Key Points
Symptomatic extensions, whether or not reaching the SFJ, are common complications of SVT.
Their frequency and associated risk of venous thromboembolic complications and medical resource consumption are reduced by fondaparinux.
Abstract
The clinical relevance of symptomatic extension of spontaneous, acute, symptomatic, lower-limb superficial-vein thrombosis (SVT) is debated. We performed a post hoc analysis of a double-blind trial comparing fondaparinux with placebo. The main study outcome was SVT extension by day 77, whether to ≤3 cm or >3 cm from the sapheno-femoral junction (SFJ). All events were objectively confirmed and validated by an adjudication committee. With placebo (n = 1500), symptomatic SVT extension to ≤3 cm or >3 cm from the SFJ occurred in 54 (3.6%) and 56 (3.7%) patients, respectively, inducing comparable medical resource consumption (eg, anticoagulant drugs and SFJ ligation); subsequent deep-vein thrombosis or pulmonary embolism occurred in 9.3% (5/54) and 8.9% (5/56) of patients, respectively. Fondaparinux was associated with lower incidences of SVT extension to ≤3 cm (0.3%; 5/1502; P < .001) and >3 cm (0.8%; 12/1502; P < .001) from the SFJ and reduced related use of medical resources; no subsequent deep-vein thrombosis or pulmonary embolism was observed in fondaparinux patients. Thus, symptomatic extensions are common SVT complications and, whether or not reaching the SFJ, are associated with a significant risk of venous thromboembolic complications and medical resource consumption, all reduced by fondaparinux. This trial was registered at www.clinicaltrials.gov: NCT00443053.
Introduction
Spontaneous, acute, symptomatic superficial-vein thrombosis (SVT) of the legs, with no deep-vein thrombosis or pulmonary embolism at presentation (ie, isolated), is now recognized as a frequent disease with the potential of leading to severe thromboembolic complications.1-4 The Comparison of Arixtra in lower LImb Superficial vein Thrombosis with placebO (CALISTO; a complete list appears in the supplemental Appendix) study showed that a 45-day subcutaneous once-daily treatment with fondaparinux 2.5 mg was associated with an 85% relative reduction in the risk of the composite of symptomatic pulmonary embolism, symptomatic deep-vein thrombosis, symptomatic SVT recurrence, or symptomatic SVT extension at 3 cm or less from the sapheno-femoral junction (SFJ), a benefit that was maintained at 30 days follow-up.5
While there is no doubt that preventing deep-vein thrombosis or pulmonary embolism is an important therapeutic goal,6 the clinical relevance of preventing SVT extensions is controversial. In CALISTO, a cutoff value of 3 cm from the SFJ was chosen for defining SVT extension because most authors and consensus groups emphasize that extensions involving the SFJ are associated with an increased risk of thrombus propagation into the deep venous system.7-12 However, few data are available describing the natural history of patients presenting with a SVT extension, whether this progressed to within ≤3 cm or to >3 cm from the SFJ, and the optimal therapeutic strategy to manage this complication remains unclear.
With a unique opportunity to use the CALISTO database, our primary objective was therefore to examine the practice management and outcome of patients with any type of symptomatic extension of spontaneous, acute, symptomatic, isolated SVT of the legs and to assess the effect of fondaparinux on such complications.
Methods
The present study is based on a post hoc analysis of the CALISTO database. The methods used in CALISTO have been described elsewhere5 and are summarized below.
Patients
Hospitalized or nonhospitalized patients aged ≥18 years with an acute, symptomatic lower-limb SVT at least 5 cm long, confirmed by standardized compression ultrasonography, were eligible for randomization. The main exclusion criteria were documented symptomatic or asymptomatic deep-vein thrombosis or documented symptomatic pulmonary embolism at presentation, SVT located within 3 cm from the SFJ, documented history of SVT within the last 3 months, deep-vein thrombosis or pulmonary embolism within the last 6 months, or active cancer within the last 6 months, and any conditions favoring bleeding.
Study design and treatments
CALISTO was an international (European), multicenter, randomized (centrally), double-blind, placebo-controlled study evaluating the efficacy and safety of fondaparinux 2.5 mg once daily for 45 days. The day of randomization was defined as day 1. Follow-up visits were scheduled at days 10 ± 2, 30 ± 2, 45 ± 2, and 75 ± 2. Routine ultrasonographic examinations were discouraged during follow-up.
Study medications were packaged in identical boxes,1 per patient, containing sufficient visually identical, prefilled, 0.5-mL single-dose syringes of either fondaparinux sodium 2.5 mg (Arixtra, GlaxoSmithKline) or placebo (sodium chloride) to allow 45-day treatment. Self-administration was encouraged, but the final decision was left to the investigator’s discretion.
The use of graduated compression stockings was encouraged for all patients. In addition, patients were free to take paracetamol (acetaminophen) or topical nonsteroidal antiinflammatory drugs as needed. Concomitant therapy interfering with the study treatments was prohibited throughout the study, in particular, the use of any other anticoagulant agent, more than 1 antiplatelet agent, aspirin at doses >325 mg per day, oral nonsteroidal antiinflammatory drugs, and topical heparins or heparinoids. The management of venous thromboembolic events occurring during follow-up was left to the investigator’s discretion.
The study was conducted according to the ethical principles stated in the Declaration of Helsinki and local regulations. An independent ethics committee approved the protocol, and written informed consent was obtained from all patients before randomization.
Outcome measures
The main outcome investigated in this post hoc analysis was ultrasonographically confirmed symptomatic extension of the index SVT reported by the investigators up to day 77 and validated by the central adjudication committee as being an extension. Extension was defined as downstream (proximal) progression of the initial thrombus by at least 2 cm. We considered 2 types of extension: those progressing to within 3 cm from the SFJ, which were a component of the composite primary efficacy outcome of the CALISTO study,5 and those not reaching the SFJ, that is, located at >3 cm from the sapheno-femoral junction.
We also included as outcomes the incidence of symptomatic pulmonary embolism and symptomatic deep-vein thrombosis occurring subsequent to a symptomatic extension, whether to <3 cm or to >3 cm from the sapheno-femoral junction, and the incidence of the composite of symptomatic pulmonary embolism, symptomatic deep-vein thrombosis, and symptomatic SVT recurrence or any symptomatic extension up to day 77. The management of symptomatic extensions was described. All outcomes were prospectively recorded during the CALISTO trial.
Symptomatic pulmonary embolism was confirmed by ventilation-perfusion scanning, helical computed tomography, pulmonary angiography, or autopsy. Symptomatic deep-vein thrombosis was confirmed by ultrasonography or venography. Recurrence was defined as a new thrombus located in a different superficial vein and not directly contiguous upstream with the index thrombus or located in the same superficial vein but separated from the index thrombus by at least 10 cm. All symptomatic extensions or symptomatic recurrences of SVT were confirmed by ultrasonography. All duplex ultrasonographic examinations were performed according to a predefined procedure standardized across all centers,13-15 summarized in the supplemental Apppendix.
Finally, all symptomatic thromboembolic complications listed above were reviewed by a central adjudication committee, the members of which were unaware of the patients’ group assignments.
Statistical analysis
All analyses were performed on data from the intention-to-treat population of CALISTO, defined as all randomized patients (n = 3002). Patients for whom an efficacy assessment was lacking (ie, those for whom no information on their status with respect to thromboembolic complications at the end of the study was recorded) were assumed not to have experienced any event. The time for evaluation in this post hoc analysis was at day 77.
Usual descriptive statistics were employed: quantitative variables were presented as mean and standard deviation or median (range) where appropriate, qualitative variables being reported as rates with corresponding 95% confidence intervals (CIs). A 2-sided Fisher exact test at the 5% significance level was performed for efficacy evaluations. The resultant P values were specified. Absolute differences and relative risks, with 95% CIs, were also presented.
Role of the funding source
GlaxoSmithKline funded the study. A steering committee was responsible for the design, conduct, and reporting of the study. Data were collected and analyzed by the study sponsor. An independent central adjudication committee managed the database of adjudicated outcomes. All authors contributed to designing this post hoc analysis; had full access to the data and analyses; contributed to interpreting the data and developing the report; agreed on the final version of the manuscript; and had final responsibility for the report content and decision to publish the findings.
Results
Study population
The CALISTO study enrolled 3002 patients between March 2007 and May 2009, of whom 1500 and 1502, respectively, were randomized to the placebo and fondaparinux groups. Demographic and clinical characteristics, prestudy medications and interventions, treatment duration and compliance, and the use of most other treatments during the study were well balanced between the 2 groups and have been described elsewhere.5 However, placebo-treated patients received anticoagulant therapy and underwent surgery for SVT more frequently than fondaparinux-treated patients during the study (6.4% vs 1.1% and 4.1% vs 1.0%, respectively).5
Symptomatic SVT extension in the placebo group
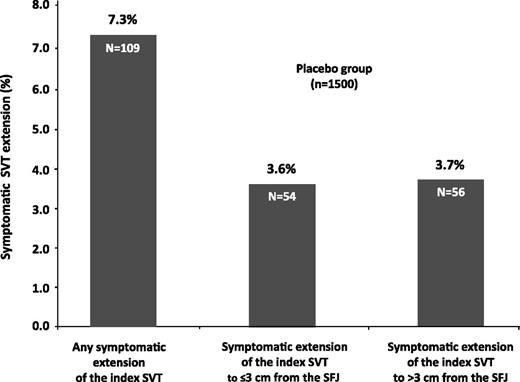
Symptomatic extension of the index SVT occurred in 109 of 1500 patients (7.3%) in the placebo group (Figure 1). The head of the thrombus extension was at ≤3 cm from the SFJ in 54 (3.6%) patients and at >3 cm from the SFJ in 56 (3.7%); 55/56 of the latter having occurred by day 47.
Symptomatic SVT extensions at day 77 in the placebo group of the CALISTO study.
Overall, placebo-treated patients with a SVT extension to >3 cm from the SFJ received as many treatments in addition to the study drug as patients with a SVT extension to ≤3 cm from the SFJ (Table 1). In particular, anticoagulants were used in more than 50% (mainly at therapeutic doses) and oral antiinflammatory agents in more than 20% of patients for both categories of SVT extension. Patients with extensions not involving the SFJ attended fewer face-to-face visits (in addition to those scheduled in the study protocol) after event diagnosis. However, these patients underwent more compression ultrasonography examinations to follow up the extension after diagnosis than patients with an extension to ≤3 cm from the SFJ. Moreover, they were less frequently hospitalized after event diagnosis or treated surgically for superficial-vein thrombosis. However, when hospitalized or operated on, the duration of hospitalization and the types of intervention (mainly ligation of the SFJ) were similar in patients with a SVT extension to >3 cm from the SFJ and those with an extension to ≤3 cm from the SFJ. Interestingly, of 6 (11.1%) placebo-treated patients with a SVT extension to ≤3 cm from the SFJ and 14 (25.0%) placebo-treated patients with a SVT extension to >3 cm from the SFJ, none received any anticoagulant treatment nor underwent surgery.
Management of patients with symptomatic SVT extension up to day 77 in the placebo group of the CALISTO study
| . | Placebo group (N = 1500) . | |
|---|---|---|
| Extension to ≤3 cm from the SFJ . | Extension to >3 cm from the SFJ . | |
| Treatment received during the study, no. (%) | ||
| Graduated compression stockings | 46 (3.1) | 51 (3.4) |
| Analgesic agents | 16 (1.1) | 22 (1.5) |
| Topical nonsteroidal antiinflammatory drugs | 17 (1.1) | 30 (2.0) |
| Topical anticoagulant drugs | 5 (0.3) | 8 (0.5) |
| Oral nonsteroidal antiinflammatory drugs/anti-cox 2 | 12 (0.8) | 13 (0.9) |
| Aspirin or other antiplatelet agents | 17 (1.1) | 15 (1.0) |
| Anticoagulant treatment* | 29 (1.9) | 30 (2.0) |
| High (therapeutic dose) | 19 (1.3) | 17 (1.1) |
| Intermediate dose | 1 (0.1) | 2 (0.1) |
| Low (prophylactic dose) | 14 (0.9) | 14 (0.9) |
| Unknown dose | 1 (0.1) | 1 (0.1) |
| Face-to-face visit after event diagnosis (in addition to those planned by the protocol), no. (%) | 34 (2.3) | 24 (1.6) |
| Compression ultrasonography to follow up this event after diagnosis, no. (%) | 19 (1.3) | 29 (1.9) |
| Hospitalization after event diagnosis, no. (%) | 33 (2.2) | 22 (1.5) |
| Duration, days (mean ± standard deviation) | 6.4 ± 6.3 | 6.6 ± 4.3 |
| Surgery to treat SVT, no. (%)† | 34 (2.3) | 20 (1.3) |
| No surgery to treat SVT, no. (%) | 19 (1.3) | 36 (2.4) |
| and no anticoagulant treatment either | 6 (0.4) | 14 (0.9) |
| . | Placebo group (N = 1500) . | |
|---|---|---|
| Extension to ≤3 cm from the SFJ . | Extension to >3 cm from the SFJ . | |
| Treatment received during the study, no. (%) | ||
| Graduated compression stockings | 46 (3.1) | 51 (3.4) |
| Analgesic agents | 16 (1.1) | 22 (1.5) |
| Topical nonsteroidal antiinflammatory drugs | 17 (1.1) | 30 (2.0) |
| Topical anticoagulant drugs | 5 (0.3) | 8 (0.5) |
| Oral nonsteroidal antiinflammatory drugs/anti-cox 2 | 12 (0.8) | 13 (0.9) |
| Aspirin or other antiplatelet agents | 17 (1.1) | 15 (1.0) |
| Anticoagulant treatment* | 29 (1.9) | 30 (2.0) |
| High (therapeutic dose) | 19 (1.3) | 17 (1.1) |
| Intermediate dose | 1 (0.1) | 2 (0.1) |
| Low (prophylactic dose) | 14 (0.9) | 14 (0.9) |
| Unknown dose | 1 (0.1) | 1 (0.1) |
| Face-to-face visit after event diagnosis (in addition to those planned by the protocol), no. (%) | 34 (2.3) | 24 (1.6) |
| Compression ultrasonography to follow up this event after diagnosis, no. (%) | 19 (1.3) | 29 (1.9) |
| Hospitalization after event diagnosis, no. (%) | 33 (2.2) | 22 (1.5) |
| Duration, days (mean ± standard deviation) | 6.4 ± 6.3 | 6.6 ± 4.3 |
| Surgery to treat SVT, no. (%)† | 34 (2.3) | 20 (1.3) |
| No surgery to treat SVT, no. (%) | 19 (1.3) | 36 (2.4) |
| and no anticoagulant treatment either | 6 (0.4) | 14 (0.9) |
Patients could have received more than 1 type or dosage regimen of anticoagulant treatment.
Patients with SVT to ≤3 cm from the SFJ: ligation of the sapheno-femoral or sapheno-popliteal junction in 31 and local thrombectomy in 3; patients with SVT to >3 cm from the SFJ: ligation of the sapheno-femoral or sapheno-popliteal junction in 18, local thrombectomy in 1, and varicose vein stripping within the great saphenous vein in 1.
In placebo-treated patients with extension, the risk of subsequent deep-vein thrombosis or pulmonary embolism was similar whether the extension progressed to ≤3 cm or >3 cm from the SFJ: 9.3% (5 of 54 patients, proximal deep-vein thrombosis in 4 patients and pulmonary embolism in 1) and 8.9% (5 of 56 patients, deep-vein thrombosis in 3 patients, proximal in 1 and distal in 2, and pulmonary embolism in 2), respectively.
Overall, symptomatic venous thromboembolic complications, that is, the composite of symptomatic deep-vein thrombosis, pulmonary embolism, and SVT recurrence or extension (irrespective of the distance of the head of the extension from the SFJ), occurred in 141 of 1500 patients (9.4%) in the placebo group.
Fondaparinux effect on the incidence, management, and thrombotic complications of symptomatic SVT extension
Symptomatic extension of the index SVT occurred in 17 of 1502 patients (1.1%) in the fondaparinux group (relative risk fondaparinux vs placebo: 0.16; 95% CI, 0.10 to 0.26; P < .001; supplemental Figure 1). Fondaparinux reduced the risk of extension to ≤3 cm and to >3 cm from the SFJ by the same magnitude, from 3.6% to 0.3% (n = 5/1502; relative risk fondaparinux vs placebo: 0.09; 95% CI, 0.04 to 0.23; P < .001) and from 3.7% to 0.8% (n = 12/1502; relative risk fondaparinux vs placebo: 0.21; 95% CI, 0.12 to 0.40; P < .001), respectively (supplemental Figure 1). Eight of the 12 episodes of symptomatic extension to >3 cm from the SFJ in the fondaparinux group had occurred by day 47.
Compared with placebo, fondaparinux was associated with reduced use of medical resources, particularly in terms of anticoagulant treatment at therapeutic dosage (0% vs 1.9%), surgery to treat SVT (0.5% vs 3.6%), and need for additional face-to-face visits (0.5% vs 3.9%) and hospitalizations (0.5% to 3.7%; supplemental Table 1). All 5 fondaparinux-treated patients with SVT extension to ≤3 cm from the SFJ underwent surgery for superficial-vein thrombosis, whereas 7 of the 12 fondaparinux-treated patients with SVT extension to >3 cm from the SFJ did not undergo anticoagulant treatment or surgery. None of the fondaparinux-treated patients presenting symptomatic SVT extension, whether to ≤3 cm or to >3 cm from the SFJ, experienced subsequent deep-vein thrombosis or pulmonary embolism (supplemental Figure 2).
Overall, fondaparinux reduced the rate of any symptomatic thromboembolic complication up to day 77 by 79%, from 9.4% (141 of 1500 patients) in the placebo group to 1.9% (29 of 1502 patients; relative risk: 0.21; 95% CI, 0.14 to 0.30; P < .001; number needed to treat: 13; Figure 2).
Discussion
The placebo group of the CALISTO study, comprising 1500 patients, represents to date the largest series of untreated patients with spontaneous, acute, symptomatic SVT of the legs, without deep-vein thrombosis or pulmonary embolism at presentation, allowing description of the natural history of the disease. Although derived from a post hoc analysis, our findings are based on prospectively collected data on objectively confirmed symptomatic events validated by a blinded adjudication committee. The main finding of the present study is that symptomatic extension of the index SVT is a common complication in these patients, associated with an increased risk of subsequent deep-vein thrombosis or pulmonary embolism and greater consumption of medical resources, irrespective of the distance of the thrombus head from the SFJ at diagnosis of the extension. Compared with placebo, fondaparinux significantly reduced the risk of symptomatic extensions and subsequent symptomatic venous thromboembolic events and was associated with lower use of additional medical resources.
Spontaneous SVT of the legs has long been regarded as a benign, self-limiting disease, usually expected to resolve spontaneously within a few weeks.7,16 During the last 3 decades, however, several studies have shown that it can be associated with a significant risk of thromboembolic complications, including deep-vein thrombosis, pulmonary embolism, and extension or recurrence of the index event, even in patients receiving anticoagulant treatments.2-4,12,17 Our study confirms these findings, with a 9.4% rate of such objectively confirmed symptomatic thromboembolic complications in untreated patients at day 77, including a 7.3% rate of symptomatic SVT extensions. These high rates were observed despite the exclusion of patients in the highest risk category for thromboembolic complications (such as those with active cancer or recent venous thromboembolism) from the CALISTO study, to avoid their potential exposure to treatment with a placebo. Moreover, as patients in the CALISTO study underwent close clinical monitoring (probably more intensive than that likely in a real-life setting), most SVT extensions were probably detected early on, that is, before their propagation into the deep venous system.
Contiguous propagation of the thrombotic process from a superficial vein into a deep vein via the SFJ, the sapheno-popliteal junction, or a perforating vein is probably the main mechanism underlying the association between SVT of the legs and deep-vein thrombosis or pulmonary embolism.7,8,17,18 As the great saphenous vein is the most frequent location of spontaneous SVT of the leg17 (more than 92% in CALISTO5 ), the SFJ connecting the great saphenous vein to the common femoral vein, extension of a SVT to within 3 cm from this junction may be considered as serious as a proximal deep-vein thrombosis. In patients with SVT extension involving the SFJ, most authors and consensus groups recommend full-dose anticoagulant therapy.7-11 However, it is also common practice in some centers to perform surgical ligation of the SFJ or thrombectomy. In accordance with these recommendations or practices, 88.9% (48/54) of placebo patients and all fondaparinux patients with an extension progressing to ≤3 cm from the SFJ received such treatments. In the placebo group, 5 (9.3%) of these patients experienced subsequent deep-vein thrombosis or pulmonary embolism vs none in the fondaparinux group.
The clinical significance of SVT extension not involving the SFJ (ie, >3 cm from the SFJ) was less clear, and its management remains controversial. Reducing the risk of symptomatic extension may be viewed as an important and valid therapeutic objective, in general, as these complications are associated with pain, impaired mobility, and additional consultations and ultrasonographic tests, as shown in our study. However, in our view, the main issue is the potential of such extensions to propagate into the deep venous system. In a retrospective study of untreated patients with SVT but no deep-vein thrombosis at presentation, progression to the deep venous system was reported in 16 (23.9%) of the 67 patients with an initial thrombus in the proximal great saphenous vein but not involving the SFJ.8 In CALISTO, investigators were free to manage thromboembolic complications of the index event according to their usual practice. The overall use of medical resources was the same whether the extension was <3 cm or >3 cm from the SFJ, particularly with respect to anticoagulant therapy, although placebo-treated patients with an extension to >3 cm from the SFJ underwent surgery less frequently and 25% (14 of 56 patients) neither underwent surgery nor received anticoagulant treatment. Subsequent deep-vein thrombosis or pulmonary embolism was reported in 5 (8.9%) of the placebo patients with an extension not involving the SFJ, corresponding to a risk of propagation into the deep venous system similar to that of patients with extensions involving the SFJ. Interestingly, although 58% (7 of 12) of fondaparinux patients with an extension progressing to >3 cm from the SFJ neither underwent surgery nor received anticoagulant treatment, none developed subsequent deep-vein thrombosis or pulmonary embolism. A possible explanation is that when an extension occurs under fondaparinux treatment, it is less severe in terms of the length of the thrombus and/or its location.
The comparable risk of subsequent deep-vein thrombosis or pulmonary embolism observed in the placebo group between patients with extensions advancing to ≤3 cm and to >3 cm from the SFJ, respectively, suggests that the 3-cm limit acknowledged as indicative of extension severity7-11 might be artificial. Our data suggest that propagation of the thrombus is a continuum and that the occurrence of thrombus extension by itself is indicative of severity compared with thrombi remaining locally confined. Patients with an extension to >3 cm and to ≤3 cm, respectively, from the SFJ are likely to have been comparable, the only difference being that the former were seen at an earlier stage of their disease because they experienced symptoms earlier. A logical reaction might be to propose monitoring of extensions using serial ultrasonography. In CALISTO, ultrasonography was permitted only to confirm symptomatic events, and systematic ultrasound examinations were discouraged. Nevertheless, we believe that serial ultrasonography is not the ideal management strategy for 2 reasons: (1) it was shown to be neither efficient nor cost effective in a series of patients with spontaneous acute symptomatic SVT of the legs, without deep-vein thrombosis or pulmonary embolism at presentation,19 and (2) in our study, fondaparinux was effective in reducing, by the same magnitude, the incidence of any type of SVT extension, the resulting morbidity, and the related use of additional medical resources, with no serial untrasonographic monitoring.
Overall, this study supports the common knowledge that extension of SVT involving the SFJ is a clinically important event. Moreover, it shows that symptomatic extensions not reaching the SFJ are common and also associated with a significant risk of subsequent deep-vein thrombosis or pulmonary embolism, suggesting that more aggressive therapeutic strategies (anticoagulant treatment and/or surgery) is needed in these patients. Fondaparinux consistently decreased the risk of symptomatic extensions irrespective of the distance of the head of the thrombus from the SFJ as well as subsequent venous thromboembolic complications. In addition, fondaparinux was associated with reduced consumption of medical resources, an additional benefit to be taken into account when evaluating the cost effectiveness of this drug in the treatment of spontaneous, acute, symptomatic SVT of the legs.
Presented in abstract form at the 53rd annual meeting of the American Society of Hematology, San Diego, CA, December 10–13, 2011.
The online version of the article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to Dr Jean-Yves Darmon (MediBridge, France) who provided drafts and editorial assistance to the authors during preparation of this manuscript, supported by funding from GlaxoSmithKline.
This study was supported by a grant from GlaxoSmithKline.
Authorship
Contribution: A steering committee was responsible for the design, conduct, and reporting of the CALISTO study; data were collected and analyzed by the study sponsor; an independent central adjudication committee managed the database of adjudicated outcomes; A.L., P.P., and H.D. served on the steering committee; F.B. served on the central adjudication committee and was chair of the compression ultrasonography validation committee; I.Q. was the national coordinator in France and enrolled patients; all authors contributed to the design of this post hoc analysis; had full access to the data and analyses; contributed to interpreting the data and developing the report; agreed on the final version of the manuscript; and vouch for the report’s accuracy and completeness.
Conflict-of-interest disclosure: A.L. reports having received research grant support from Bristol-Myers Squibb and fees for board memberships from Bayer Pharmaceuticals, Boehringer Ingelheim, GlaxoSmithKline, and Sanofi-Aventis. F.B. reports having received honoraria for his participation in the compression ultrasonography validation committee. I.Q. reports having received honoraria from GlaxoSmithKline for participation in this study as an investigator and for having presented the study data. P.P. reports having received research grant support and consultancy fees from GlaxoSmithKline. H.D. reports having received research grant support from Bayer, Daiichi-Sankyo, and GlaxoSmithKline as well as fees for board membership from Bayer, Daiichi-Sankyo, and GlaxoSmithKline. The remaining author declares no competing financial interests.
Correspondence: Alain Leizorovicz, Service de Pharmacologie Clinique, UMR 5558, Faculté de Médecine RTH Laennec, Rue Guillaume Paradin, BP 8071, 69376 Lyon cedex 08, France; e-mail: al@upcl.univ-lyon1.fr.