Abstract
Mast cell leukemia (MCL) is a very rare form of aggressive systemic mastocytosis accounting for < 1% of all mastocytosis. It may appear de novo or secondary to previous mastocytosis and shares more clinicopathologic aspects with systemic mastocytosis than with acute myeloid leukemia. Symptoms of mast cell activation—involvement of the liver, spleen, peritoneum, bones, and marrow—are frequent. Diagnosis is based on the presence of ≥ 20% atypical mast cells in the marrow or ≥ 10% in the blood; however, an aleukemic variant is frequently encountered in which the number of circulating mast cells is < 10%. The common phenotypic features of pathologic mast cells encountered in most forms of mastocytosis are unreliable in MCL. Unexpectedly, non-KIT D816V mutations are frequent and therefore, complete gene sequencing is necessary. Therapy usually fails and the median survival time is < 6 months. The role of combination therapies and bone marrow transplantation needs further investigation.
Introduction
Mastocytosis is a heterogeneous group of disorders characterized by the abnormal growth and accumulation of mast cells (MCs) in one or more organ systems.1,2 Diagnostic criteria and classification were recently updated by the WHO.1 This classification defines 7 disease variants (Table 1): cutaneous mastocytosis, indolent systemic mastocytosis (ISM), systemic mastocytosis (SM) with an associated clonal hematologic non–MC-lineage disease (SM-AHNMD), aggressive SM (ASM), MC leukemia (MCL), MC sarcoma, and extracutaneous mastocytoma. SM is defined by major and minor criteria. The diagnosis is established if at least one major and one minor or at least 3 minor criteria are present (Table 2). SM is subclassified according to MC burden, involvement of non-MC lineages, and aggressiveness of the disease (C findings).1,3 The clinical course is variable, ranging from asymptomatic with normal life expectancy to highly aggressive with poor prognosis.4-6
WHO classification of mastocytosis
| Variant . | Subvariants . |
|---|---|
| Cutaneous mastocytosis | Urticaria pigmentosa |
| Maculopapular | |
| Diffuse | |
| Solitary mastocytoma of skin | |
| Indolent systemic mastocytosis | Smoldering SM |
| Isolated bone marrow mastocytosis | |
| Systemic mastocytosis with an associated clonal hematologic non–MC-lineage disease | SM-AML |
| SM-MDS | |
| SM-MPD | |
| SM-CEL or SM-HES | |
| SM-CMML | |
| SM-NHL | |
| Aggressive systemic mastocytosis | |
| MC leukemia | |
| MC sarcoma | |
| Extracutaneous mastocytom |
| Variant . | Subvariants . |
|---|---|
| Cutaneous mastocytosis | Urticaria pigmentosa |
| Maculopapular | |
| Diffuse | |
| Solitary mastocytoma of skin | |
| Indolent systemic mastocytosis | Smoldering SM |
| Isolated bone marrow mastocytosis | |
| Systemic mastocytosis with an associated clonal hematologic non–MC-lineage disease | SM-AML |
| SM-MDS | |
| SM-MPD | |
| SM-CEL or SM-HES | |
| SM-CMML | |
| SM-NHL | |
| Aggressive systemic mastocytosis | |
| MC leukemia | |
| MC sarcoma | |
| Extracutaneous mastocytom |
Adapted from Swerdlow et al.1
MC indicates mast cell; SM, systemic mastocytosis; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasia; CEL, chronic eosinophilic leukemia; HES, hypereosinophilic syndrome; CMML, chronic myelomonocytic leukemia; and NHL, non-Hodgkin lymphoma.
WHO diagnostic criteria (2008) for systemic mastocytosis
| Criteria . |
|---|
| Major |
| Multifocal, dense infiltrates of MC (≥ 15 MC in aggregates) in BM and/or other extracutaneous organ(s) |
| Minor |
| (1) > 25% of MC in the infiltrate of biopsy sections are spindle-shaped or have atypical morphology or, of all MC in the BM aspirate smears, > 25% are immature or atypical |
| (2) Activating point mutation at codon 816 of c-KIT in BM, blood, or another extracutaneous organ |
| (3) MC in BM, blood, or extracutaneous organs express CD2 and/or CD25 in addition to normal MC markers |
| (4) Total serum tryptase persistently exceeds 20 ng/mL* |
| Criteria . |
|---|
| Major |
| Multifocal, dense infiltrates of MC (≥ 15 MC in aggregates) in BM and/or other extracutaneous organ(s) |
| Minor |
| (1) > 25% of MC in the infiltrate of biopsy sections are spindle-shaped or have atypical morphology or, of all MC in the BM aspirate smears, > 25% are immature or atypical |
| (2) Activating point mutation at codon 816 of c-KIT in BM, blood, or another extracutaneous organ |
| (3) MC in BM, blood, or extracutaneous organs express CD2 and/or CD25 in addition to normal MC markers |
| (4) Total serum tryptase persistently exceeds 20 ng/mL* |
Diagnosis of systemic mastocytosis requires the presence of the major criterion and 1 minor criterion or at least 3 minor criteria.
MC indicates mast cell; and BM, bone marrow.
Serum tryptase is not valid as a criterion if there is an associated clonal myeloid disorder.
MCL is a very rare form of ASM, involving < 0.5% of all mastocytosis patients in the French Reference Center for Mastocytosis (CEREMAST). It is characterized by the leukemic spread of MCs, with frequent and multiple organ involvement such as the liver, peritoneum, spleen, bone, and marrow. MCL shares more clinical and biologic aspects with ASM than with acute myeloid leukemia (AML).7 MCL diagnosis requires the presence of SM criteria with additional features including leukemic infiltration of bone marrow (BM) and/or blood by at least 20% high-grade MC1 as well as the infiltration of extracutaneous organs by neoplastic MC. The threshold > 20% of bone marrow mast cells (BMMCs) should be confirmed by cytologic analysis of BM smears thus avoiding false-positive diagnosis if the count is done on BM biopsies. “An aleukemic” form of MCL is frequent. This diagnosis is made if the number of circulating MCs is < 10%.1 Thus, a cytologic analysis of aspiration smear preparations, from both blood and marrow, is required to make a MCL diagnosis.7
MCL can occur de novo or secondary to SM. Lim et al, in a large cohort of 342 patients with adult SM,6 reported an overall risk of transformation of 6%, in the majority of cases to AML (86%), or to MCL (13%). Transformation occurred most often from SM-AHNMD or ASM and was correlated to advanced age, history of weight loss, anemia, thrombocytopenia, hypoalbuminemia, and an excess of BM blasts.
A comprehensive immunophenotyping study has shown that an immature BMMC phenotype (CD25+/FceR+lo/FSClo/SSClo/CD45lo) in the absence of coexisting normal MCs in the bone marrow, is correlated with multilineage hematopoietic involvement by the KIT D816V mutation which might be associated with higher risk of progression to a more aggressive disease.8 Nevertheless, the prognostic significance of these observations for predicting clinical outcome in patients with SM requires further investigation. Therapeutic options are limited and the disease is most often fatal within a few months.6,7,9
We performed a literature search using the PubMed database for all proven MCL cases, according to the WHO criteria,1 and propose here a review based on all published MCL cases since 1950 found, with 4 additional personal unpublished cases. Therefore, this review includes 51 MCL cases; in 41 cases, we had extensive clinical and biologic data which allowed us to analyze both de novo MCL (n = 30) as well as secondary MCL (n = 11) including a few cases with a previous history of pediatric mastocytosis (n = 4).
Epidemiology
MCL was described as early as 1906 by Joachim.10 It is a very rare subcategory of SM accounting for approximately 1% of American adult SM,6 and < 0.5% of all French cases of mastocytosis reported in the CEREMAST database.
The median patient age at diagnosis was 52 years (range, 5-76 years) with a female predominance (sex ratio, F/M, of 1.5). Ethnic origin was available for 21 cases and consisted of 18 white and 3 Asian patients. No familial cases have been reported so far. Eleven patients (27%) had a history of mastocytosis and were considered as secondary MCL; whereas 30 patients (73%) were considered as having de novo MCL.
In this cohort, de novo MCL patients were older than patients with secondary MCL with a median age at diagnosis of, respectively, 51 years and 35 years. This apparent younger age in the secondary MCL group is because of the 4 patients with pediatric mastocytosis which had evolved into MCL. After exclusion of these 4 pediatric cases, the median age of both groups at MCL diagnosis was 51.5 years. Women presented more frequently with de novo MCL (60%). Ethnic origin was not different between the MCL subgroups, both mostly composed of whites (85%). After a short follow-up, 66% of the patients had died with a median survival time of 6 months (1-98 months).
Clinical manifestations
MC activation symptoms (MCAS) were frequently observed (Table 3). They included flushes (60%), fever (52%), malaise (36%), diarrhea (28%), and tachycardia (20%). Pollakiuria (7%), neuropsychiatric symptoms (6%), and severe prefracturar osteoporosis (6%) were rare. Many patients suffered from asthenia (78%), severe weight loss of > 10% of total body weight (38%), and anorexia (20%). Symptoms of MC activation appeared more frequent among patients without KIT D816V mutation (85%) than in the group with this mutation (61%).
Clinical and biologic characteristics of MCL patients: literature review
| . | MCL . | MCL with pediatric mastocytosis, n = 4 . | ||
|---|---|---|---|---|
| All, N = 51 . | De novo, n = 30 . | Secondary, n = 11 . | ||
| Median age at diagnosis, y (range) | 52 (5-76) | 51.5 (18-76) | 35 (5-75) | 24 (5-53) |
| Female/Male | 30/20 | 18/11 | 5/6 | 3/1 |
| Ethnicity, n (%) | ||||
| White | 18/21 (86) | 13/15 (87) | 5/6 (83) | 2 (50) |
| Asiatic | 3/21 (14) | 2/15 (13) | 1/6 (16) | 1 (25) |
| Familial mastocytosis, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Median time from symptoms to diagnosis, mo (range) | 5 (0-108) | 3 (0-24) | 6 (0-108) | 4.5 (0-108) |
| History of mastocytosis, n (%) | 11/40 (27) | 11 (100) | 4 (100) | |
| Aleukemic MCL, n (%) | 29/47 (62) | 15 (50) | 7 (70) | 4 (100) |
| Organ involvement, n (%) | ||||
| Skin, UP | 15/50 (30) | 4 (14) | 5 (45) | 4 (100) |
| Hepatomegaly | 32/47 (68) | 21 (78) | 6 (60) | 2 (50) |
| Splenomegaly | 38/49 (65) | 23 (82) | 9 (82) | 4 (100) |
| Lymph nodes | 16/43 (37) | 11 (44) | 3 (33) | 1/2 (50) |
| Ascites | 9/50 (18) | 5 (17) | 1 (9) | 0 (0) |
| Gastroduodenal ulcer, n (%) | 11/38 (29) | 10 (38.4) | 0 (0) | 0 (0) |
| AHNMD, n (%) | 4/40 (10) | 3 (11) | 0 | 0 |
| MCAS, n (%) | 39/50 (78) | 24 (83) | 8 (73) | 3 (75) |
| KITmutation | 28/51 (55) | 15 (50) | 5 (45) | 2 (50) |
| D816V KIT mutation | 13/28 (46) | 6 (40) | 1 (20) | 0 |
| WT KIT | 2/28 (7) | 1 (6) | 1 (20) | 0 |
| 502-503 KIT mutation | 3/28 (11) | 2 (13) | 1 (20) | 1 |
| 522/560/654 KIT mutation | 3/28 (11) | 1 (6) | 2 (40) | 1 |
| Abnormal cytogenetics, n (%)* | 4/24 (17) | 3 (27) | ||
| Phenotype, n (%) | ||||
| CD2+ | 15/29 (52) | 6 (46) | 1 | 0 |
| CD25+ | 18/24 (75) | 6 (50) | 3 | 1/3 |
| CD2−/CD25+ | 3/24 | 1 | 1 | 1/3 |
| CD2−/CD25− | 9/24 | 5 | 3 | 2/3 |
| CD2+/CD25+ | 13/24 | 4 | 1 | 0 |
| CD2+/CD25− | 1/24 | 1 | 0 | 0 |
| Laboratory data, median (range) | ||||
| Tryptase, ng/L | 433 (21-2357) | 433 (21-742) | 250 (173-2357) | 173.5 (173-200) |
| Hemoglobin, g/dL | 9.9 (5.4-14) | 9 (5.4-13.7) | 11 (8.1-13.3) | 12.5 (8.1-13.3) |
| Platelets, G/L | 110 (5-318) | 82 (5-202) | 111 (30-150) | 136 (101-318) |
| PMN, G/L | 3.65 (0.99-15.3) | 6.0 (0.99-14) | 8.5 (1.7-15.3) | |
| BM mastocytes % | 50 (20-100) | 60 (20-100) | 60 (25-90) | 60 (25-75) |
| Blood mastocytes % | 5.5 (0-72) | 7 (0-72) | 0 (0-12) | 2 (one tested) |
| Outcome | ||||
| Death, n (%) | 33/48 (69) | 24 (83) | 5 (62) | 2 (50%)† |
| Median survival time, mo (range) | 6 (0.5-98) | 4 (0.5-24) | 5 (1-18) | |
| . | MCL . | MCL with pediatric mastocytosis, n = 4 . | ||
|---|---|---|---|---|
| All, N = 51 . | De novo, n = 30 . | Secondary, n = 11 . | ||
| Median age at diagnosis, y (range) | 52 (5-76) | 51.5 (18-76) | 35 (5-75) | 24 (5-53) |
| Female/Male | 30/20 | 18/11 | 5/6 | 3/1 |
| Ethnicity, n (%) | ||||
| White | 18/21 (86) | 13/15 (87) | 5/6 (83) | 2 (50) |
| Asiatic | 3/21 (14) | 2/15 (13) | 1/6 (16) | 1 (25) |
| Familial mastocytosis, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Median time from symptoms to diagnosis, mo (range) | 5 (0-108) | 3 (0-24) | 6 (0-108) | 4.5 (0-108) |
| History of mastocytosis, n (%) | 11/40 (27) | 11 (100) | 4 (100) | |
| Aleukemic MCL, n (%) | 29/47 (62) | 15 (50) | 7 (70) | 4 (100) |
| Organ involvement, n (%) | ||||
| Skin, UP | 15/50 (30) | 4 (14) | 5 (45) | 4 (100) |
| Hepatomegaly | 32/47 (68) | 21 (78) | 6 (60) | 2 (50) |
| Splenomegaly | 38/49 (65) | 23 (82) | 9 (82) | 4 (100) |
| Lymph nodes | 16/43 (37) | 11 (44) | 3 (33) | 1/2 (50) |
| Ascites | 9/50 (18) | 5 (17) | 1 (9) | 0 (0) |
| Gastroduodenal ulcer, n (%) | 11/38 (29) | 10 (38.4) | 0 (0) | 0 (0) |
| AHNMD, n (%) | 4/40 (10) | 3 (11) | 0 | 0 |
| MCAS, n (%) | 39/50 (78) | 24 (83) | 8 (73) | 3 (75) |
| KITmutation | 28/51 (55) | 15 (50) | 5 (45) | 2 (50) |
| D816V KIT mutation | 13/28 (46) | 6 (40) | 1 (20) | 0 |
| WT KIT | 2/28 (7) | 1 (6) | 1 (20) | 0 |
| 502-503 KIT mutation | 3/28 (11) | 2 (13) | 1 (20) | 1 |
| 522/560/654 KIT mutation | 3/28 (11) | 1 (6) | 2 (40) | 1 |
| Abnormal cytogenetics, n (%)* | 4/24 (17) | 3 (27) | ||
| Phenotype, n (%) | ||||
| CD2+ | 15/29 (52) | 6 (46) | 1 | 0 |
| CD25+ | 18/24 (75) | 6 (50) | 3 | 1/3 |
| CD2−/CD25+ | 3/24 | 1 | 1 | 1/3 |
| CD2−/CD25− | 9/24 | 5 | 3 | 2/3 |
| CD2+/CD25+ | 13/24 | 4 | 1 | 0 |
| CD2+/CD25− | 1/24 | 1 | 0 | 0 |
| Laboratory data, median (range) | ||||
| Tryptase, ng/L | 433 (21-2357) | 433 (21-742) | 250 (173-2357) | 173.5 (173-200) |
| Hemoglobin, g/dL | 9.9 (5.4-14) | 9 (5.4-13.7) | 11 (8.1-13.3) | 12.5 (8.1-13.3) |
| Platelets, G/L | 110 (5-318) | 82 (5-202) | 111 (30-150) | 136 (101-318) |
| PMN, G/L | 3.65 (0.99-15.3) | 6.0 (0.99-14) | 8.5 (1.7-15.3) | |
| BM mastocytes % | 50 (20-100) | 60 (20-100) | 60 (25-90) | 60 (25-75) |
| Blood mastocytes % | 5.5 (0-72) | 7 (0-72) | 0 (0-12) | 2 (one tested) |
| Outcome | ||||
| Death, n (%) | 33/48 (69) | 24 (83) | 5 (62) | 2 (50%)† |
| Median survival time, mo (range) | 6 (0.5-98) | 4 (0.5-24) | 5 (1-18) | |
MCL indicates mast cell leukemia; UP, urticaria pigmentosa; AHNMD, associated clonal hematologic non–MC-lineage disease; MCAS, mast cell activation syndrome including flushes, fever, malaise, diarrhea, and tachycardia; WT, wild type; PMN, polymorphonuclear; and BM, bone marrow.
Abnormal cytogenetics, 5q deletion (2 patients with AHNMD), t(10;16) (q22;q13q22), and t(8;21)(q22;q22) (2 patients).
No information on the other 2 patients at 4 months.
On physical examination, hepatomegaly and splenomegaly were the most frequent clinical signs and were present, respectively, in 68% and 65% of the patients with a median spleen enlargement of 21 cm (ranging from 13 to 25 cm). Lymph node enlargement was found in 37% and skin involvement was only present in 30% of all cases. Gastrointestinal manifestations frequently included gastroduodenal ulcers (29%)11-20 which were often complicated by gastrointestinal hemorrhage (64%). Gastroduodenal ulcers seem to be more frequently associated with the KIT D816V (12.5%) mutation than with other KIT mutations (0%). Ascites and portal hypertension were present in 18% and 16% of cases, respectively (Table 3).
De novo or secondary MCL
A comparison between de novo (n = 30) and secondary (n = 11) MCL subgroups revealed more frequent cutaneous manifestations (urticaria pigmentosa) in secondary MCL. In secondary MCL, this is certainly because of the previous mastocytosis. Other clinical features—including hepatosplenomegaly, lymph node enlargement, ascites, and symptoms of MC activation—were comparable between the 2 groups. Interestingly, no gastroduodenal ulcers were observed in patients with secondary MCL whereas they were found in 38% of de novo MCL cases. As gastroduodenal ulcers are linked to the release of histamine by pathologic MC,21,22 we could hypothesize that in de novo MCL, pathologic MC are highly aggressive, proliferate rapidly, infiltrate all organs (including the digestive tract), and degranulate easily, releasing large amounts of histamine, leading to gastroduodenal ulcer formation.
Mast cell leukemia and clonal hematologic non–MC-lineage disease
MCL is rarely associated with clonal hematologic non-MC disease. Only 4 cases (8%) were found in the literature. AHNMD were mainly myelodysplastic syndromes (n = 3) and a chronic myelomonocytic leukemia (n = 1). Clinical symptoms were not different from other MCL manifestations, and the aleukemic form of the disease was predominant. AHNMD was only found in the KIT D816V mutation subgroup (n = 3/7). Cytogenetic studies revealed a 5q deletion in 2 cases which was more likely related to the MDS clone rather than to MC clones. The prognosis was also poor and not different from MCL without AHNMD (Tables 3–4).
Patients' characteristics according to KIT sequencing: literature review
| . | No D816V mutation, n = 15§ . | D816V mutations, n = 13 . |
|---|---|---|
| Media age at diagnosis, y (range) | 46 (18-66) | 58 (52-76) |
| Female/Male | 10/4‡ | 8/5 |
| Ethnicity, n (%) | ||
| White | 6 (85) | 3 (75) |
| Asiatic | 1 (15) | 1 (25) |
| Familial mastocytosis | 0 (0) | 0 (0) |
| Median time from symptoms to diagnosis, mo (range) | 5 (0-108) | 5 (0-10) |
| History of mastocytosis, n (%) | 4 (33) | 1 (15) |
| Aleukemic MCL, n (%) | 10 (83) | 8 (61) |
| Organ involvement, n (%) | ||
| Skin (UP) | 4 (28) | 3 (23) |
| Hepatomegaly | 8 (57) | 8 (66) |
| Splenomegaly | 10 (71) | 10 (77) |
| Lymph nodes | 3 (25) | 3 (23) |
| Ascites | 2 (15) | 5 (38) |
| Gastroduodenal ulcer, n (%) | 0 (0) | 1 (12.5) |
| AHNMD, n (%) | 0 (0) | 3 (43) |
| MCAS, n (%) | 12 (85) | 8 (61) |
| KITmutation (%)* | ||
| D816V KIT mutation | 13 (100) | |
| WT KIT | 2 (25) | |
| Exon 9 mutation | 3 (37.5) | |
| Exon 10 mutation | 1 (12.5) | |
| Exon 11 mutation | 1 (12,5) | |
| Exon 13 mutation | 1 (12.5) | |
| Not available | 7 (46) | |
| Abnormal cytogenetics, n (%)† | 1 (12.5) | 1 (8) |
| (t(8;21)(q22;q22)) | ||
| Phenotype, n (%) | ||
| CD2+ | 4 (33) | 8 (80) |
| CD25+ | 4 (36) | 11 (92) |
| CD2−/CD25+ | 1 (8) | 1 (8) |
| CD2−/CD25− | 5 (42) | 1 (8) |
| CD2+/CD25+ | 3 (25) | 8 (66) |
| CD2+/CD25− | 1 (8) | 0 (0) |
| Biological data, median (range) | ||
| Tryptase, ng/L | 400 (173-742) | 200 (111-2357) |
| Hemoglobin, g/dL | 10 (5.4-12.5) | 10 (7-13.7) |
| Platelets, ×109/L | 117.5 (45-318) | 74 (5-210) |
| PMN, ×109/L | 1.5 (1.1-4) | 10.5 (3.5-14) |
| BM mastocytes, % (range) | 64 (23-100) | 60 (30-90) |
| Blood mastocytes, % (range) | 3.5 (1-31) | 3.5 (1-20) |
| Outcome | ||
| Death, n (%) | 9 (69) | 6 (46) |
| Median survival time, mo (range) | 5 (0.5-29) | 5.5 (2-23) |
| . | No D816V mutation, n = 15§ . | D816V mutations, n = 13 . |
|---|---|---|
| Media age at diagnosis, y (range) | 46 (18-66) | 58 (52-76) |
| Female/Male | 10/4‡ | 8/5 |
| Ethnicity, n (%) | ||
| White | 6 (85) | 3 (75) |
| Asiatic | 1 (15) | 1 (25) |
| Familial mastocytosis | 0 (0) | 0 (0) |
| Median time from symptoms to diagnosis, mo (range) | 5 (0-108) | 5 (0-10) |
| History of mastocytosis, n (%) | 4 (33) | 1 (15) |
| Aleukemic MCL, n (%) | 10 (83) | 8 (61) |
| Organ involvement, n (%) | ||
| Skin (UP) | 4 (28) | 3 (23) |
| Hepatomegaly | 8 (57) | 8 (66) |
| Splenomegaly | 10 (71) | 10 (77) |
| Lymph nodes | 3 (25) | 3 (23) |
| Ascites | 2 (15) | 5 (38) |
| Gastroduodenal ulcer, n (%) | 0 (0) | 1 (12.5) |
| AHNMD, n (%) | 0 (0) | 3 (43) |
| MCAS, n (%) | 12 (85) | 8 (61) |
| KITmutation (%)* | ||
| D816V KIT mutation | 13 (100) | |
| WT KIT | 2 (25) | |
| Exon 9 mutation | 3 (37.5) | |
| Exon 10 mutation | 1 (12.5) | |
| Exon 11 mutation | 1 (12,5) | |
| Exon 13 mutation | 1 (12.5) | |
| Not available | 7 (46) | |
| Abnormal cytogenetics, n (%)† | 1 (12.5) | 1 (8) |
| (t(8;21)(q22;q22)) | ||
| Phenotype, n (%) | ||
| CD2+ | 4 (33) | 8 (80) |
| CD25+ | 4 (36) | 11 (92) |
| CD2−/CD25+ | 1 (8) | 1 (8) |
| CD2−/CD25− | 5 (42) | 1 (8) |
| CD2+/CD25+ | 3 (25) | 8 (66) |
| CD2+/CD25− | 1 (8) | 0 (0) |
| Biological data, median (range) | ||
| Tryptase, ng/L | 400 (173-742) | 200 (111-2357) |
| Hemoglobin, g/dL | 10 (5.4-12.5) | 10 (7-13.7) |
| Platelets, ×109/L | 117.5 (45-318) | 74 (5-210) |
| PMN, ×109/L | 1.5 (1.1-4) | 10.5 (3.5-14) |
| BM mastocytes, % (range) | 64 (23-100) | 60 (30-90) |
| Blood mastocytes, % (range) | 3.5 (1-31) | 3.5 (1-20) |
| Outcome | ||
| Death, n (%) | 9 (69) | 6 (46) |
| Median survival time, mo (range) | 5 (0.5-29) | 5.5 (2-23) |
MCL indicates mast cell leukemia; UP, urticaria pigmentosa; AHNMD, associated clonal hematologic non–MC-lineage disease; MCAS, mast cell activation syndrome; PMN, polymorphonuclear; and BM, bone marrow.
Exon 9 mutation at codons 501-502-503; exon 10 mutation at codon 522, exon 11 mutation at codon 560, exon 13 mutation at codon 654; not available by KIT sequencing.
Abnormal cytogenetics, 5q deletion (2 patients with AHNMD), t(10;16) (q22;q13q22) and t(8;21)(q22;q22) (2 patients).
One missing.
Two patients with previous mast cell sarcoma and 2 patients with previous pediatric mastocytosis.
Literature review of MCL cases
| Authors . | Year of publication . | No. of patients . | Age at diagnosis, y . | WHO MCL . | De novo MCL . | Aleukemic MCL . | c-KIT mutations . | Survival time, mo . |
|---|---|---|---|---|---|---|---|---|
| Hissard et al83 | 1951 | 1 | 47 | Yes | No | Yes | NA | NA |
| Waters et al37 | 1956 | 1 | 5 | Yes | No | Yes | NA | 3 |
| Efrati et al15 | 1957 | 1 | 52 | Yes | Yes | No | NA | 2 |
| Friedman et al16 | 1958 | 1 | NA | Yes | Yes | No | NA | 2 |
| Brinkmann et al84 | 1959 | 1 | 51 | Yes | Yes | NA | NA | NA |
| Szweda et al19 | 1962 | 1 | 50 | Yes | Yes | Yes | NA | 4 |
| Mutter et al85 | 1963 | 1 | 59 | Yes | Yes | Yes | NA | 8 |
| Daniel et al14 | 1975 | 1 | 39 | Yes | Yes | No | NA | 5 |
| Clancy et al86 | 1976 | 1 | 46 | Yes | Yes | NA | NA | 4 |
| Coser at al12 | 1980 | 1 | 54 | Yes | Yes | No | NA | 1 |
| Kimura et al87 | 1979 | 1 | 39 | Yes | Yes | No | NA | NA |
| Horny et al88 | 1986 | 1 | 75 | Yes | No | No | NA | 1 |
| Dalton et al13 | 1986 | 1 | 57 | Yes | Yes | No | NA | 8 |
| Travis et al20 | 1986 | 1 | 52 | Yes | Yes | No | NA | 1 |
| Baghestanian et al11 | 1996 | 1 | 34 | Yes | Yes | No | NA | 3 |
| Le Cam et al18 | 1997 | 1 | 44 | Yes | Yes | Yes | NA | 7 |
| Escribano et al89 | 1997 | 1 | 67 | Yes | Yes | Yes | NA | 16 |
| Kyriakou et al17 | 1998 | 1 | 72 | Yes | Yes | Yes | NA | 9 |
| Pauls et al90 | 1999 | 1 | 48 | Yes | No | Negative | 7 | |
| Lin et al79 | 2002 | 1 | 25 | Yes | No | No | NA | NA |
| Horny et al91 | 2002 | 1 | 48 | Yes | Yes | Yes | Negative | NA |
| Pardanani et al65 | 2003 | 1 | NA | Yes | Yes | NA | Negative | 0, 5 |
| Chen et al82 | 2003 | 1 | 18 | Yes | Yes | Yes | Negative | |
| Akin et al92 | 2004 | 1 | 25 | Yes | No | Yes | Phe522Cys | |
| Noack et al9 | 2004 | 1 | 75 | Yes | Yes | Yes | D816V | |
| Gotlib et al73 | 2005 | 1 | 48 | Yes | Yes | No | D816V | 6 |
| Penack et al46 | 2005 | 1 | 65 | Yes | Yes | No | D816V | |
| Valentini et al7 | 2007 | 10 | 56* | Yes | No | Yes† | ‡ | 24§ |
| Krauth et al93 | 2007 | 1 | 35 | Yes | No | Yes | WT | |
| Brcic et al77 | 2007 | 1 | 5 | Yes | No | No | NA | |
| Aichberger et al72 | 2008 | 1 | 71 | Yes | Yes | No | D816V | |
| Yoshida et al94 | 2009 | 1 | 52 | Yes | Yes | No | D816V | |
| Arredondo et al24 | 2010 | 1 | 61 | Yes | No | Yes | Negative | |
| Mital et al30 | 2011 | 1 | 53 | Yes | Yes | Yes | dup 502-503 | 18 |
| Spector et al33 | 2011 | 1 | 42 | Yes | No | Yes | V654A | |
| Chantorn et al36 | 2012 | 1 | 23 | Yes | No | Yes | NA | |
| Georgin-Lavialle et al29 | 2012 | 1 | 66 | Yes | Yes | Yes | del(501;502) | |
| Georgin-Lavialle et al32 | 2012 | 1 | 42 | Yes | No | NA | val 560 gly | |
| Joris et al95 | 2012 | 1 | 52 | Yes | Yes | Yes | WT | 3 |
| Hermine et al | Unpublished | 1 | 29 | Yes | Yes | Yes | dup 501-502 | 6 |
| Damaj et al | Unpublished | 2 | 76, 58 | Yes | Yes | Yes | D816V (2) | 7, 11 |
| Authors . | Year of publication . | No. of patients . | Age at diagnosis, y . | WHO MCL . | De novo MCL . | Aleukemic MCL . | c-KIT mutations . | Survival time, mo . |
|---|---|---|---|---|---|---|---|---|
| Hissard et al83 | 1951 | 1 | 47 | Yes | No | Yes | NA | NA |
| Waters et al37 | 1956 | 1 | 5 | Yes | No | Yes | NA | 3 |
| Efrati et al15 | 1957 | 1 | 52 | Yes | Yes | No | NA | 2 |
| Friedman et al16 | 1958 | 1 | NA | Yes | Yes | No | NA | 2 |
| Brinkmann et al84 | 1959 | 1 | 51 | Yes | Yes | NA | NA | NA |
| Szweda et al19 | 1962 | 1 | 50 | Yes | Yes | Yes | NA | 4 |
| Mutter et al85 | 1963 | 1 | 59 | Yes | Yes | Yes | NA | 8 |
| Daniel et al14 | 1975 | 1 | 39 | Yes | Yes | No | NA | 5 |
| Clancy et al86 | 1976 | 1 | 46 | Yes | Yes | NA | NA | 4 |
| Coser at al12 | 1980 | 1 | 54 | Yes | Yes | No | NA | 1 |
| Kimura et al87 | 1979 | 1 | 39 | Yes | Yes | No | NA | NA |
| Horny et al88 | 1986 | 1 | 75 | Yes | No | No | NA | 1 |
| Dalton et al13 | 1986 | 1 | 57 | Yes | Yes | No | NA | 8 |
| Travis et al20 | 1986 | 1 | 52 | Yes | Yes | No | NA | 1 |
| Baghestanian et al11 | 1996 | 1 | 34 | Yes | Yes | No | NA | 3 |
| Le Cam et al18 | 1997 | 1 | 44 | Yes | Yes | Yes | NA | 7 |
| Escribano et al89 | 1997 | 1 | 67 | Yes | Yes | Yes | NA | 16 |
| Kyriakou et al17 | 1998 | 1 | 72 | Yes | Yes | Yes | NA | 9 |
| Pauls et al90 | 1999 | 1 | 48 | Yes | No | Negative | 7 | |
| Lin et al79 | 2002 | 1 | 25 | Yes | No | No | NA | NA |
| Horny et al91 | 2002 | 1 | 48 | Yes | Yes | Yes | Negative | NA |
| Pardanani et al65 | 2003 | 1 | NA | Yes | Yes | NA | Negative | 0, 5 |
| Chen et al82 | 2003 | 1 | 18 | Yes | Yes | Yes | Negative | |
| Akin et al92 | 2004 | 1 | 25 | Yes | No | Yes | Phe522Cys | |
| Noack et al9 | 2004 | 1 | 75 | Yes | Yes | Yes | D816V | |
| Gotlib et al73 | 2005 | 1 | 48 | Yes | Yes | No | D816V | 6 |
| Penack et al46 | 2005 | 1 | 65 | Yes | Yes | No | D816V | |
| Valentini et al7 | 2007 | 10 | 56* | Yes | No | Yes† | ‡ | 24§ |
| Krauth et al93 | 2007 | 1 | 35 | Yes | No | Yes | WT | |
| Brcic et al77 | 2007 | 1 | 5 | Yes | No | No | NA | |
| Aichberger et al72 | 2008 | 1 | 71 | Yes | Yes | No | D816V | |
| Yoshida et al94 | 2009 | 1 | 52 | Yes | Yes | No | D816V | |
| Arredondo et al24 | 2010 | 1 | 61 | Yes | No | Yes | Negative | |
| Mital et al30 | 2011 | 1 | 53 | Yes | Yes | Yes | dup 502-503 | 18 |
| Spector et al33 | 2011 | 1 | 42 | Yes | No | Yes | V654A | |
| Chantorn et al36 | 2012 | 1 | 23 | Yes | No | Yes | NA | |
| Georgin-Lavialle et al29 | 2012 | 1 | 66 | Yes | Yes | Yes | del(501;502) | |
| Georgin-Lavialle et al32 | 2012 | 1 | 42 | Yes | No | NA | val 560 gly | |
| Joris et al95 | 2012 | 1 | 52 | Yes | Yes | Yes | WT | 3 |
| Hermine et al | Unpublished | 1 | 29 | Yes | Yes | Yes | dup 501-502 | 6 |
| Damaj et al | Unpublished | 2 | 76, 58 | Yes | Yes | Yes | D816V (2) | 7, 11 |
MCL indicates mast cell leukemia; NA, not available; and WT, wild type.
Median age.
Seven of 10 cases were aleukemic MCL.
Six patients were D816V c-KIT positive and 2 patients were D816V c-KIT negative.
Median survival time at last reported follow-up.
Laboratory characteristics
Blood and marrow characteristics
The percentage of circulating MC ranged from 0% to 72% of all leukocytes. Aleukemic MCL was the most frequent form found, making up 62% of all cases; 70% of secondary MCL and 55% of de novo MCL. Anemia was always present at diagnosis with a median hemoglobin value of 9.9 g/dL and a lower hemoglobin level in de novo (9 g/dL) than in secondary (11 g/dL) MCL patients. Thrombocytopenia was also frequent. The median platelet count was 110 G/L in the whole group of patients with lower median platelet counts in the de novo than in the secondary MCL patients (82 vs 111 G/L).
Levels of polymorphonuclear cells were within normal values with a median count of 3.65 G/L. The median bone marrow mast cell infiltration was 50%, ranging from 20% to 100% with no difference between de novo and secondary MCL subgroups. Serum tryptase levels were always increased with a median level of 433 ng/L (Table 3).
Morphology
Normal MCs and their progenitors display 4 morphologic stages of differentiation in BM smears stained with May-Grünwald-Giemsa: (1) ungranulated but tryptase-positive blast cells, (2) metachromatic blast, (3) promastocyte, and (4) typically round mononuclear mature MC with metachromatic granules in the cytoplasm.
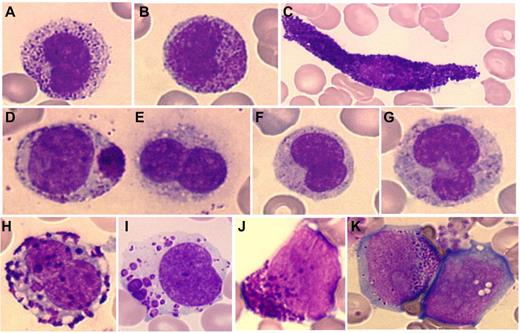
Malignant MC from MCL patients may exhibit the features classically encountered in mastocytosis with mature MC displaying morphologic abnormalities such as elongated cytoplasmic extensions (spindle-shaped MC), an eccentric oval nucleus, hypo-granulated cytoplasm, and possible focal granule accumulations (Figure 1).
Cytologic abnormalities of mast cells in MCL. Mast cells from MCL range from mature mast cells (A-D) to more immature cells with (E-I) promastocytes and (J-K) even metachromatic or (K) ungranulated blasts. (A-B) Circulating round mast cells; (C) spindle-shaped mast cell; (D) packed and polar granule aggregates; (E-G) degranulated/hypogranulated promastocytes; (H-I) coalescent granules; (I) lacunar cytoplasmic areas; (J-K) undifferentiated immature cells with metachromatic granules and prominent nucleoli.
Cytologic abnormalities of mast cells in MCL. Mast cells from MCL range from mature mast cells (A-D) to more immature cells with (E-I) promastocytes and (J-K) even metachromatic or (K) ungranulated blasts. (A-B) Circulating round mast cells; (C) spindle-shaped mast cell; (D) packed and polar granule aggregates; (E-G) degranulated/hypogranulated promastocytes; (H-I) coalescent granules; (I) lacunar cytoplasmic areas; (J-K) undifferentiated immature cells with metachromatic granules and prominent nucleoli.
MCL may also typically exhibit immature forms of MC, seldom seen in other types of mastocytosis, reminiscent of the whole morphologic differentiation chronology. Promastocytes, metachromatic blasts, and even ungranulated blasts are seen with specific abnormalities such as polarized or coalescent granules, cytoplasmic lacunar areas, prominent nucleoli, fine nuclear chromatin, and bi- or multilobed nuclei (Figure 1).1,3
MCL differs from all other forms of SM in that diagnosis can rely on cytology alone.1,7,23 In addition to qualitative features and to make the diagnosis, on cytologic analysis, MC should represent > 20% of nucleated cells in marrow aspirates or > 10% in peripheral blood. The diagnosis of an aleukemic variant of MCL is met if the count of MC on peripheral blood is < 10%.
Beyond these criteria for diagnosis, the detection of circulating mature MC is abnormal and must prompt investigation for MCL.
Immunophenotype
In mastocytosis, abnormal antigen expression on MC is identified either by flow cytometry or immunohistochemistry. Expression of CD2 and/or CD25 is the most common feature of abnormal MC and is recognized as a minor criteria for diagnosis of mastocytosis.1
MC from MCL can express both CD25 and CD2, although the expression of CD25 and particularly CD2 was not found in, respectively, 25% and 48% of cases, and one-third of MCL cases reported in the literature had a double-negative CD2/CD25 immunophenotype (Table 3).24-26 In line with the classic association of the KIT D816V mutation with the expression of CD2/CD25 on MC in SM, the positive coexpression of CD2 and CD25 was very frequent among MCL patients who were KIT D816V positive (66%) compared with those who were KIT D816V negative (25%). Thus, the common feature of pathologic MC encountered in most forms of mastocytosis is unreliable in MCL.
Recently, it has been proposed that aggressive forms of mastocytosis, including MCL, exhibit an immature phenotype, in contrast to the more indolent forms which are characterized by an activated immunophenotype.8,27 It follows that MC from MCL may express immaturity markers such as CD123, CD34, HLA-DR, and display reduced expression of CD117 and FceRI. The immature phenotype is correlated with multilineage KIT mutations in bone marrow and a poor prognosis.8,27 From this observation, arises the concept that the occurrence of a KIT mutation in an early progenitor may result in multilineage involvement, early blockade of maturation of MC, and aggressive disease. In contrast, mutations that occur in a later-stage progenitor remain MC-restricted and lead to late maturation blockade with a mature and activated MC immunophenotype.27,28 At present, the significance of the phenotypic heterogeneity according to different histologic, clinical, and genetic features remains unresolved.
Cytogenetic and molecular characteristics
Cytogenetic studies were available for 23 of 51 reported cases. A normal cytogenetic profile was found in the majority of cases (83%). Two patients (9%) had a 5q deletion, and were diagnosed as having MCL associated with myelodysplastic syndrome,7 with the deletion of 5q probably being related to the myelodysplastic clone rather than to MCL itself. Two other patients with de novo MCL had t(10;16)(q22;q13q22) and t(8;21)(q22;q22). According to these findings, there is no recurrent cytogenetic abnormality in MCL.
c-KIT mutations
The KIT D816V mutation was detected in 13 of 28 patients studied (Table 4). In 8 patients without KIT D816V, the entire KIT gene was sequenced. In 2 cases, KIT was wild type (WT), whereas in the other 6 cases, mutations were detected in exon 9 (n = 3),29,30 exon 10 (n = 1),31 exon 11 (n = 1),32 and exon 13 (n = 1).33 It is noteworthy that these mutations are also found in stromal gastrointestinal tumors, which are aggressive. Most of these non-D816V mutations have only recently been described, 1 in 2004 and 6 since 2011. This may explain why in previous literature reviews, made when the KIT gene had not been entirely sequenced, the KIT D816V mutation was described as the most frequently encountered mutation. We now know that in MCL this is not so.
In the non-KIT D816V mutation subgroup, 4 patients had a history of mastocytosis including 2 MC sarcomas and 2 with pediatric cutaneous mastocytosis, that is, 33% of the patients studied, in contrast to only 1 of 7 patients in the KIT D816V mutation group (15%).
Death occurred more frequently in the subgroup without D816V mutation (69%) than for those with the D816V mutation (46%), although the median survival time did not differ.
TET2
TET2 (Ten-Eleven-Translocation 2) is 1 of 3 homologous human proteins (TET1, TET2, and TET3) that may play a role in the epigenetic regulation of transcription. TET2 mutations have been reported in 29% of systemic mastocytosis, with 15% ISM, 40% ASM, and 35% being SM-AHNMD.34 Recent data from our group reporting on 74 patients with cutaneous and SM, including 4 with MCL, showed the TET2 mutation as a potential marker to diagnose and predict severe forms of the disease.35
Secondary MCL after pediatric mastocytosis
Interestingly, 4 patients developed MCL after pediatric mastocytosis (Table 6).30,31,36,37 In 1 case, diffuse cutaneous mastocytosis evolved into MCL, leading to death in childhood37 (KIT gene sequencing was not available at that time). In 2 other cases,30,31 maculopapular cutaneous mastocytosis occurred in childhood and developed into MCL 25 and 53 years later. Mutations in exon 9 and 10 were found, with no or low expression of CD2 and CD25. Both patients were treated with imatinib, obtained partial response, and were alive at 5 and 18 months, respectively. The last interesting case36 is that of mastocytoma diagnosed at 5 years of age. After complete surgical excision for aesthetic reasons at age 19, the patient developed aggressive systemic mastocytosis and MCL. Allogeneic stem cell transplantation was ineffective and was followed by rapid disease progression and death. This last case demonstrates that mastocytoma can evolve into MCL. The description of frequent non-D816V KIT mutations among children with cutaneous mastocytosis38 may indicate that mutations in exon 9 and 10 of KIT can lead to severe forms of mastocytosis. Taken together, these 4 cases highlight the risk of evolution of pediatric mastocytosis to MCL.
Main features of MCL cases with a previous history of pediatric mastocytosis
| Author . | Year . | Sex . | Age, mo* . | Type† . | Clinical evolution . | MC phenotype . | KIT sequencing . | Cytological features . | Treatment . |
|---|---|---|---|---|---|---|---|---|---|
| Waters37 | 1957 | M | 9 | DCM | 5 y: MCL transformation | ND | ND | Mast cells appeared as large polymorphic cells with acidophilic cytoplasm and large rounded nucleus, few metachromatic granules were discerned in the cytoplasm. | None |
| Death within 3 mo due to diffuse hemorrhage. | |||||||||
| Akin31 | 2004 | F | 5 | MPCM | 22 y: deterioration of general status | CD2neg | Phe 522 Cys | Round, highly granulated and centrally located nucleus | IFNα: no response |
| 25 y: ASM | CD25neg | (exon 10) | Imatinib: PR | ||||||
| And 6 mo later: MCL | FU: 5 mo | ||||||||
| Mital30 | 2011 | F | 12 | MPCM | 47 y: unexplained splenomegaly | CD2low | 502-503 Dup | Large mast cells | 2CDA: no response |
| 53 y: aleukemic MCL | CD25low | (exon 9) | Imatinib: PR | ||||||
| 56 y: MCL evolution | FU: 18 mo | ||||||||
| Chantorn36 | 2012 | F | 5 | Mastocytoma | 10 y: MPCM | CD2− | ND | Rare spindle shaped mast cells on bone marrow histology | poly chemotherapy, |
| 19 y: mastocytoma exeresis | CD25+ | Atypically spindle shaped morphology on BM smear | allo-SCT, Steroids | ||||||
| 23 y: ASM; MCL and death 5 mo later | Tacrolimus: | ||||||||
| No response |
| Author . | Year . | Sex . | Age, mo* . | Type† . | Clinical evolution . | MC phenotype . | KIT sequencing . | Cytological features . | Treatment . |
|---|---|---|---|---|---|---|---|---|---|
| Waters37 | 1957 | M | 9 | DCM | 5 y: MCL transformation | ND | ND | Mast cells appeared as large polymorphic cells with acidophilic cytoplasm and large rounded nucleus, few metachromatic granules were discerned in the cytoplasm. | None |
| Death within 3 mo due to diffuse hemorrhage. | |||||||||
| Akin31 | 2004 | F | 5 | MPCM | 22 y: deterioration of general status | CD2neg | Phe 522 Cys | Round, highly granulated and centrally located nucleus | IFNα: no response |
| 25 y: ASM | CD25neg | (exon 10) | Imatinib: PR | ||||||
| And 6 mo later: MCL | FU: 5 mo | ||||||||
| Mital30 | 2011 | F | 12 | MPCM | 47 y: unexplained splenomegaly | CD2low | 502-503 Dup | Large mast cells | 2CDA: no response |
| 53 y: aleukemic MCL | CD25low | (exon 9) | Imatinib: PR | ||||||
| 56 y: MCL evolution | FU: 18 mo | ||||||||
| Chantorn36 | 2012 | F | 5 | Mastocytoma | 10 y: MPCM | CD2− | ND | Rare spindle shaped mast cells on bone marrow histology | poly chemotherapy, |
| 19 y: mastocytoma exeresis | CD25+ | Atypically spindle shaped morphology on BM smear | allo-SCT, Steroids | ||||||
| 23 y: ASM; MCL and death 5 mo later | Tacrolimus: | ||||||||
| No response |
MCL indicates mast cell leukemia; M, male; DCM, diffuse cutaneous leukemia; ND, not done; F, female; MPCM, maculopapular cutaneous mastocytosis; ASM, aggressive systemic mastocytosis; IFNa, interferon alpha; PR, partial response; FU, follow-up; BM, bone marrow; and allo-SCT, allogeneic stem cell transplantation.
Age at diagnosis of pediatric mastocytosis.
Type of pediatric mastocytosis at diagnosis.
Management of mast cell burden
In general, treatments of SM can be divided into 2 broad categories: (1) those intended to control mast cell mediator-related symptoms and reduce disabilities associated with the disease and (2) those intended to limit MC burden in aggressive forms and increase survival by the use of cytoreductive therapy.39 To date, there is no approved standard therapy. For MCL, few options are available for treatment and because of the rarity of the disease very few clinical trials address the question.
Steroids
Corticosteroids are often tried, at least at the onset of the disease. High-dose corticosteroids may induce a reduction in MC burden and improve clinical symptoms including flushing, ascitis, pain, cardiac failure as well as cytopenia. However, their effect is usually transient and precautions should be taken. Proton pump inhibitor therapy should be systematically started concurrently with the introduction of corticosteroids because of the increased risk of gastrointestinal bleeding, particularly in this context.
Interferon-α
2-Chloro-deoxy-adenosine
The rationale behind the use of 2-Chloro-deoxy-adenosine (2-CdA) for the treatment of SM is based on the fact that MC are derived from hematopoietic stem cells and may share a common progenitor with monocytes,47 combined with the knowledge that the drug is particularly toxic to monocytes, both in vitro and in vivo.48
While the potential value of 2-CdA in the treatment of ASM has been confirmed in several studies,49,50 in MCL, 2-CdA has no24,30 or little7 activity, although in rare cases a prolonged partial response has been observed.9
Ten patients with a diagnosis of MCL were treated with 2-CdA. Responses were obtained in 3 of them (30%) who were still alive at 17, 24, and 48 months (with no subsequent therapy for 2 patients).7,24,33,51
In our experience, when 68 patients with the diagnosis of SM were treated with single-agent Cladribine at a dose of 0.14 mg/kg/d for 5 or 7 consecutive days, no significant effects were observed in the subgroup of 3 patients with MCL. Combination therapies with high-dose cytarabine (O.H., oral communication at second annual meeting of reference centers, September 16, 2012), mTOR inhibitors,52 or tyrosine kinase inhibitor (TKI)53 need to be evaluated in patients with MCL.
Targeted therapies or tyrosine kinase inhibitors
The mutation in c-KIT codon 816 found in adult human mastocytosis causes constitutive activation of the KIT kinase.54 Different classes of KIT activating mutations respond differentially to KIT inhibitors depending on the site and the type of mutation.55-58 The presence and/or the type of KIT mutation have clinical prognostic importance.59 Thus, data detailing mutations are useful in predicting drug resistance and supporting individualization of therapy based on the response of specific mutant proteins to specific drugs.
Imatinib.
The rationale behind the use of imatinib in SM stems from its in vitro inhibitory activity of both WT and certain (eg, V560G; F522C) but not all D816V classes of constitutively active KIT receptors.31,60-65 In October 2006, these data led the US Food and Drug Administration to approve the use of imatinib for adult patients with ASM lacking the D816V KIT mutation, or with unknown KIT mutational status, at a dose of 400 mg daily.
Nine patients with a diagnosis of MCL and treated by imatinib have so far been reported. Four (2 adults and 2 children) of them obtained a partial response (44%). The KIT mutations were Phe522Cys and Dup 502-503 in 2 responding patients and another responder had WT KIT. The fourth responding patient had the D816V KIT-positive mutation and was alive after 48 months on imatinib alone.7,30,31,33,65
Masitinib.
Masitinib (AB1010) is a tyrosine kinase inhibitor which, in vitro, potently and selectively inhibits the mutated form in the juxtamembrane region of the KIT receptor and the KIT WT receptor. It also inhibits the PDGF receptor and the mutated fibroblast growth factor receptor (FGFR3). At the cellular level, masitinib is a more selective inhibitor of KIT WT-dependent cell proliferation (IC50 of 0.2μM), than imatinib mesilate (IC50 of 0.6μM). Masitinib is in general well-tolerated at the doses of 7.5-12 mg/kg/d used for oncology indications, and up to 6 mg/kg/d in nononcology indications.66 The most frequent adverse events—nausea/vomiting, edema, rash, and diarrhea—are manageable, mild to moderate, and mostly resolve under treatment; nevertheless, 1.3% of patients experienced severe neutropenia that might be because of hypersensitivity of neutrophils to KIT inhibition.
Data on MCL patients is scarce. Two MCL patients not carrying the D816V KIT mutation were treated with masitinib.29 One of them obtained partial response, relapsed 7 months after initiation of masatinib, and died from disease progression at 11 months. This drug needs to be further evaluated in this setting.
Dasatinib.
Dasatinib is an oral bioavailable SRC/ABL inhibitor that has activity against multiple imatinib-resistant BCR-ABL isoforms in vitro and in vivo. It demonstrates significant inhibitory activity, in the nanomolar range, against both wild-type KIT and the KIT D816V mutation67 as well as against autophosphorylation of the juxtamembrane domain of mutant KIT and KIT-dependent activation of downstream pathways68 in in vitro and cell-based kinase assays. Growth inhibition of a human mastocytosis cell line carrying KIT D816V has also been achieved with this drug.67 However, its activity has only been modest in clinical settings.51,69-71
PKC 412.
PKC412 (Midostaurin; Novartis), a small molecule inhibitor of multiple type III TK receptors involved in hematopoiesis and leukemia, inhibits all major isoforms of protein kinase C as well as the TK associated with the vascular endothelial growth factor receptor. It can also exert inhibitory activity on other mutated tyrosine kinases implicated in a variety of diseases, including KIT (systemic mast cell disease, gastrointestinal stromal tumors, PDGFR, or FGFR1 myeloproliferative disorders). Midostaurin has shown strong inhibitory activity on neoplastic human MC carrying the KIT D816V mutation in preclinical and clinical settings.53,73,74 However, data reporting on the efficacy of midostaurin in MCL is scarce.
Our experience with PKC-412 is based on 21 patients treated for advanced SM. Four of them had been diagnosed with MCL (n = 2) or MCL with AHNMD (n = 2). Partial responses were obtained in 2 patients (Damaj G.D., and O.H. and O.C., unpublished data). Preliminary results from the phase 2 study of KIT inhibitor midostaurin showed an overall response rate in, advanced SM, of 60% with the majority being major responses (52.5%). Of the 7 patients with MCL (3 patients with AHNMD), 4 (57%) patients achieved major responses, including 3 ongoing incomplete remissions (19+ months in 2 patients and 32+ months in 1 patient). The overall survival in MCL patients was 22.6 months.75 These results are encouraging and combination strategies with chemotherapy or other targeted therapies could be of interest (Figure 2).
Chemotherapy
Various cytostatic drugs have been shown to induce apoptosis and inhibit proliferation of the human MCL cell line HMC-1.76 However, whether patients with MCL may benefit from combination polychemotherapy remains unknown. AML-type induction chemotherapy has been used in 6 of 51 reported MCL patients. However, the response rate was not available for all patients, the median survival time was 7 months, and all patients died between 2 and 29 months from progression or multiorgan failure.77-79 These results suggest a lack of efficacy of AML-type induction polychemotherapy in MCL patients.
Bone marrow transplantation
Allogeneic stem cell transplantation (allo-SCT) could play a potential curative role in MCL. Some reports have shown a positive role of the graft compared with the mast cell effect in decreasing MC burden after the transplant. However, allo-SCT has limited ability to completely eradicate the disease.80,81
Rapid engraftment of donor MCs has been documented after allo-SCT. Thus, caution should be applied when evaluating the neoplastic MC burden after allo-SCT, and MC should be analyzed carefully to differentiate reactive donor MC from the potential persistence of the neoplastic MC clone.82
Seven cases of patients who received allo-SCT have been reported. Sustained remission was not achieved in any of them. One patient, however, obtained a response but died 23 months after transplantation in complete remission.7,18,36,81,82
Recently, Ustun et al (oral communication, European Competence Network on Mastocytosis meeting, September 2012), reported on a retrospective series of 23 patients with SM who received allo-SCT either after myeloablative conditioning (n = 14) or a fludarabine-based reduced-intensity regimen. The best responses were obtained in SM-AHNMD (AML/MDS) and the worst were in MCL patients. They confirmed the results of a previous report in which only rarely was CR obtained after transplantation. Therefore, in the absence of sufficient data in MCL patients, allo-SCT remains to be proven and further evaluation of this strategy in these patients is needed, alone or in combination with drugs to obtain greater reduction in disease burden, which may translate to better long-term control of the disease (Figure 2).
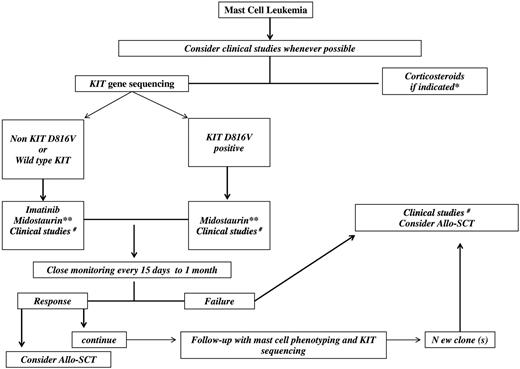
Proposed algorithm for MCL treatment. *Fever, bone pain, flushing, mast cell activation symptoms, cardiac failure, pleural effusion, ascites, cytopenia. **If available through a compassionate patient-named program. #Examples of drugs for clinical studies: cytarabine + 2-chlorodeoxyadenosine (2-CdA); CLAG (clofarabine, aracytine, granulocyte stimulating agents); FLAG (fludarabine, aracytine, granulocyte stimulating agents); tyrosine kinase inhibitors (midostaurin, masitinib, ponatinib, sorafenib), or Aurora kinase inhibitors in combination with other drugs; mammalian target of rapamicin (m-TOR) inhibitors; deoxy nucleotide methyl-transferase inhibitors (azacytidine, decitabine).
Proposed algorithm for MCL treatment. *Fever, bone pain, flushing, mast cell activation symptoms, cardiac failure, pleural effusion, ascites, cytopenia. **If available through a compassionate patient-named program. #Examples of drugs for clinical studies: cytarabine + 2-chlorodeoxyadenosine (2-CdA); CLAG (clofarabine, aracytine, granulocyte stimulating agents); FLAG (fludarabine, aracytine, granulocyte stimulating agents); tyrosine kinase inhibitors (midostaurin, masitinib, ponatinib, sorafenib), or Aurora kinase inhibitors in combination with other drugs; mammalian target of rapamicin (m-TOR) inhibitors; deoxy nucleotide methyl-transferase inhibitors (azacytidine, decitabine).
Conclusion
MCL is a rare form of systemic mastocytosis with poor prognosis and very few therapeutic options. It can appear de novo or after previous mastocytosis. It shares more clinicobiologic aspects with ASM than with AML. Hepatosplenomegaly, anemia, and thrombocytopenia are usually present and aleukemic forms are frequent. The serum tryptase level is usually high. MCs are usually CD2 and/or CD25 positive. Wild-type or exon 9 to 13 KIT mutations are not rare and complete gene sequencing is needed. Therapy usually fails and the median survival time is < 6 months. New combination therapies including KIT inhibitors, chemotherapy, 2-CdA, and bone marrow transplantation should in the near future improve the prognosis of this still devastating disease.
Acknowledgments
The authors thank Alison Foote (Grenoble Clinical Research Center) for critically editing the manuscript with particular attention to language usage.
Authorship
Contribution: S.G.-L., L.L., and G.D. reviewed and analyzed the literature data; S.G.-L., O.H., and G.D. conceived and designed the study; P.D. M.-O.C., O.H., and G.D. provided unpublished patients' data; and all authors wrote the manuscript and gave final approval of the manuscript.
Conflict-of-interest disclosure: O.H. and P.D. are co-founders of, consultants to, and stockholders in AB Science (Paris, France). The remaining authors declare no competing financial interests.
Correspondence: Gandhi Damaj, MD, Department of Hematology, CHU d'Amiens, Hôpital Sud, Avenue Laennec, 80054, Amiens, France; e-mail: damaj.gandhi@chu-amiens.fr; or Prof Olivier Hermine, Service d'Hématologie Adultes, CNRS UMR 8147 et Centre de Référence sur les Mastocytoses, Hôpital Necker-Enfants Malades, Université Paris Descartes, AP-HP, 149 Rue des Sèvres, 75743 Paris Cedex 15, France; e-mail: ohermine@gmail.com.