Key Points
This is the first report about the detection of human Tregs specific for minor histocompatibility antigens.
We detected, quantified, and cloned mHA-specific Tregs and expanded these potent Tregs in sufficient numbers for use in human transplantation.
Abstract
Alloreactive donor T cells against host minor histocompatibility antigens (mHAs) cause graft-versus-host disease (GVHD) after marrow transplantation from HLA-identical siblings. We sought to identify and expand regulatory CD4 T cells (Tregs) specific for human mHAs in numbers and potency adequate for clinical testing. Purified Tregs from normal donors were stimulated by dendritic cells (DCs) from their HLA-matched siblings in the presence of interleukin 2, interleukin 15, and rapamycin. Male-specific Treg clones against H-Y antigens DBY, UTY, or DFFRY-2 suppressed conventional CD4 T cell (Tconv) response to the specific antigen. In the blood of 16 donors, we found a 24-fold (range, 8-fold to 39-fold) excess Tconvs over Tregs reactive against sibling mHAs. We expanded mHA-specific Tregs from 4 blood samples and 4 leukaphereses by 155- to 405-fold. Cultured Tregs produced allospecific suppression, maintained demethylation of the Treg-specific Foxp3 gene promoter, Foxp3 expression, and transforming growth factor β production. The rare CD4 T conv and CD8 T cells in the end product were anergic. This is the first report of detection and expansion of potent mHA-specific Tregs from HLA-matched siblings in sufficient numbers for application in human transplant trials.
Introduction
Graft-versus-host disease (GVHD) remains the main cause of treatment-related mortality after allogeneic bone marrow transplantation.1,2 GVHD is mediated by donor CD4+ and CD8+ T cells, which inflict damage to the recipient target organs including the skin, intestines, liver, and lung.3 In the case of HLA-identical sibling transplants, the risk of acute GVHD is increased in male recipients of female grafts, denoting pathogenic alloreactivity against male-associated H-Y antigens. These and other minor histocompatibility antigens (mHAs) produce antigenic peptides presented by HLA molecules on recipient or donor antigen-presenting cells (APCs) that sensitize alloreactive donor T cells and cause GVHD.4-7
Regulatory T cells (Tregs) are naturally occurring or induced during a tolerogenic immune response.8 Tregs are distinguished by constitutive expression of the interleukin 2 (IL-2) receptor α chain (CD25)9 and the transcription factor Foxp3.10,11 Their potent, antigen-driven immune suppression and their dominant role in transplantation tolerances have made Tregs an attractive candidate for adoptive immunotherapy.12 Studies in rodents with adoptive transfer of in vitro–expanded natural or induced Tregs have shown prevention of lethal GVHD13,14 and, in most examples, preservation of graft-versus-tumor responses.15,16 Tregs express a T-cell receptor repertoire that enables them to recognize self-antigens17 or alloantigens.15,18 Given their low frequency in human blood, several groups have explored ex vivo Treg expansion for therapeutic application and cultured Treg-retained suppressive activity.10,11,19-21 In contrast to polyspecific Tregs, antigen-specific Tregs produce selective suppression of alloresponses with no effect on third-party responses and facilitate alloantigen-specific tolerance after marrow transplantation and organ grafting in rodents.14,15,22-24 Previously, we measured the frequency, growth requirements, and functional phenotype of ex vivo–expanded human Tregs against disparate HLA.18 While CD8 T cells specific for mHAs expressed on leukemic cells were isolated, expanded in vitro, and infused into allogeneic bone marrow transplant recipients to prevent or treat leukemia relapse,25 there are no reports on the identification of mHA-specific Tregs in humans.
In the present study, we have detected mHA-specific, functional CD4 Tregs and cloned them. We measured the blood frequency of mHA-specific Tregs against HLA-identical siblings and used good manufacturing practice (GMP) for expanding mHA-specific Tregs in numbers sufficient for therapeutic application. The expanded Tregs maintained viability, antigen-specific suppression, transforming growth factor β (TGF-β) production, demethylation of the Treg-specific Foxp3 demethylation region (TSDR), and Foxp3 expression. The contaminating CD8 and CD4 conventional T cells in the final product were rare and anergic in response to specific antigen. With these data in hand, we have planned a first-in-humans phase 1 study for the prevention of acute GVHD in HLA-identical sibling transplants.
Methods
Cell sources and CD25 separation
Eligible for the study were sibling pairs matched for HLA-A, B, C, DRB1, and DQB1. Typing for HLA-DPB1 was not performed because the probability of a DQB/DPB recombination is less than 1%. The study protocol was approved by the University of South Florida institutional review board. Subjects donated 100 mL of blood or cytapheresis after providing written informed consent in accordance with the Declaration of Helsinki. Tregs were isolated from blood samples using the CD4+CD25+CD127− Treg isolation kit II (Miltenyi Biotech), involving negative selection of CD4+CD127− T cells followed by positive selection of CD25. For some experiments, CD4+CD25+CD127− Tregs were instead isolated on a BD FACSAria II high-speed cell sorter (BD Biosciences) under sterile conditions. Purified Treg populations obtained from magnetic or flow sort methods were 95% to 99% pure. For large-scale expansion, CD25+ Tregs were purified from leukapheresis products by single-step CD25-specific beads (catalog #274-01) using CliniMACS (Miltenyi Biotech) in the Cell Therapy Core Facility at the Moffitt Cancer Center. The CD25-specific beads were optimized by the manufacturer to select 6 × 109 highly expressing CD25 cells from 40 × 109 white blood cells from leukapheresis in a volume of 380 mL. Cells were washed with isotonic saline supplemented with EDTA and 0.5% human serum albumin, incubated with paramagnetic Fe3O4tagged CD25 monoclonal antibodies at 4°C to 8°C for 15 minutes, and loaded onto the CliniMACS for automated separation. The tagged cells were pooled in the collection bag, washed, and resuspended in RPMI medium supplemented with 10% human AB-positive serum.
Alloresponses of mHA-specific Tregs
For small-scale experiments, monocytes were separated by CD14 immunoabsorption, whereas for large-scale experiments, monocytes were enriched by plastic adherence. Monocyte-derived DCs were produced by culture in X-VIVO 15 (Lonza) or CellGenix DC serum-free medium for 6 days in 1000 U/mL GM-CSF (R&D Systems) and 1000 U/mL IL-4 (R&D Systems). DCs were γ-irradiated (3000 cGy). Although the potential contaminating conventional CD4 T cells (Tconvs) are sensitive to rapamycin, Tregs express PTEN, a negative regulator of the PI3-K/AKT/mTOR pathway, and are relatively resistant to rapamycin.26 The additive effect of IL-15 and IL-2 is critical for maximal Treg expansion, bcl-2 expression, and survival in the presence of rapamycin.18 Freshly isolated Tregs and HLA-matched sibling DCs or self-DCs were seeded at a 1:1 ratio in the presence of IL-2 (10 U/mL; R&D Systems), IL-15 (10 ng/mL; R&D Systems), and rapamycin (100 ng/mL; Millipore). The optimal DC:Treg ratio was determined in titration experiments (data not shown) and found to be between 2:1 and 1:2 at optimal cell density. Culture medium was RPMI 1640 plus glutamine supplemented with 0.02 mM pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated human pooled AB serum (SeraCare or Omega Scientific). Cells were cultured at 37°C, 95% humidity, and 5% CO2 and in 96-well round- or flat-bottom plates (Nunc) or 225 cm2 tissue culture flask, as indicated. For small-scale mHA-specific Treg expansion, approximately 1 × 105 Tregs were cultured with 1 × 105 irradiated HLA-matched recipient DCs in 96-well flat-bottom plates. Treg cultures were fed with fresh medium containing cytokines plus rapamycin on days 6, 8, and 10. To determine expansion kinetics of mHA-specific Tregs, the frequency of antigen-specific Tregs was calculated by limiting dilution analysis (LDA) at the start (day 0) and end (day 12) of the culture.
Assays for proliferation, cytokine release, anergy, and suppression
Proliferation was analyzed by 3H-thymidine incorporation using a gas scintillation counter (Matrix 96 β counter; Canberra Packard). Cells were pulsed with 1 μCi per well 3H-thymidine for the last 18 hours in culture and harvested on day 6 to measure proliferation. Results are expressed in counts per minute (CPM) of at least triplicate measurements. Where indicated, culture supernatants were collected and analyzed for TGF-β and tumor necrosis factor (TNF) levels by enzyme-linked immunosorbent assay (ELISA; eBioscience). To determine the fraction of replicating cells, cells were cultured in a glucose-free formulation of RPMI 1640 supplemented with the addition of [6, 6-2H2] glucose (Cambridge Isotope Laboratories, Cambridge, MA). Deuterium-labeled glucose is taken up by replicating cells, metabolized through the pentose-phosphate pathway to phosphoribose-pyrophosphate, and incorporated into nucleic acids.27,28 After 12 days, genomic DNA-associated deuterium was quantified by mass spectrometry as described elsewhere.29 To assess for anergy, end-of-culture or sorted subpopulations were sorted with a fluorescence-activated cell sorter and cultured with 1 × 103 irradiated allogeneic DCs or self-DCs in the presence or absence of IL-2 (10 U/mL) for 6 days. Culture supernatants were collected and tested for TGF-β or TNF, and remaining cells were pulsed with 3H-thymidine to measure proliferation. Suppression by Tregs was assayed in a mixed leukocyte culture assay. Expanded Tregs were titrated and mixed with 1 × 105 effectors (CD4+CD25− T cells) and mixed with 1 × 104 irradiated DCs from an original or third-party stimulator. Proliferation was analyzed by 3H-thymidine incorporation.
LDA and T-cell cloning
The frequency of mHA-specific Tregs or Tconvs in blood or apheresis samples was determined by LDA as described previously.20 Briefly, T cells were seeded at various numbers increasing by 2-fold from 6 to 15 000 per well and cultured with a fixed concentration of DCs (2 × 103) in a minimum of 10 replicates in U-bottomed 96-well plates. Treg cultures were supplemented with IL-2 (10 U/mL) and IL-15 (10 ng/mL) cytokines with rapamycin (100 ng/mL) and Tconv cultures with IL-2 alone. Wells were considered positive if CPM (or cytokine concentration) was greater than the mean plus 3 standard deviations (SDs) over the sum of negative control responder and stimulator cells, with each cultured alone. Online extreme LDA (http://bioinf.wehi.edu.au/software/elda/index.html) was used to calculate the frequency and 95% confidence interval (95% CI) of replicating T cells. We previously validated LDA by testing carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled Tregs sorted 8 days after alloantigen stimulation using DCs from HLA-incompatible donors.18 LDA showed enrichment of alloreactive Tregs among CFSE-negative (replicated) compared with CFSE-positive (resting) Tregs, but only 9% of CFSE-negative Tregs were alloreactive. Because CFSE estimates approach the frequency of antigen-specific T cells detected by HLA:peptide tetramers and γ-IFN release, the sensitivity of LDA may be as low as 9%.30 T-cell cloning was performed as detailed in supplemental “Materials.” The H-Y–associated genes DBY, UTY, SMCY, or DFFRY-2, encoded by the Y chromosome, were cloned into a Moloney murine leukemia virus–based retroviral vector LZRS with green fluorescent protein tag and kindly provided by M. Griffioen and J. H. Falkerburg (Leiden University Medical Center, Netherlands).
Flow cytometry
Purity of Tregs and viability testing by Live/Dead Yellow (Life Technologies) were analyzed before and after Treg expansion using multicolor flow cytometry (LSR II; BD Biosciences). Cells were surface stained with CD3-PB, CD4-A700, CD127-A647, CD25-APC-Cy7, CD62L-PE-Cy7, CD45RA-PerCP-5.5, and CCR7-PE (BD Biosciences). As noted, cells were stained with CD3-PB, CD4-A700, CD127-A647, and CD25-PE-Cy7 and then fixed and permeabilized using the Fix and Fix/Perm buffer (eBioscience) according to the manufacturer’s instructions and stained with Foxp3-PE (BD Biosciences) or BCL-2-PE and Foxp3-A488. A separate panel included staining with CD3-APC-Cy7, CD8-PE, CD16-A700, CD56-PerCP5.5, CD19-PE-Cy7, CD14-PB, and CD11c-APC. DC purity was checked with CD3-APC-Cy7, CD8-PE, CD16-A700, CD56-PerCP5.5, CD19-PE-Cy7, CD14-PB, and CD11c-APC. Data were analyzed with FlowJo software version 9.3 (Tree Star). Isotype controls or fluorescence minus one was used for markers or gate settings.
Foxp3 TSDR demethylation
This highly conserved region within the human Foxp3 gene is demethylated exclusively on Tregs and not in any other blood-cell or non–blood-cell types. The percentage FoxP3 demethylation of naïve or expanded Tregs was measured using quantitative polymerase chain reaction (qPCR) as described by Wieczorek et al in 2009, with minor modification.31 Details of the assay are provided in supplemental “Materials.”
Statistical analysis
Differences in frequency or proliferation of alloreactive T cells were analyzed by the 2-tailed Student t test, and P < .05 was considered significant. A 2-way analysis of variance (ANOVA) model was used to calculate the specificity of the suppressive effect of each Treg clone.
Results
Detection of mHA-specific Tregs and isolation of H-Y–specific Treg clones
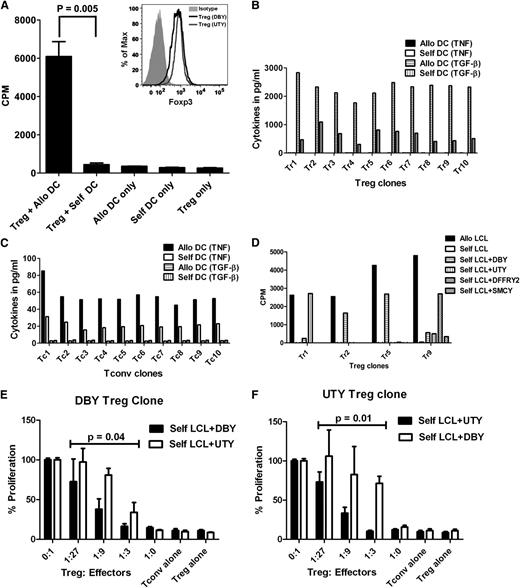
We sought to characterize the responses of purified Tregs from normal individuals against mHAs expressed by their HLA-identical sibling APCs. CD4+/CD25+/CD127− Tregs from volunteer donors were enriched by CD25 immunoabsorption, and cultured with the sibling DC, in the presence of IL-2, IL-15, and rapamycin. Under these conditions, we detected a 15- to 50-fold higher 3H-TdR uptake at 6 days in Treg cultures stimulated by HLA-identical, mHA-incompatible sibling DCs compared with self-DCs (Figure 1A).
mHA-specific Treg proliferative response. (A) Tregs were purified from a normal donor and stimulated with γ-irradiated dendritic cells from an HLA-matched sibling in the presence of rapamycin (100 ng/mL), IL-2 (10 U/mL), and IL-15 (10 ng/mL). Each bar represents mean + SD of 3H-thymidine uptake (in CPM) on day 6 of culture. The inset shows Foxp3 expression of DBY-specific (bold line) and UTY-specific (thin line) Treg clones by flow cytometry. (B,C) Identification of mHA-specific T-cell clones. Treg (B) and Tconv (C) cells were obtained from a female, purified, and stimulated with γ-irradiated dendritic cells from an HLA-matched brother; wells positive for TGF-β release were pooled and cloned by limiting dilution. After 14 days, wells containing Tconv or Treg clones were split into 2 and restimulated with allogeneic (male) or self- (female) DCs for 24 hr. Each bar represents TGF-β or TNF released by mHA-specific T-cell clones in the culture supernatant after restimulation with allogeneic DCs or self-DCs. (D) mHA-specific Treg clones were tested for proliferative response to allogeneic LCL, self-LCL, or self-LCL transduced with DBY, UTY, DFFRY-2, or SMCY. Results shown here are CPM and are representative of 2 experiments. (E) The DBY-specific Treg clone was cultured with a different ratio of autologous-DBY–specific Tconvs in the presence of DBY-transduced autologous LCL, with autologous-UTY–specific Tconvs in the presence of UTY-transduced auto-LCL as specificity control. Mean CPM of DBY- or UTY-specific Tconvs with the stimulators alone was 3919 and 4914, respectively. (F) The UTY-specific autologous Treg clone was cultured with a different ratio of autologous-UTY–specific Tregs in the presence of UTY-transduced autologous LCL, with autologous-DBY–specific Tconvs in the presence of DBY-transduced autologous LCL as specificity control. Mean CPM of UTY- or DBY-specific Tconvs with the stimulators alone was 4906 and 4053, respectively. P values refer to the difference in suppression of specific vs control Tconvs by the ANOVA model.
mHA-specific Treg proliferative response. (A) Tregs were purified from a normal donor and stimulated with γ-irradiated dendritic cells from an HLA-matched sibling in the presence of rapamycin (100 ng/mL), IL-2 (10 U/mL), and IL-15 (10 ng/mL). Each bar represents mean + SD of 3H-thymidine uptake (in CPM) on day 6 of culture. The inset shows Foxp3 expression of DBY-specific (bold line) and UTY-specific (thin line) Treg clones by flow cytometry. (B,C) Identification of mHA-specific T-cell clones. Treg (B) and Tconv (C) cells were obtained from a female, purified, and stimulated with γ-irradiated dendritic cells from an HLA-matched brother; wells positive for TGF-β release were pooled and cloned by limiting dilution. After 14 days, wells containing Tconv or Treg clones were split into 2 and restimulated with allogeneic (male) or self- (female) DCs for 24 hr. Each bar represents TGF-β or TNF released by mHA-specific T-cell clones in the culture supernatant after restimulation with allogeneic DCs or self-DCs. (D) mHA-specific Treg clones were tested for proliferative response to allogeneic LCL, self-LCL, or self-LCL transduced with DBY, UTY, DFFRY-2, or SMCY. Results shown here are CPM and are representative of 2 experiments. (E) The DBY-specific Treg clone was cultured with a different ratio of autologous-DBY–specific Tconvs in the presence of DBY-transduced autologous LCL, with autologous-UTY–specific Tconvs in the presence of UTY-transduced auto-LCL as specificity control. Mean CPM of DBY- or UTY-specific Tconvs with the stimulators alone was 3919 and 4914, respectively. (F) The UTY-specific autologous Treg clone was cultured with a different ratio of autologous-UTY–specific Tregs in the presence of UTY-transduced autologous LCL, with autologous-DBY–specific Tconvs in the presence of DBY-transduced autologous LCL as specificity control. Mean CPM of UTY- or DBY-specific Tconvs with the stimulators alone was 4906 and 4053, respectively. P values refer to the difference in suppression of specific vs control Tconvs by the ANOVA model.
To demonstrate that Tregs are specific for mHAs, we selected H-Y antigens as targets because they are biologically relevant and the frequency of H-Y–specific Tconvs is high,4-7 and we surmised that H-Y-specific Tregs would be detectable. Blood Tregs were purified from a female donor, and Treg lines were expanded by stimulation with DCs from her HLA-identical brother. Responding T cells that secrete TGF-β were pooled and cloned by limiting dilution. We obtained 10 stable Treg clones that retained TGF-β secretion in response to the brother’s DCs, but not self-DCs, and produced only small amounts of TNF (Figure 1B). Conversely, Tconv clones secreted TNF, but not TGF-β, in response to the allogeneic DCs (Figure 1C). To assess the specificity, we tested cloned Tregs against self–Epstein-Barr virus–transformed B lymphoblastoid cell lines (self-LCLs) transduced with one of the H-Y-associated genes DBY, UTY, DFFRY-2, SMCY, or an empty vector. Of the 10 Treg clones tested, 2 were specific for DBY, 1 for UTY, and 1 for DFFRY-2, while the specificity of the other clones remains unknown (Figure 1D). Cloned Tregs expressed high levels of Foxp3 (Figure 1A inset) and demonstrated mHA-specific suppression (Figure 1E,F). For the first time, we demonstrated that it is possible to detect mHA-specific Treg alloresponses in humans.
Frequency of mHA-specific Tregs and Tconvs
We employed LDA to measure the precursor frequency of mHA-specific CD4 Tregs and CD4 Tconvs after stimulation of the purified subsets by HLA-identical sibling DCs. Both Tregs and Tconvs showed specific alloreactivity against sibling DCs when compared with self-DCs (Figure 2A,B). The median precursor frequency of mHA-specific Tregs is 1.6 (range, 0.4-8.4) cells per million total blood CD4 T cells, while the median frequency of mHA-specific Tconvs is 25.4 (range, 8.7-89) per million total blood CD4 T cells (Table 1). Among 16 HLA-identical sibling pairs tested in any gender combination, there was a 24-fold (range, 8-fold to 39-fold) excess of mHA-specific Tconvs over mHA-specific Tregs in the donor blood (Table 1), and the mHA-specific Tconv:Treg ratio had poor correlation (R2 = 0.24) with the total Tconv:Treg ratio (not shown). The predominance of alloreactive Tconvs over Tregs likely drives the alloimmune responses causing GVHD and organ graft rejection, and the mHA-specific Tconv:Treg ratio may be a more precise predictor of alloreactivity than the total Tconv:Treg ratio.
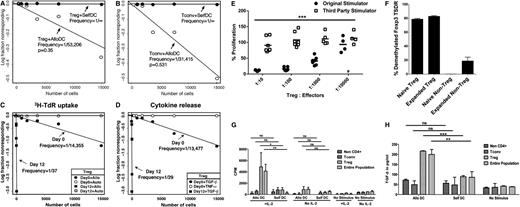
Characterization of expanded mHA-specific Tregs. LDA of mHA-specific blood Tregs or Tconvs stimulated by DCs from HLA-matched siblings. Plots represent (y-axis) the log-fraction of nonresponding Treg (A) or Tconv (B) responses against allogeneic or autologous DCs vs (x-axis) the number of T cells per well. The slopes represent frequency estimates validated by the likelihood ratio test of single-hit model. Not shown for clarity are the 95% confidence estimates. Each LDA was performed with a minimum of 10 replicates per cell number. (C) Tregs were purified from leukapheresis and cultured ex vivo for 12 days with HLA-identical sibling DCs, IL-2, IL-15, and rapamycin. LDA of allospecific Tregs was performed on day 0 and 12 of culture. Results shown are 3H-TdR incorporation on day 6 of LDA. Frequencies of Tregs against sibling DCs on day 0 are marked by a full circle and on day 12 by a full square. Tregs tested with autologous DCs on day 0 are marked by an empty circle and day 12 by an empty square. Each regression line significantly fit the data. (D) Cytokine release of mHA-specific Tregs in response to sibling DCs by LDA. TGF-β and TNF released by mHA-specific Tregs were measured in the culture supernatant after stimulation with allogeneic DCs. TGF-β levels by Tregs tested against allo-DCs on day 0 (full circle) and day 12 (full square). None of the wells stimulated with allogeneic DC showed positivity for TNF levels on day 0 (empty circle) or day 12 (data not shown). (E) mHA-specific suppressive function of cultured Tregs was tested at different ratios to self-Tconvs in the presence of DCs from the original sibling or an HLA-incompatible third party. Results shown are from 7 independent experiments. ***P < .001 by ANOVA. (F) Foxp3 gene promoter demethylation was assessed by PCR primer for methylated and demethylated Treg-specific Foxp3 promoter sequence in Tregs or Tconvs before and after expansion. Results are the representative of 3 individual experiments. (G,H) mHA-specific Treg proliferation and TGF-β release. The entire end-of-culture population and sorted Tregs were tested for proliferation by 3H-TdR incorporation (G) and TGF-β release (H) on day 6, in response to HLA-identical sibling DCs, self-DCs, or no stimulus in the presence or absence of IL-2. TGF-β and TNF levels in the culture supernatant were measured by ELISA. Each bar represents mean + SD of CPM or cytokine levels in response to stimulus. Results shown are 1 experiment representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001. ns, nonsignificant.
Characterization of expanded mHA-specific Tregs. LDA of mHA-specific blood Tregs or Tconvs stimulated by DCs from HLA-matched siblings. Plots represent (y-axis) the log-fraction of nonresponding Treg (A) or Tconv (B) responses against allogeneic or autologous DCs vs (x-axis) the number of T cells per well. The slopes represent frequency estimates validated by the likelihood ratio test of single-hit model. Not shown for clarity are the 95% confidence estimates. Each LDA was performed with a minimum of 10 replicates per cell number. (C) Tregs were purified from leukapheresis and cultured ex vivo for 12 days with HLA-identical sibling DCs, IL-2, IL-15, and rapamycin. LDA of allospecific Tregs was performed on day 0 and 12 of culture. Results shown are 3H-TdR incorporation on day 6 of LDA. Frequencies of Tregs against sibling DCs on day 0 are marked by a full circle and on day 12 by a full square. Tregs tested with autologous DCs on day 0 are marked by an empty circle and day 12 by an empty square. Each regression line significantly fit the data. (D) Cytokine release of mHA-specific Tregs in response to sibling DCs by LDA. TGF-β and TNF released by mHA-specific Tregs were measured in the culture supernatant after stimulation with allogeneic DCs. TGF-β levels by Tregs tested against allo-DCs on day 0 (full circle) and day 12 (full square). None of the wells stimulated with allogeneic DC showed positivity for TNF levels on day 0 (empty circle) or day 12 (data not shown). (E) mHA-specific suppressive function of cultured Tregs was tested at different ratios to self-Tconvs in the presence of DCs from the original sibling or an HLA-incompatible third party. Results shown are from 7 independent experiments. ***P < .001 by ANOVA. (F) Foxp3 gene promoter demethylation was assessed by PCR primer for methylated and demethylated Treg-specific Foxp3 promoter sequence in Tregs or Tconvs before and after expansion. Results are the representative of 3 individual experiments. (G,H) mHA-specific Treg proliferation and TGF-β release. The entire end-of-culture population and sorted Tregs were tested for proliferation by 3H-TdR incorporation (G) and TGF-β release (H) on day 6, in response to HLA-identical sibling DCs, self-DCs, or no stimulus in the presence or absence of IL-2. TGF-β and TNF levels in the culture supernatant were measured by ELISA. Each bar represents mean + SD of CPM or cytokine levels in response to stimulus. Results shown are 1 experiment representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001. ns, nonsignificant.
Frequency of HLA-identical sibling mHA-specific CD4 T cells in peripheral blood
| HLA-Id sibling pair . | Treg . | Tconv . | mHA-specific Tconv/Treg ratio . | ||||
|---|---|---|---|---|---|---|---|
| % of total CD4* . | Frequency† . | 95% CI . | % of total CD4* . | Frequency† . | 95% CI . | ||
| 1 | 7.5 | 0.5 | 0.2-0.7 | 92.5 | 20.4 | 12.0-34.2 | 39 |
| 2 | 3.6 | 0.4 | 0.1-1.4 | 96.4 | 8.7 | 2.9-22.2 | 24 |
| 3 | 7.9 | 0.9 | 0.4-2.0 | 92.1 | 23.0 | 13.8-35.9 | 26 |
| 4 | 6.8 | 0.8 | 0.3-1.9 | 93.2 | 25.2 | 14.9-42.9 | 31 |
| 5 | 7.7 | 0.9 | 0.3-2.9 | 92.3 | 24.9 | 15.7-40.6 | 27 |
| 6 | 14.3 | 2.0 | 0.9-4.6 | 85.7 | 23.1 | 13.7-37.7 | 12 |
| 7 | 4.9 | 1.1 | 0.4-2.5 | 95.1 | 34.2 | 15.2-77.0 | 32 |
| 8 | 3.5 | 0.9 | 0.6-1.6 | 96.5 | 25.1 | 14.5-42.5 | 27 |
| 9 | 5.2 | 1.6 | 0.7-3.7 | 94.8 | 41.7 | 19.9-87.2 | 27 |
| 10 | 9.4 | 3.0 | 1.6-5.6 | 90.6 | 25.4 | 13.6-48.9 | 8 |
| 11 | 6.6 | 2.8 | 2.5-4.3 | 93.4 | 42.0 | 25.2-71.0 | 15 |
| 12 | 6.4 | 3.3 | 1.9-5.4 | 93.6 | ND | — | — |
| 13 | 7.7 | 5.4 | 3.2-9.0 | 92.3 | 68.3 | 41.5-113.5 | 13 |
| 14 | 9.2 | 8.4 | 5.2-13.5 | 90.8 | 89.0 | 55.4-141.6 | 11 |
| 15 | 8.3 | 1.6 | 0.7-3.7 | 91.7 | 29.3 | 14.7-58.7 | 19 |
| 16 | 14.7 | 6.7 | 3.7-12.8 | 85.3 | 63.1 | 37.5-104.1 | 9.3 |
| Median | 7.6 | 1.6 | 92.4 | 25.4 | 24 | ||
| Range | 3.5-14.7 | 0.4-8.4 | 85.3-96.5 | 8.7-89 | 8-39 | ||
| HLA-Id sibling pair . | Treg . | Tconv . | mHA-specific Tconv/Treg ratio . | ||||
|---|---|---|---|---|---|---|---|
| % of total CD4* . | Frequency† . | 95% CI . | % of total CD4* . | Frequency† . | 95% CI . | ||
| 1 | 7.5 | 0.5 | 0.2-0.7 | 92.5 | 20.4 | 12.0-34.2 | 39 |
| 2 | 3.6 | 0.4 | 0.1-1.4 | 96.4 | 8.7 | 2.9-22.2 | 24 |
| 3 | 7.9 | 0.9 | 0.4-2.0 | 92.1 | 23.0 | 13.8-35.9 | 26 |
| 4 | 6.8 | 0.8 | 0.3-1.9 | 93.2 | 25.2 | 14.9-42.9 | 31 |
| 5 | 7.7 | 0.9 | 0.3-2.9 | 92.3 | 24.9 | 15.7-40.6 | 27 |
| 6 | 14.3 | 2.0 | 0.9-4.6 | 85.7 | 23.1 | 13.7-37.7 | 12 |
| 7 | 4.9 | 1.1 | 0.4-2.5 | 95.1 | 34.2 | 15.2-77.0 | 32 |
| 8 | 3.5 | 0.9 | 0.6-1.6 | 96.5 | 25.1 | 14.5-42.5 | 27 |
| 9 | 5.2 | 1.6 | 0.7-3.7 | 94.8 | 41.7 | 19.9-87.2 | 27 |
| 10 | 9.4 | 3.0 | 1.6-5.6 | 90.6 | 25.4 | 13.6-48.9 | 8 |
| 11 | 6.6 | 2.8 | 2.5-4.3 | 93.4 | 42.0 | 25.2-71.0 | 15 |
| 12 | 6.4 | 3.3 | 1.9-5.4 | 93.6 | ND | — | — |
| 13 | 7.7 | 5.4 | 3.2-9.0 | 92.3 | 68.3 | 41.5-113.5 | 13 |
| 14 | 9.2 | 8.4 | 5.2-13.5 | 90.8 | 89.0 | 55.4-141.6 | 11 |
| 15 | 8.3 | 1.6 | 0.7-3.7 | 91.7 | 29.3 | 14.7-58.7 | 19 |
| 16 | 14.7 | 6.7 | 3.7-12.8 | 85.3 | 63.1 | 37.5-104.1 | 9.3 |
| Median | 7.6 | 1.6 | 92.4 | 25.4 | 24 | ||
| Range | 3.5-14.7 | 0.4-8.4 | 85.3-96.5 | 8.7-89 | 8-39 | ||
HLA-Id, HLA-identical; ND, not determined.
Percentage Treg (CD4+CD25+CD127−) or Tconv (CD4+CD25−CD127+) was calculated based on flow cytometry analysis of unseparated blood samples.
Treg ands Tconvs were enriched to >95% purity before the assay. Values shown are the frequency of mHA-specific Tregs or Tconvs per million total CD4 T cells with 95% confidence intervals. mHA-specific Treg or Tconv frequencies were obtained from LDA of purified population in culture with DCs from HLA-identical siblings in the presence of IL-2, IL-15, and rapamycin (Tregs) or IL-2 alone (Tconvs).
Ex vivo expansion of mHA-specific Tregs
Tregs were enriched from blood samples of normal donors to >95% purity and expanded in culture for 12 days by stimulation with HLA-identical sibling DCs in the presence of IL-2, IL-15, and rapamycin. To determine the degree of expansion of mHA-specific Tregs, we measured their frequency by LDA in the start and end of culture populations. The frequency estimate of stimulator-specific Tregs was 1/14, 355 (95% CI, 1/24, 075-1/8,559) in the starting population and 1/37 (95% CI, 1/56-1/24) in the end population (Figure 2C). LDA results by 3H-TdR uptake and TGF-β release showed similar estimates of mHA-specific Treg frequency, and there was 98% concordance for culture positivity by 3H-TdR uptake and TGF-β. No TNF production was detected (Figure 2D). In 7 of 8 experiments, there was contraction of the total cell numbers by end of culture; however, the frequency of mHA-specific Tregs increased so the total numbers of mHA-specific Tregs increased a median of 330-fold (range, 155-fold to 405-fold; see Table 2). The ratio of mHA-specific Tconvs to Tregs in blood varies between 8 and 39 (median, 24; Table 1), so the magnitude of mHA-specific Treg expansion described here should allow exceeding the starting blood content of mHA-specific Tconvs.
Expansion of mHA-specific Tregs
| . | . | mHA-specific Treg FPM* . | . | |
|---|---|---|---|---|
| Sibling pair . | Samples from blood/apheresis . | Before culture . | After culture . | mHA-specific Treg fold expansion . |
| 1 | Blood | 28 | 18 182 | 405 |
| 2 | Blood | 50 | 11 111 | 167 |
| 3 | Blood | 91 | 14 925 | 155 |
| 4 | Blood | 30 | 14 225 | 358 |
| Median | Blood | 40 | 14 575 | 263 |
| Range | (28-91) | (11 111-18 182) | (155-405) | |
| 5 | Apheresis | 70 | 27 248 | 324 |
| 6 | Apheresis | 19 | 8475 | 405 |
| 7 | Apheresis | 46 | 17 762 | 336 |
| 8 | Apheresis | 74 | 15 765 | 212 |
| Median | Apheresis | 58 | 16 764 | 330 |
| Range | (19-74) | (8475-27 248) | (212-405) | |
| . | . | mHA-specific Treg FPM* . | . | |
|---|---|---|---|---|
| Sibling pair . | Samples from blood/apheresis . | Before culture . | After culture . | mHA-specific Treg fold expansion . |
| 1 | Blood | 28 | 18 182 | 405 |
| 2 | Blood | 50 | 11 111 | 167 |
| 3 | Blood | 91 | 14 925 | 155 |
| 4 | Blood | 30 | 14 225 | 358 |
| Median | Blood | 40 | 14 575 | 263 |
| Range | (28-91) | (11 111-18 182) | (155-405) | |
| 5 | Apheresis | 70 | 27 248 | 324 |
| 6 | Apheresis | 19 | 8475 | 405 |
| 7 | Apheresis | 46 | 17 762 | 336 |
| 8 | Apheresis | 74 | 15 765 | 212 |
| Median | Apheresis | 58 | 16 764 | 330 |
| Range | (19-74) | (8475-27 248) | (212-405) | |
FPM, frequency per million.
mHA-specific Treg frequencies were calculated by LDA of purified Tregs before and 12 days after culture.
Phenotype and functional characterization of expanded Tregs
In coculture suppression assay, expanded Tregs were titrated in various ratios with naïve autologous CD4+CD25− Tconvs and stimulated by the original HLA-identical sibling DCs or HLA-incompatible third-party DCs. Tconv proliferation was reduced by 50% at a ratio of ∼1:5000 and by >90% at a Treg:Tconv ratio of ∼1:10. No suppressive effects were observed in the presence of a third-party stimulator (Figure 2E). Thus, Tregs maintained antigen-specific suppressive function after large-scale ex vivo expansion under GMP conditions as intended for in vivo use. To investigate whether expanded Tregs had maintained demethylation of the Foxp3 gene promoter TSDR that is required for stable Foxp3 expression, we sorted CD4+CD127−CD25+ Tregs and CD4+CD127+CD25− Tconvs by flow cytometry before or after expansion and assessed the TSDR by qPCR. We found that Treg TSDR at the end culture was demethylated to the same degree as before culture (Figure 2F).
To assess whether the expanded Treg-enriched populations included immune-competent T cells, we flow-sorted Tregs, CD4 Tconvs, and CD8 T cells (termed “non-CD4 cells” in Figure 2G,H) and stimulated the entire end-of-culture population or each purified subset with allogeneic DCs or self-DCs in the presence or absence of IL-2. There was no significant response to antigen in any of the populations tested in the absence of IL-2. In the presence of IL-2, only the entire end-of-culture population and the purified Tregs responded to alloantigens by 3H-TDR uptake and TGF-β release (Figure 2G,H). We concluded from these experiments that contaminating CD4 T conv and CD8 T cells were tolerized to the specific mHAs while in culture with Tregs and rapamycin.
Expanded Tregs retained high expression of CD25 that is required for expansion in response to IL-2, Foxp3 that is required for suppressive function, BCL-2 that is required for survival, chemokine receptor CCR7, and lymphoid homing receptor CD62L, predicting that they can migrate and home to lymphoid tissue in vivo (Figure 3).
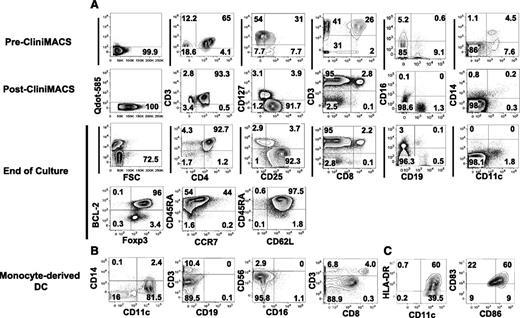
Phenotypic characterization of Tregs and monocyte-derived DCs from leukapheresis. (A) Phenotype of Tregs from donor leukapheresis before and after enrichment and after large-scale expansion. Mononuclear cells were obtained from blood cell apheresis followed by positive selection with CD25 paramagnetic beads for immunoabsorption on column by CliniMACS. The top row (pre-CliniMACS) shows phenotype of mononuclear cells after donor apheresis. The second row (post-CliniMACS) shows viability, phenotype, and purity of Tregs after CliniMACS separation. The third and fourth data rows show Treg viability, phenotype, and purity after 12-day ex vivo expansion using DCs from an HLA-identical sibling, IL-2, IL-15, and rapamycin. Viability was assessed by Qdot 585-A exclusion, and viable cells were gated for marker studies. CD3+/CD4+ double-positive cells were gated for assessment of CD25 and CD127 expression. CD25+/CD127− cells were gated for assessment of Foxp3, BCL-2, CD45RA, and CCR7 expression. Cells other than CD4 from the end-of-culture population were also tested. Cell separation and culture was performed in the cell therapy core facility at Moffitt Cancer Center. (B,C) Surface phenotype of cells enriched for monocyte-derived DCs before coculture with Tregs. All flow-cytometric plots are gated on live cells, and HLA-DR, CD86, and CD83 plots are also gated on CD11c+/CD14− cells.
Phenotypic characterization of Tregs and monocyte-derived DCs from leukapheresis. (A) Phenotype of Tregs from donor leukapheresis before and after enrichment and after large-scale expansion. Mononuclear cells were obtained from blood cell apheresis followed by positive selection with CD25 paramagnetic beads for immunoabsorption on column by CliniMACS. The top row (pre-CliniMACS) shows phenotype of mononuclear cells after donor apheresis. The second row (post-CliniMACS) shows viability, phenotype, and purity of Tregs after CliniMACS separation. The third and fourth data rows show Treg viability, phenotype, and purity after 12-day ex vivo expansion using DCs from an HLA-identical sibling, IL-2, IL-15, and rapamycin. Viability was assessed by Qdot 585-A exclusion, and viable cells were gated for marker studies. CD3+/CD4+ double-positive cells were gated for assessment of CD25 and CD127 expression. CD25+/CD127− cells were gated for assessment of Foxp3, BCL-2, CD45RA, and CCR7 expression. Cells other than CD4 from the end-of-culture population were also tested. Cell separation and culture was performed in the cell therapy core facility at Moffitt Cancer Center. (B,C) Surface phenotype of cells enriched for monocyte-derived DCs before coculture with Tregs. All flow-cytometric plots are gated on live cells, and HLA-DR, CD86, and CD83 plots are also gated on CD11c+/CD14− cells.
Characterization of cell phenotype under GMP
To assess the feasibility of Treg expansion to a clinical scale, we obtained mononuclear cells by apheresis from 3 donors. In each of the experiments, donor cells were incubated with GMP-grade anti-CD25 immunomagnetic beads in the cell therapy laboratory and enriched to a 76% to 82% purity using CliniMACS. The Tregs yield was a mean of 25% of the starting Tregs and only 3% of the starting mononuclear cells. Flow cytometry analyses of cells before and after CliniMACS purification and after ex vivo culture are shown in Figure 3A. We found contamination by CD4 Tconv (mean 7%), CD8 (mean 1.4%), and CD19 (mean 4%) cells after purification (Table 3). After freezing and thawing, the Treg recovery was a mean of 64% and the Treg phenotype and the frequencies of contaminants were unchanged. Before coculture, GMP-grade monocyte-derived DCs had an average purity of 59% (range, 30%-82%) based on a CD11c+/CD14− phenotype and expressed HLA-DR, CD83, and CD86 (Figure 3B,C). A fraction of the purified Tregs were cultured with irradiated (30 Gy) DCs from an HLA-matched sibling at an approximate 1:1 ratio, with IL-2, IL-15, and rapamycin. After 12 days, the mean viability of the cells in culture was 89% and the mean Treg purity was 78% by flow cytometry. There was contamination by a mean of 6.9% CD4 T convs, 1.2% CD8 T cells, and 2.4% B cells from the original Treg-enriched population and 1.4% natural killer cells and 1.5% DCs from the irradiated APCs, and there were no detectable monocytes. The mHA-specific Tregs of the 3 pairs tested expanded by 281- to 405-fold, in each case exceeding the numbers of mHA-specific Tconvs in the starting apheresis (Table 4).
Composition of T cells and DCs obtained via apheresis before and after Treg enrichment and after expansion culture under GMP
| Mononuclear cells . | DC* (n=3) . | Treg . | |||
|---|---|---|---|---|---|
| Before column (n = 3) . | After column (n = 3) . | Before culture† (n = 3) . | After culture (n = 4) . | ||
| Viable | |||||
| N × 106 | 94 (57.2-127.8) | 27 718 (21 778-37 000) | 257.2 (139-477) | 101.7 (65-146.9) | 69.9 (50.5-97.2) |
| % | 88 (79-99.1) | 99.9 (99.9-100) | 99.9 (99.8-100) | 94.5 (90.7-97.7) | 88.8 (72.5-99.3) |
| CD4+CD127−CD25+Foxp3+ | |||||
| N × 106 | ND | 799.6 (424.6-1265) | 206.6 (114.5-386.1) | 87.7 (55.2-132.9) | 63.36 (41.5-97.1) |
| % | ND | 2.8 (1.9-3.4) | 79.9 (76.4-82.4) | 80.5 (76.3-83) | 78.3 (72.5-85.2) |
| CD4+CD127−CD25+Foxp3− | |||||
| N × 106 | ND | 234.5 (34-581.8) | 10.9 (1.5-22.2) | 2.7 (0.3-5.7) | 2.8 (0.2-6.2) |
| % | ND | 0.7 (0.1-1.6) | 3.8 (1.1-5.8) | 3.1 (0.2-5.8) | 3.5 (0.2-6.8) |
| CD4+CD127−CD25− | |||||
| N × 106 | ND | 1082 (369.4-1821) | 3.6 (1.2-5.7) | 2.0 (0.1-3.4) | 1.0 (0.7-1.4) |
| % | ND | 3.6 (1.7-4.9) | 1.5 (0.9-2.5) | 1.6 (0.1-2.5) | 1.3 (1.1-1.4) |
| CD4+CD127+ | |||||
| N × 106 | 11.4 (0.5-22.8)‡ | 9871 (3248-20 370) | 17.6 (9.0-31.3) | 6.2 (4.6-7.8) | 5.8 (3.3-8.3) |
| % | 10.3 (0.7-23.3) | 32.0 (13.3-55.1) | 7.0 (6.5-8) | 6.2 (3.8-8.0) | 6.9 (6.0-9.1) |
| CD8+ | |||||
| N × 106 | 3.2 (0.5-6.4) | 5205 (684-2562) | 5.2 (1.0-13.3) | 1.7 (0.3-3.5) | 1.2 (0.4-2.9) |
| % | 2.5 (0.8-4) | 17.1 (9.3-26.3) | 1.4 (0.7-2.8) | 1.3 (0.6-2.2) | 1.2 (0.8-2.2) |
| CD19+ | |||||
| N × 106 | 14 (0.1-24.6) | 1704 (684.5-2562) | 7.1 (6.1-9.0) | 2.4 (0.5-4.4) | 1.7 (0.8-2.7) |
| % | 18.2 (0.1-37) | 6.2 (3.1-10.5) | 3.8 (1.3-5.8) | 2.2 (0.7-4.5) | 2.4 (0.6-3.2) |
| CD16+or CD56+ | |||||
| N × 106 | 8.5 (6.6-12.2) | 2362 (8.7-3,589) | 0.4 (0-1.0) | 0.1 (0-0.3) | 1.5 (0.2-4.3) |
| % | 9.8 (4.1-18.3) | 8 (0-14.3) | 0.1 (0-0.2) | 0.1 (0-0.3) | 1.4 (0.5-3.2) |
| CD14+ | |||||
| N × 106 | 16.9 (4.1-24.7) | 5836 (2087-8882) | 1.8 (0.1-4.8) | 0.6 (0.1-1.4) | 0.2 (0-0.5) |
| % | 20.2 (2.5-32.9) | 24 (5.6-36.4) | 0.4 (0.1-1) | 0.6 (0.1-1.4) | 0.2 (0-0.6) |
| CD14−CD11c+ | |||||
| N × 106 | 68 (29-132) | 2081 (1613-2812) | 0.7 (0.2-1.4) | 0.2 (0.1-0.4) | 1.3 (0.8-2.5) |
| % | 58.8 (30-81.6) | 7.5 (7.4-7.6) | 0.2 (0.1-0.3) | 0.3 (0.1-0.6) | 0.2 (1-1.9) |
| Mononuclear cells . | DC* (n=3) . | Treg . | |||
|---|---|---|---|---|---|
| Before column (n = 3) . | After column (n = 3) . | Before culture† (n = 3) . | After culture (n = 4) . | ||
| Viable | |||||
| N × 106 | 94 (57.2-127.8) | 27 718 (21 778-37 000) | 257.2 (139-477) | 101.7 (65-146.9) | 69.9 (50.5-97.2) |
| % | 88 (79-99.1) | 99.9 (99.9-100) | 99.9 (99.8-100) | 94.5 (90.7-97.7) | 88.8 (72.5-99.3) |
| CD4+CD127−CD25+Foxp3+ | |||||
| N × 106 | ND | 799.6 (424.6-1265) | 206.6 (114.5-386.1) | 87.7 (55.2-132.9) | 63.36 (41.5-97.1) |
| % | ND | 2.8 (1.9-3.4) | 79.9 (76.4-82.4) | 80.5 (76.3-83) | 78.3 (72.5-85.2) |
| CD4+CD127−CD25+Foxp3− | |||||
| N × 106 | ND | 234.5 (34-581.8) | 10.9 (1.5-22.2) | 2.7 (0.3-5.7) | 2.8 (0.2-6.2) |
| % | ND | 0.7 (0.1-1.6) | 3.8 (1.1-5.8) | 3.1 (0.2-5.8) | 3.5 (0.2-6.8) |
| CD4+CD127−CD25− | |||||
| N × 106 | ND | 1082 (369.4-1821) | 3.6 (1.2-5.7) | 2.0 (0.1-3.4) | 1.0 (0.7-1.4) |
| % | ND | 3.6 (1.7-4.9) | 1.5 (0.9-2.5) | 1.6 (0.1-2.5) | 1.3 (1.1-1.4) |
| CD4+CD127+ | |||||
| N × 106 | 11.4 (0.5-22.8)‡ | 9871 (3248-20 370) | 17.6 (9.0-31.3) | 6.2 (4.6-7.8) | 5.8 (3.3-8.3) |
| % | 10.3 (0.7-23.3) | 32.0 (13.3-55.1) | 7.0 (6.5-8) | 6.2 (3.8-8.0) | 6.9 (6.0-9.1) |
| CD8+ | |||||
| N × 106 | 3.2 (0.5-6.4) | 5205 (684-2562) | 5.2 (1.0-13.3) | 1.7 (0.3-3.5) | 1.2 (0.4-2.9) |
| % | 2.5 (0.8-4) | 17.1 (9.3-26.3) | 1.4 (0.7-2.8) | 1.3 (0.6-2.2) | 1.2 (0.8-2.2) |
| CD19+ | |||||
| N × 106 | 14 (0.1-24.6) | 1704 (684.5-2562) | 7.1 (6.1-9.0) | 2.4 (0.5-4.4) | 1.7 (0.8-2.7) |
| % | 18.2 (0.1-37) | 6.2 (3.1-10.5) | 3.8 (1.3-5.8) | 2.2 (0.7-4.5) | 2.4 (0.6-3.2) |
| CD16+or CD56+ | |||||
| N × 106 | 8.5 (6.6-12.2) | 2362 (8.7-3,589) | 0.4 (0-1.0) | 0.1 (0-0.3) | 1.5 (0.2-4.3) |
| % | 9.8 (4.1-18.3) | 8 (0-14.3) | 0.1 (0-0.2) | 0.1 (0-0.3) | 1.4 (0.5-3.2) |
| CD14+ | |||||
| N × 106 | 16.9 (4.1-24.7) | 5836 (2087-8882) | 1.8 (0.1-4.8) | 0.6 (0.1-1.4) | 0.2 (0-0.5) |
| % | 20.2 (2.5-32.9) | 24 (5.6-36.4) | 0.4 (0.1-1) | 0.6 (0.1-1.4) | 0.2 (0-0.6) |
| CD14−CD11c+ | |||||
| N × 106 | 68 (29-132) | 2081 (1613-2812) | 0.7 (0.2-1.4) | 0.2 (0.1-0.4) | 1.3 (0.8-2.5) |
| % | 58.8 (30-81.6) | 7.5 (7.4-7.6) | 0.2 (0.1-0.3) | 0.3 (0.1-0.6) | 0.2 (1-1.9) |
Data are presented as mean (range).
DCs were γ-irradiated (3000 cGy) before coculture with Tregs to prevent contamination.
A fraction of the purified Tregs were cultured with DCs from an HLA-matched sibling at a 1:1 ratio with IL-2, IL-15, and rapamycin.
This population was not tested for CD127 expression.
Quantification of mHA-specific Tconvs and Tregs in donor apheresis after Treg enrichment and after expansion under GMP
| . | Donor . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| Total blood volume processed by apheresis (mL) | 21 358 | 10 270 | 24 046 |
| Total live mononuclear cells × 108 | 370 | 218 | 244 |
| No. of Tconvs × 108 (% total) | 204 (55%) | 60 (28%) | 32 (13%) |
| mHA-specific Tconvs* | |||
| Frequency (%) | 0.009 | 0.003 | 0.007 |
| n | 1 861 326 | 190 823 | 238 834 |
| No. of Tregs × 108 | |||
| Before immunoabsorption (% total) | 12.7 (3.4%) | 4.2 (1.9%) | 7.1 (2.9%) |
| After immunoabsorption (% yield) | 3.9 (31%) | 1.2 (28%) | 1.1 (16%) |
| After freezing and thawing (% yield) | 2.1 (17%) | 0.83 (20%) | 1.33 (16%) |
| Total Tregs used for expansion × 108 | |||
| Before culture† | 1.33 | 0.75 | 0.55 |
| After culture | 0.97 | 0.65 | 0.50 |
| mHA-specific Tregs*before culture† | |||
| Frequency (%) | 0.007 | 0.002 | 0.005 |
| n | 11 285 | 1842 | 3084 |
| mHA-specific Tregs after culture | |||
| Frequency (%) | 2.7 | 0.65 | 1.8 |
| n | 3 651 226 | 745 763 | 1 035 524 |
| Fold expansion | |||
| Required to match CD4 T effector cells at a 1:1 ratio | 165 | 104 | 77 |
| Measured | 324 | 405 | 336 |
| . | Donor . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| Total blood volume processed by apheresis (mL) | 21 358 | 10 270 | 24 046 |
| Total live mononuclear cells × 108 | 370 | 218 | 244 |
| No. of Tconvs × 108 (% total) | 204 (55%) | 60 (28%) | 32 (13%) |
| mHA-specific Tconvs* | |||
| Frequency (%) | 0.009 | 0.003 | 0.007 |
| n | 1 861 326 | 190 823 | 238 834 |
| No. of Tregs × 108 | |||
| Before immunoabsorption (% total) | 12.7 (3.4%) | 4.2 (1.9%) | 7.1 (2.9%) |
| After immunoabsorption (% yield) | 3.9 (31%) | 1.2 (28%) | 1.1 (16%) |
| After freezing and thawing (% yield) | 2.1 (17%) | 0.83 (20%) | 1.33 (16%) |
| Total Tregs used for expansion × 108 | |||
| Before culture† | 1.33 | 0.75 | 0.55 |
| After culture | 0.97 | 0.65 | 0.50 |
| mHA-specific Tregs*before culture† | |||
| Frequency (%) | 0.007 | 0.002 | 0.005 |
| n | 11 285 | 1842 | 3084 |
| mHA-specific Tregs after culture | |||
| Frequency (%) | 2.7 | 0.65 | 1.8 |
| n | 3 651 226 | 745 763 | 1 035 524 |
| Fold expansion | |||
| Required to match CD4 T effector cells at a 1:1 ratio | 165 | 104 | 77 |
| Measured | 324 | 405 | 336 |
The frequency of mHA-specific Treg or Tconv was obtained from limiting dilution analysis.
A fraction of the purified Tregs were cultured with DCs from an HLA-matched sibling at a 1:1 ratio with IL-2, IL-15, and rapamycin.
Deuterium labeling of Tregs
We employed a second GMP-compliant assay to measure the fraction of Tregs replicating in response to antigen stimulation. Tregs were cultured in media that contained enriched [6, 6-2H2] glucose, and the fraction of Treg-associated DNA that had incorporated deuterium was measured by mass spectrometry at end of culture.27-29 We detected 4.4% and 10.2% deuterium enrichment in the DNA of the cultured Tregs in 2 large-scale expansion experiments, well over the 0.1% background signal in unlabeled culture and representing 7.1% and 16.5% newly divided cells, respectively. These data are in corroboration with the LDA results showing expansion, as evidenced by enrichment of mHA-specific Tregs up to 0.8% and 1.8% by the end of culture. Deuterium labeling did not affect Treg expansion as measured by LDA, suppressive activity, or Foxp3 demethylation. Deuterium-labeled Tregs secreted TGF-β and minimal TNF in the presence of allogeneic DCs (Figure 4). An advantage of this approach to measure DNA replication is that trace-labeled cells can be detected after in vivo transfer: T-cell subsets from blood or other tissues are sorted by flow cytometry, and DNA is extracted and tested by mass spectrometry for deuterium enrichment.
Lack of effect on Treg function of ex vivo deuterium labeling with [6, 6-2H2] glucose. Purified Tregs were cultured in RPMI medium containing [6, 6-2H2] glucose or natural glucose in the presence of sibling DC, IL-2, IL-15, and rapamycin. After 12 days, Tregs cultured in 2 culture conditions were tested for suppressive function, Foxp3 Treg-specific demethylation region analysis, and TGF-β cytokine production. (A,B) Cultured Tregs were tested at different ratios to self-Tconvs in the presence of DCs from the original sibling or an HLA-incompatible third party. (C) Foxp3 demethylation region was assessed by qPCR. Each bar represents mean + SD of triplicate measurements. TGF-β and TNF release of expanded Tregs. Sorted Tregs or Tconvs were restimulated with allogeneic or self-DCs or phorbol myristate acetate (PMA) and ionomycin (IO) for 24 hours. Culture supernatant was tested for TGF-β (D) or TNF (E) production by ELISA. *P < .05; ***P < .001.
Lack of effect on Treg function of ex vivo deuterium labeling with [6, 6-2H2] glucose. Purified Tregs were cultured in RPMI medium containing [6, 6-2H2] glucose or natural glucose in the presence of sibling DC, IL-2, IL-15, and rapamycin. After 12 days, Tregs cultured in 2 culture conditions were tested for suppressive function, Foxp3 Treg-specific demethylation region analysis, and TGF-β cytokine production. (A,B) Cultured Tregs were tested at different ratios to self-Tconvs in the presence of DCs from the original sibling or an HLA-incompatible third party. (C) Foxp3 demethylation region was assessed by qPCR. Each bar represents mean + SD of triplicate measurements. TGF-β and TNF release of expanded Tregs. Sorted Tregs or Tconvs were restimulated with allogeneic or self-DCs or phorbol myristate acetate (PMA) and ionomycin (IO) for 24 hours. Culture supernatant was tested for TGF-β (D) or TNF (E) production by ELISA. *P < .05; ***P < .001.
Discussion
In this article, we identified, quantified, and cloned Tregs specific against human mHAs and developed a GMP-compliant protocol to expand sufficient numbers of mHA-specific Tregs for clinical trial testing. To our knowledge, this is the first report of mHA-specific human Tregs. Because mHAs drive transplantation responses, the technology reported here might be applied to prevent GVHD and organ graft rejection and adapted to control immune responses against pathogenic antigens in autoimmune disorders.
Among many human mHAs already identified,4-7,32 we selected H-Y antigens to test the specificity of Treg clones because it has been unequivocally demonstrated that H-Y antigens are responsible for pathogenic transplant reactions of females against males and they elicit a broad immune response.33,34 We isolated Treg clones specific for H-Y–associated DBY,6 UTY,5,35 and DFFRY4 mHAs. The Treg clones against DBY or UTY showed significantly more suppression against Tconvs specific for the same mHA compared with Tconvs specific for another. Therefore, we envision that antigen-specific Tregs may be exploited to regulate GVHD-causing responses against ubiquitous mHAs, while selectively sparing antitumor responses against lineage-restricted mHAs or tumor-associated antigens. In contrast, polyspecific Tregs expanded with artificial APCs are expected to produce broader immune suppression and may be more likely to suppress antitumor immunity.15,22
Several cytokines have been implicated in Treg-mediated suppression, including TGF-β, IL-10, and IL-3536 . IL-35 is expressed by a subset of ICOS-positive inducible Tregs,37,38 whereas our investigation has focused on natural Tregs. Our initial experiments18 found that TGF-β production separates more clearly alloreactive Tregs from helper T cells than IL-10, and therefore in this study we adopted TGF-β production as a selection criterion for alloreactive Treg clones.
The observation that Treg clones totally inhibit responses by Tconv clones at the ratio of 1:3 (Figure 1E,F) is consistent with murine data demonstrating that adoptive transfer of polyspecific Tregs at a Treg:Tconv ratio of approximately 1:1 is necessary for preventing GVHD.13,14,19 These data predict that the minimum number of mHA-specific Tregs effective for the prevention of GVHD has to approximate the number of mHA-specific Tconvs in the stem cell graft. A few studies estimated the frequencies of alloreactive Tconvs in the blood of hematopoietic cell transplant donors against the graft recipient.39,40 In HLA-identical siblings, Theobald et al41 determined that the precursor frequency of mHA-specific, IL-2–secreting donor T cells in the blood is 1 per 100 000. Using a more sensitive and rapid (6 days vs 14 days) limiting dilution assay that employs DCs as APCs, we found that the frequency of mHA-specific, TNF-producing proliferative Tconvs is 2.5 per 100 000 blood CD4 T cells (Table 1). We report here that the ratio of mHA-specific Tregs:Tconvs in normal donor blood is on average 1:24. These findings are largely applicable to mobilized blood stem cell grafts, because granulocyte-colony stimulating factor, which is used to mobilize stem cells, barely favors Treg over Tconv mobilization.42 Our data are consistent with the hypothesis that the predominance of alloreactive Tconv over Treg in the graft drives the immune response to cause GVHD and that the expansion of mHA-specific Tregs by an average of 24-fold in a blood cell graft may be adequate to prevent GVHD. Others computed that much greater expansion of polyspecific Tregs is required for effective immunotherapy.21,43
A high degree of Treg purity can be attained by multistep immunoabsorption on magnetic columns using multiple antibodies or fluorescence-activated cell sorting.18,44-46 Although these procedures are highly effective in the research laboratory, the cost of multiple GMP-compatible immunobeads as used by Di Ianni et al,46 or the lack of broadly available cell sorters for clinical use as proposed by Sagoo et al,44 would make the routine clinical application unfeasible. Therefore, we elected to employ a single-step purification using clinical-grade CD25-specific paramagnetic beads that attained an average 80% Treg purity (range: 75%-82%; Table 3) and relied upon the subsequent T cell culture with alloantigen, excess Tregs, and rapamycin to tolerize contaminating Tconvs and CD8 (Figure 2 G,H).47 By the end of culture, the Treg purity met previously accepted specifications48 and the Tconv and CD8 cells appeared totally anergic in response to alloantigen and IL-2. A carefully titrated dose-escalation phase 1 study of GVHD prophylaxis is planned to test the in vivo safety of alloreactive Tregs grown under the conditions described here (NCT01795573).
We employed 2 GMP-compliant techniques to measure Treg expansion against mHAs: LDA and DNA labeling with deuterium-containing glucose. We18 and others49,50 found that LDA underestimates the frequencies of allospecific Tregs and Tconvs. However, by measuring frequencies before and after culture, the LDA has the ability to providing an accurate measure of the expansion. Although in most cases the total number of cells contracted slightly by the end of culture, the frequency of mHA-specific Tregs increased by 155- to 405-fold (Table 2). Large-scale expansion experiments in the GMP-compliant Cell Therapy Core Laboratory at Moffitt showed that numbers of mHA-specific Tregs in the final product consistently exceeded the numbers of mHA-specific Tconvs in the start apheresis (Table 4) and the numbers of Treg that we have planned to administer for GVHD prophylaxis. DNA labeling with deuterium has major advantages over CFSE or 5-bromo-2'-deoxyuridine to be GMP compliant, because it is a stable, naturally occurring isotope and it incorporates into the DNA, providing a safe method to measure cell replication in vitro down to 0.1% sensitivity. Also, deuterium allows subsequent in vivo cell tracking for months.29 The fraction of deuterium-labeled Tregs correlated with the mHA frequency measured by LDA, and labeled cells had preserved suppressive function (Figure 4). We plan to use deuterium labeling for assessing cell replication in culture and Treg longevity after transfer.
After expansion, Tregs retained potent antigen-specific suppression, with 50% inhibition of T-effector responses at ratios approaching 1:2500 and 90% inhibition at 1:100. The suppression was selective for the specific alloantigens, because inhibition of third-party responses was 1000 times less effective. Expanded Tregs produced TGF-β and minimal amounts of TNF when restimulated with allogeneic DCs and exhibited high expression of CD25, which is required for the exquisite sensitivity to IL-2, and Foxp3,21 which is required for the suppressive function. Under stimulation with phorbol ester and ionomycin, cultures released higher amounts of TNF, presumably secreted by monocytes and monocyte-derived DCs. However, the ratio TGF-β/TNF was significantly higher in the supernatant of cultures containing Tregs compared with Tconvs (Figure 4 D,E; P = .01). As previously shown with allogeneic-HLA–specific Tregs, a sizeable fraction of expanded Tregs expressed CD45RA, because Treg conversion from CD45RA to CD45RO after T-cell receptor activation is inhibited by rapamycin.51,52 This is important because CD45RA-positive Tregs retain Foxp3 TSDR demethylation and more potent suppressive function.45,53 In addition, we found cultured Treg expression of lymphoid homing receptor CD62L and chemokine receptor CCR7 that predicted the ability of Tregs to migrate to lymphoid tissues in vivo.54 A highly conserved region within the human Foxp3 gene is demethylated exclusively on Tregs and not in any other blood-cell or non–blood-cell type, and Tregs require persistent demethylation of this region to express Foxp3 and maintain suppression function.55 The levels of TSDR demethylation were similar in the Tregs before and after expansion, predicting lineage stability after transfer.
In the present study, we used allogeneic recipient-type DCs to present mHAs to establish proof of principle for their ability to stimulate specific donor Tregs. However, DCs from patients would also present mHAs associated with hematopoietic malignancies, and therefore methods for loading patient mHAs from GVHD target organ to donor DCs will have to be developed for clinical translation so as to minimize the risk of stimulating donor Tregs that suppress graft-versus-leukemia responses.
This report demonstrates that mHA-specific Tregs can be expanded ex vivo to large scale under GMP conditions and retain sufficient purity, potency, and immune attributes that are sufficient to proceed to testing in clinical trials for GVHD prevention.
Presented in abstract form at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 8, 2012, and at the 2013 BMT Tandem Meetings of the American Society for Blood and Marrow Transplantation, Salt Lake City, UT, February 13, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the research subjects who volunteered for this study; Ashur-Dee Brown for identifying sibling pairs; Darlene Rhan for the leukapheresis collections; Renee Smilee, Albert J. Ribickas, Emily Hopewell, and Andrea Carney for CD25 separation using CliniMACS; Anupama Shastry and Sabine S. Ellwanger for large-scale Treg expansion under GMP in the cellular therapy laboratory at Moffitt Cancer Center; and Mark Fitch (Department of Nutritional Science and Toxicology, University of California, Berkeley) for measurement of deuterium enrichments by mass spectrometry.
This work was supported in part by grants from the National Institutes of Health: National Cancer Institute; National Heart, Lung, and Blood Institute; and National Institute of Allergy and Infectious Diseases (CA132197 and HL114994) (C.A.), (3P30CA076292) (W.J.), (AI43866) (M.H.), (CA118116) (X.-Z.Y.); the American Cancer Society (MRSG-11-149-01-LIB) (J.P.); and was facilitated by the flow cytometry, molecular biology, and cell therapy core facilities at the Moffitt Cancer Center.
Authorship
Contribution: A.V. participated in the design and performed most of the experiments; F.B. performed the flow cytometry studies; W.J. directed the large-scale Treg expansion experiments; M.H directed the experiments of measuring deuterium enrichment; A.V., F.B., B.B., J.T., J.K, and C.A. analyzed the results and produced figures; A.V., J.P., W.J., X.-Z.Y, and C.A. designed the research; A.V. and C.A. wrote the manuscript; and all authors read and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudio Anasetti, Moffitt Cancer Center, FOB3-BMT 12902 Magnolia Dr, Tampa, FL 33612; e-mail: claudio.anasetti@moffitt.org

![Figure 4. Lack of effect on Treg function of ex vivo deuterium labeling with [6, 6-2H2] glucose. Purified Tregs were cultured in RPMI medium containing [6, 6-2H2] glucose or natural glucose in the presence of sibling DC, IL-2, IL-15, and rapamycin. After 12 days, Tregs cultured in 2 culture conditions were tested for suppressive function, Foxp3 Treg-specific demethylation region analysis, and TGF-β cytokine production. (A,B) Cultured Tregs were tested at different ratios to self-Tconvs in the presence of DCs from the original sibling or an HLA-incompatible third party. (C) Foxp3 demethylation region was assessed by qPCR. Each bar represents mean + SD of triplicate measurements. TGF-β and TNF release of expanded Tregs. Sorted Tregs or Tconvs were restimulated with allogeneic or self-DCs or phorbol myristate acetate (PMA) and ionomycin (IO) for 24 hours. Culture supernatant was tested for TGF-β (D) or TNF (E) production by ELISA. *P < .05; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/13/10.1182_blood-2013-03-492397/4/m_2251f4.jpeg?Expires=1765912898&Signature=AouVOQdRxojoiUXISpUeusbSWin66lMPdW5r7pODd1fTNqIpyoAex~RzW3tx-~4rfAKKbj14MQJjcCt8rHQMWI4Y09zFi~Loh98cm7I4sHVOUtF3CLb0rsaouVxY0gCBGUJbByJFmZM03Np3bc7B7z~pM-88p6HsT4J89wXOHrfGGz2pgzSHS8scuLC-wKmt5KYjvi7chiCk35Z712XsIA17OFsSeFeDJrvLduT8KhqNKKd1XtUG~XOzlGed7Zbrr0YxCulh6stbdFt-SMVH2SDddM6twiZjzTMMrNu7ofKNLcodCgK0U59p8flaoVZo-XN-BbGSa524w3SFzdRRVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal