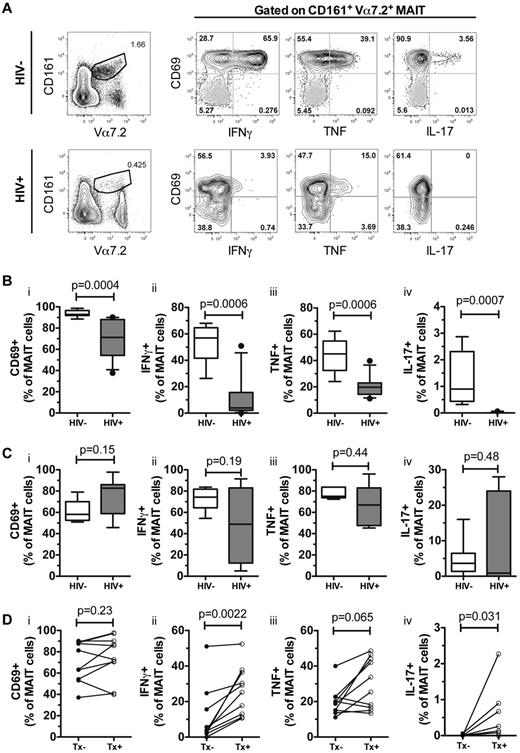
We next investigated whether MAIT-cell activation and cytokine production after bacterial stimulation is affected in chronic HIV-1 infection. We stimulated PBMCs from selected patients who maintained sufficient numbers of MAIT cells (n = 10), with fixed E coli in the presence of anti-CD28, and measured MAIT-cell activation and cytokine production (Figure 2A). In uninfected healthy individuals, we observed that MAIT cells almost universally expressed CD69 after overnight E coli stimulation, and expressed high levels of IFNγ and TNF, but had relatively weak production of IL-17 (n = 10, Figure 2B). MAIT-cell cytokine production was markedly reduced in the presence of anti-MR1 mAb (supplemental Figure 2), supporting the notion that MAIT-cell activation is MR1-dependent. HIV-1–infected patients, however, displayed significantly lower MAIT-cell activation as assessed by CD69 up-regulation (P = .0004), and IFNγ (P = .0006), TNF (P = .0006), and IL-17 (P = .0007) production after overnight E coli + anti-CD28 stimulation (Figure 2Bi-iv). In contrast, MAIT-cell activation and cytokine production after 6 hours of PMA/ionomycin stimulation was not significantly different between the groups (Figure 2Ci-Civ).
MAIT cells are functionally impaired in chronically HIV-infected patients. (A) The gating strategy for fixed Escherichia coli–activated MAIT cells in blood from HIV-1–uninfected and HIV-infected individuals. Gray dots represent unstimulated controls from the same donors. CD69, IFNγ, TNF, and IL-17 expression were determined in 10 uninfected and untreated HIV-infected individuals after an overnight stimulation with fixed E coli (B) and a 6 hour PMA/ionomycin stimulation (C). MAIT-cell activation and cytokine production from these HIV-infected patients were then assessed using an overnight bacterial stimulation after commencing cART (D). Box and whisker plots show median, IQR, and the 10th to 90th percentile. Tx indicates therapy.
MAIT cells are functionally impaired in chronically HIV-infected patients. (A) The gating strategy for fixed Escherichia coli–activated MAIT cells in blood from HIV-1–uninfected and HIV-infected individuals. Gray dots represent unstimulated controls from the same donors. CD69, IFNγ, TNF, and IL-17 expression were determined in 10 uninfected and untreated HIV-infected individuals after an overnight stimulation with fixed E coli (B) and a 6 hour PMA/ionomycin stimulation (C). MAIT-cell activation and cytokine production from these HIV-infected patients were then assessed using an overnight bacterial stimulation after commencing cART (D). Box and whisker plots show median, IQR, and the 10th to 90th percentile. Tx indicates therapy.
After long-term (median 64 months, IQR 28-84) effective virologic control by cART, MAIT-cell IFNγ, and IL-17 production in response to E coli stimulus was partially restored (Figure 2Dii-iv), but remained lower compared with uninfected controls. In contrast, we did not observe significant recovery of CD69 up-regulation or TNF production after long-term cART (Figure 2Di-iii). Taken together, these results indicate that chronic HIV-1 infection leads to defective MAIT-cell activation and cytokine production, which is only partially restored by long-term cART.
High levels of MAIT-cell immune activation are associated with MAIT-cell loss
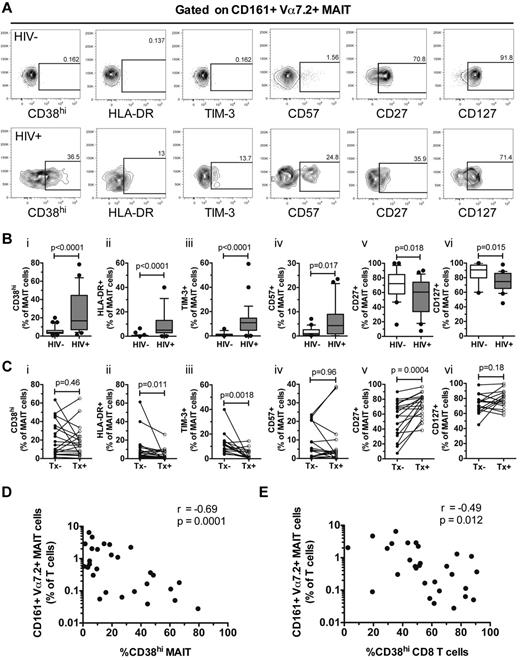
MAIT cells in blood from healthy donors expressed low levels of CD38, HLA-DR, and TIM-3, but these markers were significantly increased in chronically HIV-infected patients (CD38hiP < .0001; HLA-DR P < .0001; TIM-3 P < .0001; Figure 3A and Bi-iii). Long-term effective cART significantly decreased HLA-DR and TIM-3 expression (P = .011 and .0018, respectively), but CD38 expression remained elevated (P = .46; Figure 3Ci-iii). In addition, CD57 expression was increased in MAIT cells from HIV+ donors (P = .017; Figure 3Biv), and this was not changed by treatment (P = .96; Figure 3Civ). In contrast, the percentage of MAIT cells expressing the costimulatory molecule CD27 and CD127 (IL-7Rα) were reduced in HIV-infected individuals (CD27 P = .018; CD127 P = .015; Figure 3Bv-vi). CD27 expression recovered after long-term cART (P = .0004), but CD127 expression remained lower (Figure 3Cv-vi).
MAIT cells express activation phenotypes in chronically HIV-infected patients. (A) The gating strategy for CD38hi, HLA-DR, TIM-3, CD27, CD57, and CD127 expression on MAIT cells in HIV-1–uninfected and HIV-infected individuals. The expression of these markers was determined in 33 uninfected and 29 untreated HIV-infected individuals (B), and in 20 HIV-infected individuals before and after commencing cART (C). Spearman rank correlation between CD38hi-expressing MAIT cells (D) and total CD8 T cells (E) with the frequency of MAIT cells in the peripheral blood of 29 untreated HIV-infected individuals. Box and whisker plots show median, IQR and the 10th and 90th percentile. Tx indicates therapy.
MAIT cells express activation phenotypes in chronically HIV-infected patients. (A) The gating strategy for CD38hi, HLA-DR, TIM-3, CD27, CD57, and CD127 expression on MAIT cells in HIV-1–uninfected and HIV-infected individuals. The expression of these markers was determined in 33 uninfected and 29 untreated HIV-infected individuals (B), and in 20 HIV-infected individuals before and after commencing cART (C). Spearman rank correlation between CD38hi-expressing MAIT cells (D) and total CD8 T cells (E) with the frequency of MAIT cells in the peripheral blood of 29 untreated HIV-infected individuals. Box and whisker plots show median, IQR and the 10th and 90th percentile. Tx indicates therapy.
We next investigated the relationship between the expression of immune activation markers and the loss of MAIT cells in the HIV-infected patient cohort. MAIT-cell levels correlated inversely with CD38 expression on MAIT cells as well as on total CD8 T cells (r = −0.69, P = .0001 and r = −0.49, P = .012, respectively; Figure 3D-E). There was no association between CD38 expression on peripheral blood MAIT cells in HIV-infected patients and CD4 counts (r = 0.07, P = .73), viral loads (r = 0.21, P = .31) or time since HIV-1 diagnosis (r = 0.28, P = .16). Taken together, our data indicate that MAIT cells in peripheral blood are highly activated and exhibit abnormal phenotypes in chronic HIV-1 infection.
Accumulation of CD161− Vα7.2+ T cells
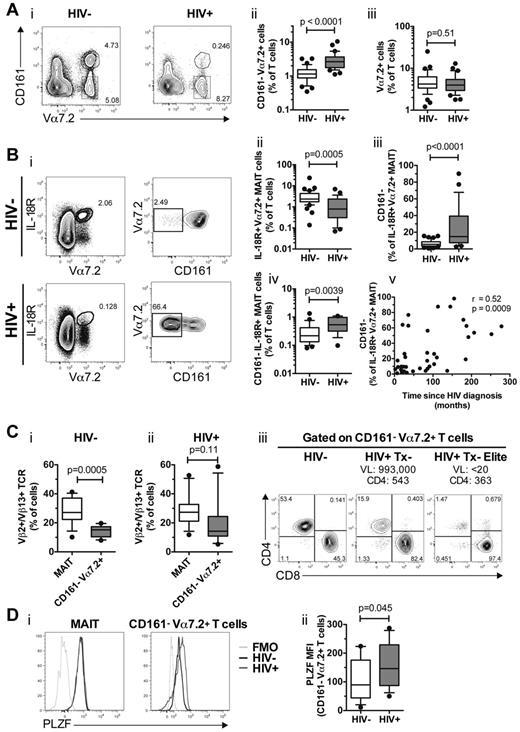
In detailed analysis of the data, we observed that the CD161− Vα7.2+ T-cell population was significantly increased in HIV-infected patients compared with uninfected controls (P < .0001; Figure 4Ai-ii). Interestingly, the total percentage of Vα7.2-expressing CD3+ cells was not significantly different in peripheral blood between the HIV-infected patients and uninfected controls (P = .51; Figure 4Aiii), suggesting that the accumulation of CD161− Vα7.2+ T cells in chronic HIV-1 infection is not simply because of the disappearance of CD161+ Vα7.2+ MAIT cells. Furthermore, CD161− Vα7.2+ T cells in both uninfected and HIV-infected individuals lacked expression of Ki67, suggesting this is a nonproliferating subset (data not shown).
Accumulation of CD161− Vα7.2+ T cells in the peripheral blood of chronically HIV-infected patients. (A) Identification and frequency of CD161− Vα7.2+ T cells in 34 HIV-1–uninfected and 33 untreated HIV-infected individuals. The frequency of total Vα7.2+ T cells is shown in subpanel iii. (B) Peripheral blood MAIT cells that were defined as IL-18R+ Vα7.2+ T cells (Bi left), and the proportion of IL-18R+ Vα7.2+ MAIT cells that did not express CD161 (Bi right) was determined in HIV-1 uninfected and HIV-1 infected individuals (iii). The frequency of CD161− IL-18R+ Vα7.2+ MAIT cells as a percentage of CD3+ T cells is shown in subpanel iv. The relationship between proportion of IL-18R+ Vα7.2+ MAIT cells that did not express CD161 and time since HIV diagnosis was assessed using Spearman rank correlation (v). (C) The frequency of Vβ2/Vβ13.1/Vβ13.2/Vβ13.6+ CD161+ Vα7.2+ MAIT cells and CD161− Vα7.2+ T cells in 13 uninfected controls and 12 HIV-infected patients (i-ii). The CD4 and CD8 distribution of CD161− Vα7.2+ T cells in a representative uninfected control, a viremic, and an elite controller HIV-infected patient (iii). (D) A representative figure of the transcription factor PLZF expression in CD161+ Vα7.2+ MAIT (Di left) and CD161− Vα7.2+ T cells (Di right). Black lines denote uninfected control and gray lines untreated HIV-infected patient. Light gray lines denote FMO controls. The PLZF mean fluorescence intensity (MFI) from 12 uninfected controls and 19 HIV-infected patients is shown in subpanel ii. Box and whisker plots show median, IQR and the 10th to 90th percentile. VL indicates viral load; and Tx, therapy.
Accumulation of CD161− Vα7.2+ T cells in the peripheral blood of chronically HIV-infected patients. (A) Identification and frequency of CD161− Vα7.2+ T cells in 34 HIV-1–uninfected and 33 untreated HIV-infected individuals. The frequency of total Vα7.2+ T cells is shown in subpanel iii. (B) Peripheral blood MAIT cells that were defined as IL-18R+ Vα7.2+ T cells (Bi left), and the proportion of IL-18R+ Vα7.2+ MAIT cells that did not express CD161 (Bi right) was determined in HIV-1 uninfected and HIV-1 infected individuals (iii). The frequency of CD161− IL-18R+ Vα7.2+ MAIT cells as a percentage of CD3+ T cells is shown in subpanel iv. The relationship between proportion of IL-18R+ Vα7.2+ MAIT cells that did not express CD161 and time since HIV diagnosis was assessed using Spearman rank correlation (v). (C) The frequency of Vβ2/Vβ13.1/Vβ13.2/Vβ13.6+ CD161+ Vα7.2+ MAIT cells and CD161− Vα7.2+ T cells in 13 uninfected controls and 12 HIV-infected patients (i-ii). The CD4 and CD8 distribution of CD161− Vα7.2+ T cells in a representative uninfected control, a viremic, and an elite controller HIV-infected patient (iii). (D) A representative figure of the transcription factor PLZF expression in CD161+ Vα7.2+ MAIT (Di left) and CD161− Vα7.2+ T cells (Di right). Black lines denote uninfected control and gray lines untreated HIV-infected patient. Light gray lines denote FMO controls. The PLZF mean fluorescence intensity (MFI) from 12 uninfected controls and 19 HIV-infected patients is shown in subpanel ii. Box and whisker plots show median, IQR and the 10th to 90th percentile. VL indicates viral load; and Tx, therapy.
We next used IL-18R as an alternative marker that, in combination with Vα7.2, also identifies the MAIT-cell population.26,27 In agreement with the results shown in Figure 1B, the IL-18R+ Vα7.2+ MAIT cells were significantly reduced in blood of HIV-infected individuals (P = .0005, Figure 4Bi-ii). We next investigated whether there was a selective loss of CD161 expression in the MAIT-cell population in HIV-infected patients. Interestingly, although the majority of IL-18R+ Vα7.2+ MAIT cells in uninfected control subjects coexpressed CD161, a significant proportion of IL-18R+ Vα7.2+ MAIT cells in HIV-infected patients had lost their CD161 expression (P < .0001; Figure 4Biii). In line with this, we observed an increased frequency of CD161− IL-18R+ Vα7.2+ MAIT cells in the HIV-infected patients (P = .0039; Figure 4Biv). The loss of CD161 expression within the IL-18R+ Vα7.2+ MAIT-cell population was significantly associated with time since HIV diagnosis (r = 0.52, P = .0009; Figure 4Bv).
We next examined the TCR Vβ2/Vβ13 usage in CD161− Vα7.2+ T cells to investigate the possibility that CD161− Vα7.2+ T cells in HIV-infected patients are enriched by MAIT cells that have down-regulated CD161 expression. In 13 uninfected controls, CD161+ Vα7.2+ MAIT cells had a preferential TCR Vβ2 and Vβ13 usage (28.1% ± 2.45%; Figure 4Ci) in agreement with a recent study.28 However, CD161− Vα7.2+ T cells had no Vβ2/Vβ13 bias and their Vβ usage was significantly different from MAIT cells (14.34% ± 1.11%, P = .0005; Figure 4Ci), and they also had a similar CD4/CD8 distribution compared with total CD3+ T cells (Figure 4Ciii). In contrast, in 12 HIV-infected patients there was no difference in Vβ2/Vβ13 usage between CD161+ Vα7.2+ MAIT cells and CD161− Vα7.2+ T cells (P = .11; Figure 4Cii). In addition, the CD4/CD8 distribution in the CD161− Vα7.2+ T-cell population in both progressor and elite controller HIV-infected patients resembled that of MAIT cells (Figure 4Ciii).
Human MAIT cells express the transcription factor PLZF (ZBTB16).26,28 CD161+ Vα7.2+ MAIT cells from HIV-1 uninfected and infected individuals equally expressed PLZF (Figure 4Di left panel). CD161− Vα7.2+ T cells from uninfected controls had low PLZF expression, similar to Vα7.2− T cells (Figure 4Di right panel and not shown). However, CD161− Vα7.2+ T cells from HIV-infected patients had significantly higher PLZF expression (P = .045; Figure 4Dii). Together, these data suggest that a CD161− Vα7.2+ T-cell population expands, in the absence of proliferation, in patients with chronic HIV-1 infection.
CD161− Vα7.2+ T cells are hyper-activated and can be generated by E coli stimulation
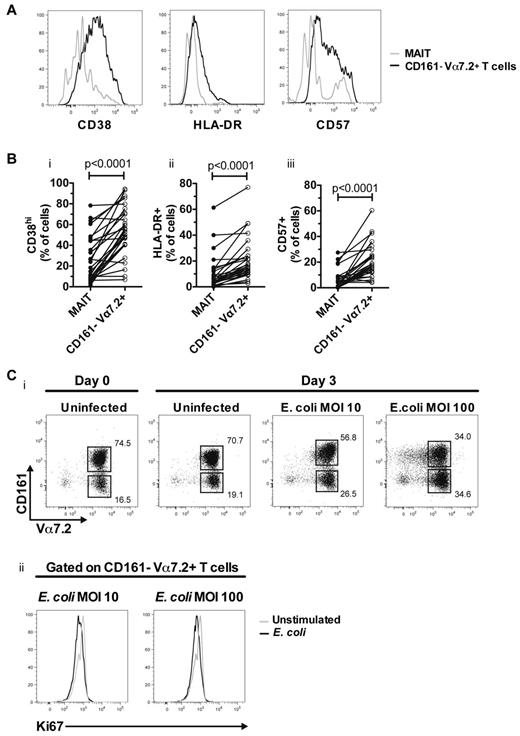
Given the elevated levels of activation in MAIT cells in HIV-1 infected subjects, we were next interested to assess the expression of activation markers in the CD161− Vα7.2+ T-cell population in blood. These cells were highly activated and expressed significantly higher levels of CD38, HLA-DR, and CD57 compared with MAIT cells from the same patients (n = 29, all P < .0001; Figure 5A-B). Furthermore, the CD161− Vα7.2+ T cells showed no detectable IFNγ, TNF, or IL-17 production in response to overnight stimulation with E coli (data not shown).
CD161− Vα7.2+ T cells in chronic HIV-1 patients are highly activated and can be generated by stimulation with E coli. (A) Representative CD38, HLA-DR and CD57 staining on MAIT (gray line) and CD161− Vα7.2+ T cells (black line) from the same HIV-1 patient. (B) The expression of these markers was determined in 29 untreated HIV-infected individuals. (C) Vα7.2+ cells were purified by MACS-sorting and cocultured for 3 days with autologous unstimulated or fixed E coli–fed monocytes at indicated MOI (i). Ki67 expression was determined in CD161− Vα7.2+ T cells (ii). Gray lines represent unstimulated and black lines represent fixed E coli–fed cultures. A representative figure from 3 independent HIV-1–uninfected donors is shown.
CD161− Vα7.2+ T cells in chronic HIV-1 patients are highly activated and can be generated by stimulation with E coli. (A) Representative CD38, HLA-DR and CD57 staining on MAIT (gray line) and CD161− Vα7.2+ T cells (black line) from the same HIV-1 patient. (B) The expression of these markers was determined in 29 untreated HIV-infected individuals. (C) Vα7.2+ cells were purified by MACS-sorting and cocultured for 3 days with autologous unstimulated or fixed E coli–fed monocytes at indicated MOI (i). Ki67 expression was determined in CD161− Vα7.2+ T cells (ii). Gray lines represent unstimulated and black lines represent fixed E coli–fed cultures. A representative figure from 3 independent HIV-1–uninfected donors is shown.
Because MAIT cells respond to diverse microbial products, and partially down-regulate CD161 after in vitro E coli stimulation of PBMCs (Figure 2A), we next investigated whether CD161− Vα7.2+ T cells can be generated from MAIT cells after bacterial stimulation in vitro. CD161− Vα7.2+ T cells expanded during a 3 days culture with relatively high levels of E coli (Figure 5Ci), in the absence of Ki67 expression (Figure 5Cii), and overt T-cell death (as determined by Trypan-blue exclusion assay; data not shown). Furthermore, in MAIT cells purified by FACS-sorting, there was clear down-regulation of CD161 already at day 1 of culture with E coli (supplemental Figure 3). Taken together, these data suggest that CD161− Vα7.2+ T cells have a hyper-activated phenotype in chronic HIV-1 infection, and that these cells may be derived from MAIT cells as a consequence of exposure to bacterial products.
MAIT cells in rectal mucosa show similar changes as in blood but are numerically better preserved
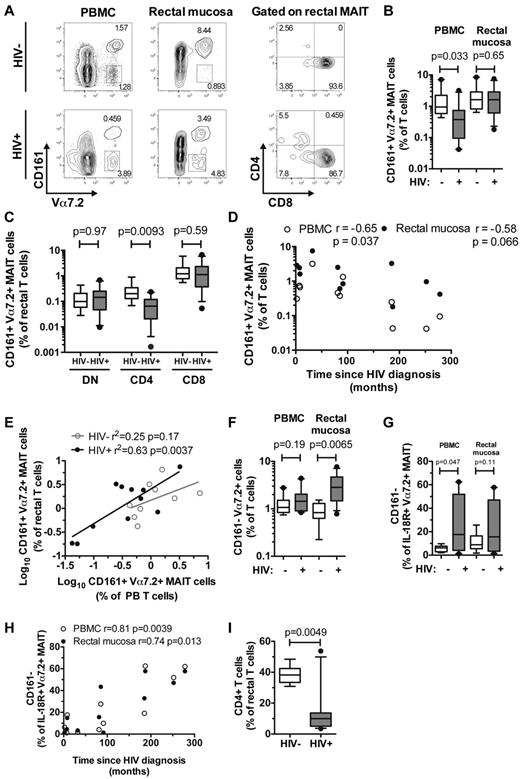
Because of the central role of the intestinal immune system in HIV pathogenesis, we next investigated the MAIT-cell population in rectal mucosa of 9 HIV-uninfected controls and 11 chronically HIV-infected patients recruited at the SFGH (Table 1; Figure 6A). Interestingly, there was no significant loss of MAIT cells from the rectal mucosa as a percentage of total T cells in HIV-1–infected patients (P = .65; Figure 6B), despite a significant reduction in peripheral blood MAIT cells (P = .033; Figure 6B, in agreement with Figure 1B). Although rectal DN and CD8 MAIT-cell subsets were unaffected in HIV-infected patients (P = .97 and .59, respectively; Figure 6C), there was a significant decrease in rectal CD4 MAIT-cell frequency compared with uninfected controls (P = .0093; Figure 6C). Similar to the pattern in the Stockholm cohort, there was an inverse correlation between time since HIV diagnosis and levels of MAIT cells in blood (r = −0.65, P = .037; Figure 6D, in agreement with Figure 1D), and this correlation was also present in rectal MAIT cells (r = −0.58, P = .066; Figure 6D).
MAIT cells and CD161− Vα7.2+ T cells in rectal mucosa of uninfected and HIV-infected individuals. Identification, frequency, and distribution of CD4 and CD8 subsets of rectal MAIT cells in 9 HIV-1–uninfected and 11 HIV-infected individuals (A-C). Spearman correlation between peripheral blood and rectal MAIT cells with time since HIV diagnosis (D), and a simple linear regression analysis of the relationship between frequency of blood MAIT cells and rectal MAIT cells (both are log10-transformed) in uninfected and infected individuals (E). The frequency of CD161− Vα7.2+ T cells (F) and IL-18R+ Vα7.2+ MAIT cells that did not express CD161 (G) in blood and rectal mucosa of HIV-1 infected and uninfected individuals. (H) Spearman correlation between the proportion of IL-18R+ Vα7.2+ MAIT cells that did not express CD161 and time since HIV diagnosis. (I) Frequency of CD4 T cells in the rectal mucosa. Open circles represent peripheral blood and closed circles represent rectal mucosa. Box and whisker plots show median, IQR and the 10th to 90th percentile.
MAIT cells and CD161− Vα7.2+ T cells in rectal mucosa of uninfected and HIV-infected individuals. Identification, frequency, and distribution of CD4 and CD8 subsets of rectal MAIT cells in 9 HIV-1–uninfected and 11 HIV-infected individuals (A-C). Spearman correlation between peripheral blood and rectal MAIT cells with time since HIV diagnosis (D), and a simple linear regression analysis of the relationship between frequency of blood MAIT cells and rectal MAIT cells (both are log10-transformed) in uninfected and infected individuals (E). The frequency of CD161− Vα7.2+ T cells (F) and IL-18R+ Vα7.2+ MAIT cells that did not express CD161 (G) in blood and rectal mucosa of HIV-1 infected and uninfected individuals. (H) Spearman correlation between the proportion of IL-18R+ Vα7.2+ MAIT cells that did not express CD161 and time since HIV diagnosis. (I) Frequency of CD4 T cells in the rectal mucosa. Open circles represent peripheral blood and closed circles represent rectal mucosa. Box and whisker plots show median, IQR and the 10th to 90th percentile.
Because MAIT cells are depleted in the blood of HIV-infected patients, we next predicted that the levels of MAIT cells in peripheral blood might be associated with the levels of rectal MAIT cells. This was indeed the case (r2 = 0.63, P = .0037; Figure 6E), although this prediction did not hold up in uninfected control subjects (r2 = 0.25, P = .17; Figure 6E). Levels of CD161− Vα7.2+ T cells were increased in the rectal mucosa (P = .0065; Figure 6F), but not in blood, of these 11 HIV-infected patients (P = .19; Figure 6F). There was a significant increase of IL-18R+ CD161− Vα7.2+ MAIT cells in peripheral blood in the SFGH HIV cohort (P = .047; Figure 6G), and a trend toward a similar pattern in the rectal mucosa (P = .11; Figure 6G). Furthermore, a significant correlation between the proportion of IL-18R+ CD161− Vα7.2+ MAIT cells and the time since HIV diagnosis was present in both blood (r = 0.81, P = .0039), and rectal mucosa (r = 0.74, P = .013) in these patients (Figure 6H). As expected, the overall CD4 T-cell frequency was significantly lower in rectal mucosa of the HIV-infected patients compared with uninfected control subjects (P = .0049; Figure 6I). These data are consistent with a model where MAIT cells in the rectal mucosa are better preserved than MAIT cells in blood during chronic HIV-1 infection.
Discussion
MR1-restricted MAIT cells are believed to be an important and evolutionarily conserved component of the T-cell response against microbes. In this study, we investigated this T-cell population in HIV-1 infection and found that levels of MAIT cells are severely reduced in circulation in patients with chronic untreated HIV-1 infection. Residual MAIT cells are highly activated and functionally exhausted. The decline of the MAIT population is associated with time since diagnosis, activation levels, and the concomitant expansion of hyper-activated CD161− Vα7.2+ T cells. Interestingly, this cell population can be generated in vitro by culture in the presence of E coli. Notably, whereas the function of residual MAIT cells is restored by cART, the levels of these cells in peripheral blood are not. Finally, we observed that MAIT cells in rectal mucosa are relatively preserved compared with peripheral blood, although some of the changes seen in blood are recapitulated in the mucosa.
The decline in MAIT-cell level and function in chronic HIV-1 infection may be a serious insult to the ability to mount immune responses to microbial pathogens. MAIT cells respond rapidly to a range of microbes that are clinically relevant in HIV-1 infection, including M tuberculosis and Candida albicans.27,32,33,38 Because of the breadth of responsiveness to many microbes, MAIT-cell exhaustion may affect immunity to most of these, irrespective of which microbes are involved in generating this exhausted state. The apparent polymicrobial specificity of this lymphocyte population thus can become a problem for the host. For example, mycobacterial infections represent a severe clinical challenge in untreated HIV-1 infection. The decline in MAIT cells may affect the ability to control mycobacterial as well as other opportunistic infections. It should be noted that we observed no significant recovery of MAIT-cell numbers in blood after several years of effective cART. This observation adds yet another argument for initiating cART early during HIV-1 infection. In contrast to the lack of numerical recovery, MAIT-cell function recovered at least partly on cART. Thus, treatment benefits this immune cell compartment even if the effects are modest. It is unclear how rapid the insult to the MAIT-cell compartment may be during primary HIV-1 infection, and if early treatment may rescue MAIT-cell numbers as well as their function. Future studies should address these issues.
MAIT-cell levels in rectal mucosa appear to be better preserved compared with those in blood. One possible reason for this could be recruitment of MAIT cells to intestinal mucosa from blood. Interestingly, MAIT-cell levels are reduced in the blood, but enriched in the lungs, of individuals with active M tuberculosis infection.27,32 Already in early HIV-1 infection the mucosal immune system and mucosal barrier functions are compromised,8-13 and MAIT-cell recruitment is a plausible way for the immune system to try to compensate for the lack of CD4 helper T cells to defend barrier integrity in the HIV-1 infected mucosa. As MAIT cells are primarily CD8+ they may be more resilient in this environment. Interestingly, whereas in blood there is no preferential loss of any subset of MAIT cells, CD4+ MAIT cells appear to be preferentially lost from rectal mucosa. In addition, despite the observation that MAIT-cell levels are not significantly lower in rectal mucosa in HIV-1 infected subjects than in uninfected controls, the levels of mucosal MAIT cells correlate with levels of MAIT cells in blood. In addition, MAIT-cell levels in rectal mucosa tended to correlate inversely with time since HIV diagnosis. This overall pattern is consistent with a model where MAIT cells are eventually lost at this mucosal site, but significant losses may only be seen after peripheral blood MAIT cells are severely depleted. In fact, the severe loss of CD4 T cells in rectal mucosa may lead to an underestimation of the degree of MAIT-cell loss at this site. Further studies will be required to ascertain if this is the case.
Our data indicate that MAIT-cell loss in blood is paired and linked with the appearance of a CD161− Vα7.2+ population of cells. We observe that MAIT cells express elevated levels of several activation markers in HIV-1–infected patients. The loss of MAIT cells correlates with the activation levels of MAIT cells, and the CD161− Vα7.2+ T-cell population express even higher levels of activation markers. Furthermore, T cells with a CD161− Vα7.2+ phenotype are generated by culture of MAIT cells with E coli. These observations are consistent with a model where continuous exposure to bacterial antigens contributes to the exhaustion and depletion of MAIT cells from circulation in HIV-1–infected patients. The exhausted MAIT cells seem, however, to persist at least partly in the form of the CD161− Vα7.2+ T-cell population. Interestingly, in NK cells CD161 has been shown to be down-regulated by engagement by its ligand, the lectin-like transcript 1 (LLT1).39 The observation that LLT1 can be induced in APCs on exposure to microbes opens a possibility for further study, as well as for therapeutic intervention.40 The identification of possible means to restore the function and MAIT character of these cells by immunotherapy is a high priority. Our observation that at least some of the CD161− Vα7.2+ T cells retain expression of PLZF and IL-18R suggests that cytokines should be evaluated for their ability to rescue these cells. Interestingly, the loss of CD161 expression in IL-18R+ Vα7.2+ MAIT cells in the rectal mucosa correlated directly with time since HIV diagnosis, suggesting that it might be possible to restore the rectal MAIT population by immunotherapeutic intervention.
MAIT cells respond to E coli stimulation by high-level production of IFNγ and TNF, and they also express some IL-17. However, the residual MAIT-cell population in chronically HIV-1–infected patients show severely suppressed production of IFNγ and TNF, and IL-17 is undetectable. In particular the loss of IL-17 production is striking and may have important implications given the role of this cytokine in defense against extracellular pathogens.41 The mechanisms of this unresponsiveness is at present unclear, but the cells appear to largely retain the ability to respond to PMA/ionomycin, suggesting that the defect may reside in the triggering and signal transduction pathway. The functionality of the residual MAIT-cell population recovers partly on effective cART, supporting this notion. We observe alterations in surface expression of several activation-associated receptors in MAIT cells in HIV-1–infected patients, and most of these do not revert when patients start cART. However, the levels of the inhibitory TIM-3 receptor decrease and the costimulatory CD27 increase after long-term effective cART. Future studies should evaluate the role that these receptors may play in MAIT-cell dysfunction in HIV-1 infection. Another possible avenue to explore in this regard is the functionality of APCs in HIV-1–infected subjects with regard to MR1-mediated antigen presentation and expression of costimulatory ligands. Numerous immune evasion pathways have been described for HIV-1 including down-modulation of phagocytosis,42-44 and the interference with MHC class I and CD1d antigen presentation.45,46 In particular, the parallel with the CD1d-restricted invariant natural killer T cells is interesting as this population shares many similarities with MAIT cells and displays a similar functional impairment in HIV-1–infected patients.47
In summary, we found that the MAIT-cell compartment is severely compromised in chronic HIV-1 infection. Given the size of this T-cell subset and their role in recognition of bacterial and fungal pathogens, it is reasonable to believe that this observation may have clinical significance. The relative preservation of MAIT cells in rectal mucosa is interesting and may suggest preferential homing to the intestinal mucosal barrier. Future studies should aim to address this possibility, to examine the role of MAIT cells in the control of opportunistic pathogens, to investigate the mechanisms of MAIT-cell exhaustion, as well as the ability of early cART to rescue the MAIT-cell compartment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Olivier Lantz for the biotinylated Vα7.2 TCR mAb (3C10), and Dr Ted Hansen for the MR1 mAb (26.5). The authors also thank Salah Zangenah for technical assistance.
This research was supported by grants from the Swedish Research Council (J.K.S., M.M.), the Swedish Cancer Foundation (J.K.S.), the Stockholm County Council (J.K.S., A.S., J.A.). Furthermore, research performed at UC Davis was supported by the NIH/NIAID grant R01-AI057020 (B.L.S.). The SCOPE cohort was supported in part by NIH/NIAID (R01-AI087145, K24-AI069994), the UCSF CFAR (P01-AI027763), the UCSF CTSI (UL1-RR024131), the Cleveland Immunopathogenesis Consortium (R01-AI076174), and the CFAR Network of Integrated Systems (R24-AI067039). E.L. is a Swedish Institute Postdoctoral Fellow. J.K.S. is a Senior Research Fellow of the Swedish Research Council.
National Institutes of Health
Authorship
Contribution: E.L. and J.K.S. conceived and designed the experiments, and wrote the paper; E.L. and A.G. performed the experiments and analyzed data; M.F.Q., M.M., M.S., A.S., S.G.D., J.N.M., and P.W.H. identified patients, acquired and processed critical samples; B.L.S. provided critical material, supervised work at UC Davis, and wrote the paper; M.M., A.S., J.A., M.F.Q., and P.W.H. contributed to paper preparation; and JKS supervised the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edwin Leeansyah or Johan Sandberg, Center for Infectious Medicine, Dept of Medicine, Karolinska Institutet Karolinska University Hospital Huddinge, F59, 14186 Stockholm, Sweden; e-mail: edwin.leeansyah@ki.se or johan.sandberg@ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal