Key Points
Caloric restriction reduces Mcl-1 expression and sensitizes lymphoma cells to ABT-737 in vivo.
Caloric restriction mimetics can sensitize lymphomas to ABT-737–induced death independently of p53 and of the main BH3-only proteins.
Abstract
Caloric restriction (CR) is proposed to decrease tumorigenesis through a variety of mechanisms including effects on glycolysis. However, the understanding of how CR affects the response to cancer therapy is still rudimentary. Here, using the Eµ-Myc transgenic mouse model of B-cell lymphoma, we report that by reducing protein translation, CR can reduce expression of the prosurvival Bcl-2 family member Mcl-1 and sensitize lymphomas to ABT-737–induced death in vivo. By using Eµ-Myc lymphoma cells lacking p53, we showed that CR mimetics such as 2-deoxyglucose led to a decrease in Mcl-1 expression and sensitized lymphoma cells to ABT-737–induced death independently of p53. In keeping with this, Eµ-Myc lymphoma cells lacking the BH3-only proapoptotic members Noxa, Puma, or Bim were also sensitized by CR mimetics to ABT-737–induced death. Remarkably, neither the loss of both Puma and Noxa, the loss of both Puma and Bim, nor the loss of all three BH3-only proteins prevented sensitization to ABT-737 induced by CR mimetics. Thus, CR can influence Mcl-1 expression and sensitize cells to BH3 mimetic–induced apoptosis, independently of the main BH3-only proteins and of p53. Exploiting this may improve the efficiency of, or prevent resistance to, cancer therapy.
Introduction
In addition to cell-autonomous changes that drive a cancer cell to proliferate and contribute to tumorigenesis, alterations in whole-organism metabolism have been observed to be associated with heightened risks for a variety of cancers.1 Intriguingly, although excess calories are associated with human cancer and shortened lifespan, caloric restriction (CR) is known to prolong lifespan and decrease tumorigenesis.2 However, the mechanism by which CR limits tumors and how it can affect responses to chemotherapy are still poorly understood.
Most DNA-damaging chemotherapeutic drugs kill tumor cells through the so-called intrinsic (or mitochondrial) apoptotic pathway. Upon cytotoxic insult, the mitochondrial outer membrane will be permeabilized (MOMP) by the proapoptotic members of the Bcl-2 family, which leads to the release of apoptotic proteins from the intermembrane space, resulting in the activation of caspases.3 Bcl-2 family members have classically been grouped into three classes.4 The prosurvival class (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, Bcl-B, and A1) inhibits apoptosis; the proapoptotic class consists of Bax, Bak, and Bok, with Bax and Bak being critical for the induction of MOMP and the subsequent release of apoptogenic molecules.5 The third class includes the proapoptotic BH3-only proteins (Bim, Puma, Noxa, Bad, Bmf, Bid, Bik, and Hrk), which promote apoptosis by regulating the antiapoptotic Bcl-2 proteins.4
The overexpression of antiapoptotic Bcl-2 protein family members is among the best-characterized alterations (along with p53 mutations) that are associated with cancer. In addition, therapeutic strategies have been developed to inhibit the antiapoptotic proteins, including the class of BH3 mimetic molecules, such as ABT-737.6 Although efficient in some settings,7 the low affinity of ABT-737 for Mcl-1 or A1 limits its cytotoxic effects in cells with high endogenous levels of Mcl-1, such as aggressive forms of non-Hodgkin B lymphomas.8 Several strategies have been developed by us and others to reduce Mcl-1 levels to thereby increase the cytotoxic effects of ABT-737.9-11 Among them, targeting of glycolytic metabolism12-14 has been shown to decrease Mcl-1 translation, thereby sensitizing cells to apoptosis.15-17
The prominent tumor suppressor p53 is found to be eliminated or mutated in approximately 50% of sporadic human cancers.18 In addition to its role in cell cycle control, the loss of p53 tends to favor glycolysis.19,20 Importantly, the loss of p53 function also increases the resistance of tumor cells to a broad range of cancer therapeutics. Therefore, the development of new strategies that can kill tumor cells in a p53-independent manner are a priority.
The link between metabolism and the expression of Bcl-2 family members is poorly understood. By using the Eµ-Myc transgenic mouse model, in which all animals spontaneously develop clonal pre-B or B-cell lymphoma,21 we have demonstrated that restriction of caloric intake by 25% can significantly reduce the translation of Mcl-1 through the AMPK/mTOR pathway and restore the sensitivity of lymphomas to ABT-737 in vivo. By using genetic approaches to define the impact of p53 and key BH3-only proteins, we established that Eµ-Myc lymphoma cells lacking p53 or Noxa, Puma, or Bim; or lacking both Puma and Noxa or both Puma and Bim; or lacking all three BH3-only proteins (Noxa, Puma, and Bim) were also sensitized by CR mimetics to ABT-737–induced death.
Unexpectedly, our results suggested that CR and CR mimetics (2-deoxyglucose [2DG] and lonidamine [LND]) can sensitize lymphomas in vivo or lymphoma cells in vitro, respectively, to ABT-737–induced apoptosis, regardless of the presence of p53 or the absence of the BH3-only proteins. These data support the metabolic control of Mcl-1 expression as a key event in this setting.
Methods
CR experiment
All animal experiments were performed according to the guidelines of the Institutional Animal Care and Use Committee and of the regional ethics committee (approval reference NCE/2011-35). Eµ-Myc/wild-type (WT) mice were obtained from the Jackson Laboratory. We observed that WT C57BL/6 mice age 6 to 8 weeks eat an average of 3 g of food per day. WT syngenic C57BL/6 mice were intravenously injected with 0.2 × 106 Eµ-Myc cells and were then fed normally or in CR mode (75% of normal dose is 2.25 g per day per mouse). Upon the appearance of lymphoma, mice were intraperitoneally injected daily for 10 days with vehicle or ABT-737 (75 mg/kg). At the end of the ABT-737 treatment, all mice were fed normally. Animals were euthanized as soon as they showed signs of illness. After 9 days of the dietary study, glycemia was measured by using a freestyle Optium blood glucose monitoring device. The number of white blood cells (WBCs) was counted 2 days after the end of the ABT-737 treatment by using a Hemavet 950FS (Drew Scientific, Inc., Le Rheu, France).
Cell culture and in vitro treatments
MEF Mcl-1−/− cells (and controls) were a kind gift of Dr. Opferman. MEF cells from Bak/Bax double-knockout mice (and controls) were a kind gift of Dr. Korsmeyer. Eµ-Myc/WT lymphoma cells were harvested from enlarged lymph nodes of C57BL/6 transgenic Eµ-Myc mice isolated and cultured as described.22 Eµ-Myc lymphomas generated to lack specific BH3-only genes or p53 have been previously described23-25 : mice were intercrossed with Eµ-Myc mice to produce single and compound-deficient lymphomas, as described in Happo et al.26 rv-MYC/Puma−/−Bim−/− double-knockout and rv-MYC/Noxa−/−Puma−/−Bim−/− triple-knockout lymphoma cell lines have been previously described.26 Next, 0.8 × 106 cells per milliliter were treated for 20 hours with various doses of ABT-737 in the presence or absence of 2DG or LND.
Statistics
The data are expressed as mean ± standard deviation. For in vivo experiments, differences in the calculated mean values (Gaussian law) between the groups were assessed by one-way analysis of variance, followed by a Fisher test, and in cases in which significant differences were detected, a Tukey honestly significant difference test was used. Kaplan-Meier survival analyses were performed, and survival curves were compared by using log-rank tests. For in vitro experiments, differences in the calculated mean values (binomial law) between the groups were assessed by two-way analysis of variance followed by a χ2 test. A P value less than .05 was considered significant. Additional methods can be found in the supplemental Data on the Blood Web site.
Results
CR reduces Mcl-1 expression through inhibition of protein translation and sensitizes Eµ-Myc mice to ABT-737 treatment
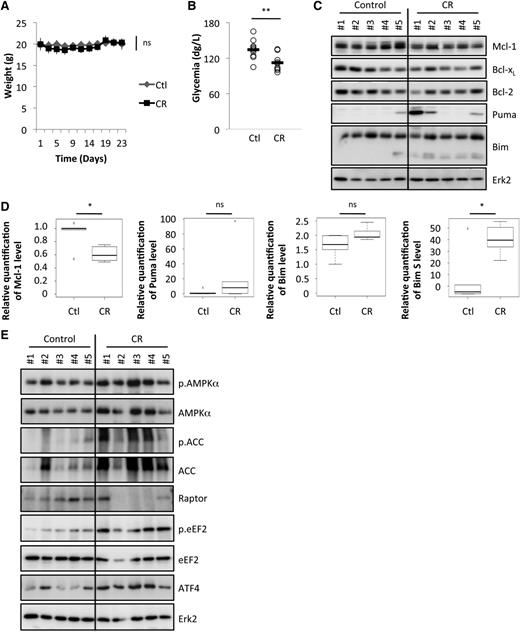
We sought to determine the impact of CR on the expression of key Bcl-2 family members. To that end, we reduced the dietary intake of the mice by 25%, which had no significant effect on the body weights of C57BL/6 mice over the duration of the experiment (Figure 1A), but which significantly reduced the glycemia of these mice compared with the control group (fed ad libitum; Figure 1B).27 Upon CR, syngeneic C57BL/6 mice were intravenously injected with Eµ-Myc/WT primary cells; 24 days later, mice from control and CR groups were euthanized in order to analyze the level of expression of several Bcl-2 members in lymphoma tissue by immunoblots (Figure 1C). Mcl-1 levels were reduced (by 39% ± 10%; P < .05; Figure 1D), but no other antiapoptotic Bcl-2 family members tested (supplemental Figure 1). In contrast, Bim S seemed to be enhanced in response to CR (P < .05; Figure 1D).
CR decreases Mcl-1 expression. WT C57BL/6 syngenic mice were injected intravenously with Eµ-Myc lymphoma cells and fed ad libitum or under CR conditions (CR, 75% of normal dose; see “Methods”). (A) Average weight of the mice that were fed for 23 days ad libitum (Ctl, control) or under CR conditions (Ctl, n = 10; CR, n = 9). (B) Glycemia was measured after 9 days of dietary study (Ctl, n = 10; CR, n = 9). **P < .01. (C) Lymph nodes bearing lymphoma were harvested from 5 independent mice after 24 days under CR or ad libitum feeding (control) and lysates were prepared. Bcl-2 family member expression was analyzed by immunoblots. (D) Average quantification of Mcl-1, Puma, Bim, and Bim S levels compared with Erk2 levels (used as a loading control) from samples in (C) (n = 5 per group). Reduction of Mcl-1, 39% ± 10%. *P < .05. (E) Lymph nodes bearing lymphoma were harvested from 5 independent mice as in (C) and the AMPK/mTOR pathway was analyzed by immunoblots. ns, nonsignificant.
CR decreases Mcl-1 expression. WT C57BL/6 syngenic mice were injected intravenously with Eµ-Myc lymphoma cells and fed ad libitum or under CR conditions (CR, 75% of normal dose; see “Methods”). (A) Average weight of the mice that were fed for 23 days ad libitum (Ctl, control) or under CR conditions (Ctl, n = 10; CR, n = 9). (B) Glycemia was measured after 9 days of dietary study (Ctl, n = 10; CR, n = 9). **P < .01. (C) Lymph nodes bearing lymphoma were harvested from 5 independent mice after 24 days under CR or ad libitum feeding (control) and lysates were prepared. Bcl-2 family member expression was analyzed by immunoblots. (D) Average quantification of Mcl-1, Puma, Bim, and Bim S levels compared with Erk2 levels (used as a loading control) from samples in (C) (n = 5 per group). Reduction of Mcl-1, 39% ± 10%. *P < .05. (E) Lymph nodes bearing lymphoma were harvested from 5 independent mice as in (C) and the AMPK/mTOR pathway was analyzed by immunoblots. ns, nonsignificant.
We and others previously established that inhibition of protein translation via the AMPK/mTOR pathway was an efficient way to decrease Mcl-1 protein levels.12,15-17 It is well established that when nutrient availability is compromised, mTORC1 is inactivated, in part through AMPK,28 thereby blocking protein synthesis, the most energy-demanding process in the cell.
As presented in Figure 1E, CR induced the expression and activation of AMPK (and the phosphorylation of ACC, one of its targets). We also observed a decrease in raptor expression, which is in line with mTORC1 destabilization and inhibition. eEF2 is a key regulator of protein translation that is inhibited upon CR via AMPK activation and mTOR inhibition.29 We confirmed that eEF2 was inactivated (phosphorylated) in lymphoma cells upon CR. Finally, translation inhibition upon CR was further supported by ATF4 overexpression, since it is one of the rare proteins induced upon general inhibition of the translation in cells.30
Altogether, our data indicate that upon CR, there is an AMPK activation and reduction of mTORC1, resulting in a decrease in protein translation. Since the Mcl-1 protein has a short half-life, a block in translation results in the reduction of its expression.12,15-17
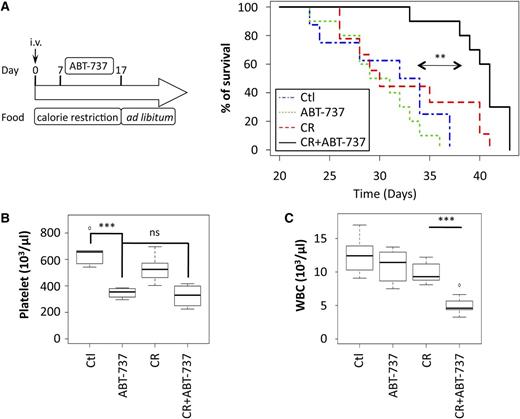
It is well established that Eµ-Myc lymphomas are resistant to ABT-737 treatment, at least in part because they express high levels of Mcl-1.12,31 Therefore, we evaluated whether CR could sensitize Eµ-Myc lymphomas to this BH3 mimetic. We intravenously injected Eµ-Myc lymphoma cells into syngeneic mice and fed the mice either ad libitum (control) or according to CR conditions. One week later, mice were treated with 75 mg/kg ABT-737 or vehicle for 10 days; mice were euthanized when they became unwell due to progressive lymphoma (Figure 2A). Although neither treatment with ABT-737 alone nor CR increased survival of mice over that of control mice, the combination of ABT-737 and CR did so. Indeed, median survival was 30 days in the control group, 33 days in the ABT-737 group, 30 days in the CR group, and 41 days in the CR + ABT-737 group (P < .001 for ABT-737 [n = 8] vs CR + ABT-737 [n = 10]).
CR increases the lifespan of mice that receive ABT-737 treatment. (A) Syngeneic C57BL/6 mice were intravenously injected with lymphoma cells that were isolated from Eµ-Myc/WT mice and fed ad libitum or under CR for 17 days. Seven days after intravenous injection, mice were treated or not for 10 days with 75 mg/kg ABT-737. Next, all mice were fed ad libitum until the time of ethical euthanasia (see the experimental procedure scheme, left panel). Lifespans of the mice from the beginning of each treatment regimen are indicated for each group (control vs CR + ABT-737: median survival, 30 and 41 days, respectively; n = 10; P = .00005; CR vs CR + ABT-737: median survival, 30 and 41 days, respectively; n = 9 and n = 10, respectively; P = .0061; ABT-737 vs CR + ABT-737: median survival, 33 and 41 days, respectively; n = 8 and n = 10, respectively; P = .00048). (B) Numbers of platelets were measured in each group at 14 days after the intravenous injection of Eµ-Myc cells (control, n = 5; CR and CR + ABT-737, n = 4 for ABT-737). (C) Numbers of WBCs were analyzed in each group 21 days after the intravenous injection of Eµ-Myc cells (control, n = 7; ABT-737 and CR + ABT-737, n = 8; CR, n = 9). In vivo experiments were conducted twice with similar results. **P < .01; ***P < .001. i.v., intravenous.; ns, nonsignificant
CR increases the lifespan of mice that receive ABT-737 treatment. (A) Syngeneic C57BL/6 mice were intravenously injected with lymphoma cells that were isolated from Eµ-Myc/WT mice and fed ad libitum or under CR for 17 days. Seven days after intravenous injection, mice were treated or not for 10 days with 75 mg/kg ABT-737. Next, all mice were fed ad libitum until the time of ethical euthanasia (see the experimental procedure scheme, left panel). Lifespans of the mice from the beginning of each treatment regimen are indicated for each group (control vs CR + ABT-737: median survival, 30 and 41 days, respectively; n = 10; P = .00005; CR vs CR + ABT-737: median survival, 30 and 41 days, respectively; n = 9 and n = 10, respectively; P = .0061; ABT-737 vs CR + ABT-737: median survival, 33 and 41 days, respectively; n = 8 and n = 10, respectively; P = .00048). (B) Numbers of platelets were measured in each group at 14 days after the intravenous injection of Eµ-Myc cells (control, n = 5; CR and CR + ABT-737, n = 4 for ABT-737). (C) Numbers of WBCs were analyzed in each group 21 days after the intravenous injection of Eµ-Myc cells (control, n = 7; ABT-737 and CR + ABT-737, n = 8; CR, n = 9). In vivo experiments were conducted twice with similar results. **P < .01; ***P < .001. i.v., intravenous.; ns, nonsignificant
We confirmed that the ABT-737 treatment was active because it induced thrombocytopenia (Figure 2B), as previously described.32 The degree of thrombocytopenia was not different between the ABT-737 and CR + ABT-737 groups (Figure 2B), consistent with Mcl-1 levels not having an impact on platelet survival. Finally, while neither ABT-737 nor CR alone modulated the number of circulating WBCs (Figure 2C), the combination of ABT-737 and CR did so, suggesting that the combination could sensitize both circulating lymphoma cells and normal WBCs to apoptosis. This reduction in WBCs is consistent with the protective effects seen following combination therapy of mice bearing lymphomas (Figure 2A). These results imply that CR can modulate Mcl-1 levels and sensitize lymphomas in mice to treatment with ABT-737.
CR mimetics sensitize cells to ABT-737, even in the absence of p53
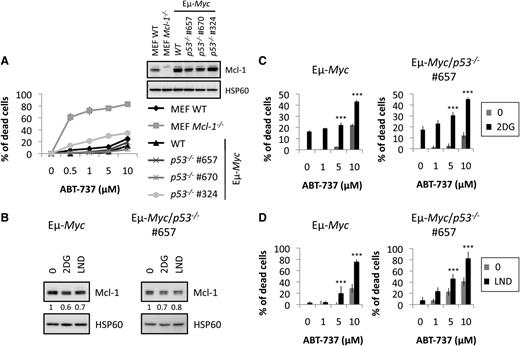
Because p53 is frequently mutated in tumors and because it is a key regulator of glycolysis, which can control Mcl-1 levels, we tested whether the benefits of co-treatment were dependent on p53 status. We performed ex vivo experiments using Eµ-Myc lymphoma cells isolated from Eµ-Myc mice that were either WT or heterozygous for p53 (during lymphomagenesis, Eµ-Myc/p53+/− lymphoma cells undergo obligatory loss of the WT allele; supplemental Figure 2). Unlike MEF cells isolated from Mcl-1 knockout pups, Eµ-Myc/p53−/− cells were not sensitive to ABT-737–induced death, indicating that BH3-mimetic treatment of Eµ-Myc lymphoma was ineffective, regardless of p53 status (Figure 3A), in accordance with a previous study.33
CR mimetics restore the sensitivity of primary Eµ-Myc cells to ABT-737, independently of p53. (A) The indicated MEF and Eµ-Myc lymphoma cells were treated with increased concentrations of ABT-737 for 20 hours. Cell death was determined by propidium iodide (PI) staining and analyzed by fluorescence-activated cell sorter (FACS). The insert shows Mcl-1 expression. (B) Eµ-Myc/WT or Eµ-Myc/p53−/− cells were incubated with 2DG 100 µg/mL or LND 100 µM for 20 hours, and the level of Mcl-1 was analyzed by immunoblot. In (A) and (B), HSP60 was used as a loading control. Quantifications of Mcl-1 over HSP60 levels are indicated. (C-D) Indicated cells were treated with 1, 5, or 10 µM ABT-737 with or without 75 µg/mL 2DG (C) or 100 µM LND (D). The percentage of PI-positive dead cells was measured as in (A). The results are expressed as the mean ± standard deviation (SD) from 3 independent experiments. Each experiment was performed on cells from 3 independently derived lymphomas. ***P < .001.
CR mimetics restore the sensitivity of primary Eµ-Myc cells to ABT-737, independently of p53. (A) The indicated MEF and Eµ-Myc lymphoma cells were treated with increased concentrations of ABT-737 for 20 hours. Cell death was determined by propidium iodide (PI) staining and analyzed by fluorescence-activated cell sorter (FACS). The insert shows Mcl-1 expression. (B) Eµ-Myc/WT or Eµ-Myc/p53−/− cells were incubated with 2DG 100 µg/mL or LND 100 µM for 20 hours, and the level of Mcl-1 was analyzed by immunoblot. In (A) and (B), HSP60 was used as a loading control. Quantifications of Mcl-1 over HSP60 levels are indicated. (C-D) Indicated cells were treated with 1, 5, or 10 µM ABT-737 with or without 75 µg/mL 2DG (C) or 100 µM LND (D). The percentage of PI-positive dead cells was measured as in (A). The results are expressed as the mean ± standard deviation (SD) from 3 independent experiments. Each experiment was performed on cells from 3 independently derived lymphomas. ***P < .001.
To analyze ex vivo the role of CR in ABT-737–induced cell death, we used 2DG and LND, two classic CR mimetics (see Kang and Wang34 for a review). First, we verified that 2DG and LND sensitized lymphoma cells to ABT-737–induced death through a caspase-dependent mechanism (ie, apoptosis; supplemental Figure 3). Next, we established that 2DG and LND led to reduced Mcl-1 expression in both Eµ-Myc/WT and Eµ-Myc/p53−/− cells (Figure 3B). In addition, by using m7-GTP (guanosine 5′-triphosphate) Sepharose beads that mimic the messenger RNA cap structure, we verified that 2DG or LND led to a block in protein translation in lymphoma cells (supplemental Figure 4A) and to a decrease in cyclin D1, a protein with a short half-life (supplemental Figure 4B).
It appeared that both Eµ-Myc/WT and Eµ-Myc/p53−/− lymphoma cells were resistant to ABT-737 (Figure 3A) and were similarly sensitized to ABT-737–induced death in the presence of 2DG or LND (Figure 3C-D). Results were reproduced in independent Eµ-Myc/p53−/− lymphoma cells (supplemental Figure 6A). Thus, ex vivo studies support the sensitization by CR mimetics of lymphoma cells to ABT-737–induced death, independently of p53 expression status.
c-MYC–driven lymphomas that lack Bim, Puma, and Noxa are sensitized to ABT-737–induced death by CR mimetics
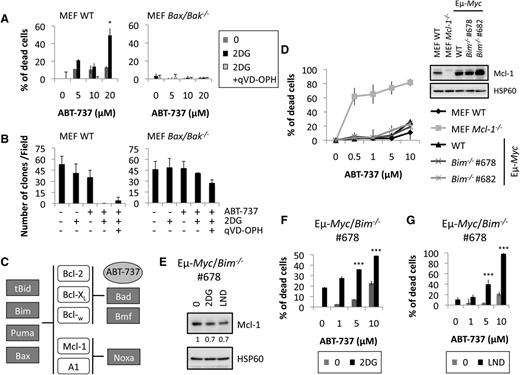
First, we investigated the role of MOMP in sensitization to ABT-737 in the presence of CR mimetics. We treated WT or Bax/Bak double-knockout MEF cells with ABT-737, with or without 2DG (Figure 4A). While WT MEF cells were sensitized by 2DG to ABT-737–induced apoptosis, this effect was not observed in Bax/Bak double-knockout MEF cells. We then showed that most Bax/Bak double-knockout MEF cells were able to preserve clonogenic survival upon treatment (Figure 4B).
Loss of Bim does not prevent the CR-mediated sensitization to ABT-737–induced cell death in primary Eµ-Myc cells. (A) WT or Bak/Bak double-knockout MEF cells were incubated with indicated amount of ABT-737 for 20 hours with or without 500 µg/mL 2DG with or without 20 µM qVD-OPH, and cell death was measured as in Figure 3A. (B) Indicated cells were treated with 20 µM ABT-737 ± 500 µg/mL 2DG ± 20 µM qVD-OPH. After 4 days, the number of clones was counted. (C) Schematic representation of the interaction between the pro- and antiapoptotic members of the Bcl-2 family. (D) The indicated MEF and Eµ-Myc cells were incubated with 0.5, 1, 5, or 10 µM ABT-737. The percentage of PI-positive cells was determined by FACS. Insert: immunoblot of Mcl-1 expression. (E) Eµ-Myc/Bim−/− cells were incubated with 2DG 100 µg/mL or LND 100 µM for 20 hours, and the levels of Mcl-1 were analyzed by immunoblot. HSP60 was used as a loading control. Quantifications of Mcl-1 over HSP60 levels are indicated. (F-G). Eµ-Myc/Bim−/− cells were treated with the indicated doses of ABT-737 in the presence or not of 2DG 75 µg/mL (F) or LND 100 µM (G) for 20 hours. Cell death was measured as in Figure 3A. Results shown are mean ± SD from 3 independent experiments. Each experiment was performed on cells from 2 independently derived lymphomas. ***P < .001.
Loss of Bim does not prevent the CR-mediated sensitization to ABT-737–induced cell death in primary Eµ-Myc cells. (A) WT or Bak/Bak double-knockout MEF cells were incubated with indicated amount of ABT-737 for 20 hours with or without 500 µg/mL 2DG with or without 20 µM qVD-OPH, and cell death was measured as in Figure 3A. (B) Indicated cells were treated with 20 µM ABT-737 ± 500 µg/mL 2DG ± 20 µM qVD-OPH. After 4 days, the number of clones was counted. (C) Schematic representation of the interaction between the pro- and antiapoptotic members of the Bcl-2 family. (D) The indicated MEF and Eµ-Myc cells were incubated with 0.5, 1, 5, or 10 µM ABT-737. The percentage of PI-positive cells was determined by FACS. Insert: immunoblot of Mcl-1 expression. (E) Eµ-Myc/Bim−/− cells were incubated with 2DG 100 µg/mL or LND 100 µM for 20 hours, and the levels of Mcl-1 were analyzed by immunoblot. HSP60 was used as a loading control. Quantifications of Mcl-1 over HSP60 levels are indicated. (F-G). Eµ-Myc/Bim−/− cells were treated with the indicated doses of ABT-737 in the presence or not of 2DG 75 µg/mL (F) or LND 100 µM (G) for 20 hours. Cell death was measured as in Figure 3A. Results shown are mean ± SD from 3 independent experiments. Each experiment was performed on cells from 2 independently derived lymphomas. ***P < .001.
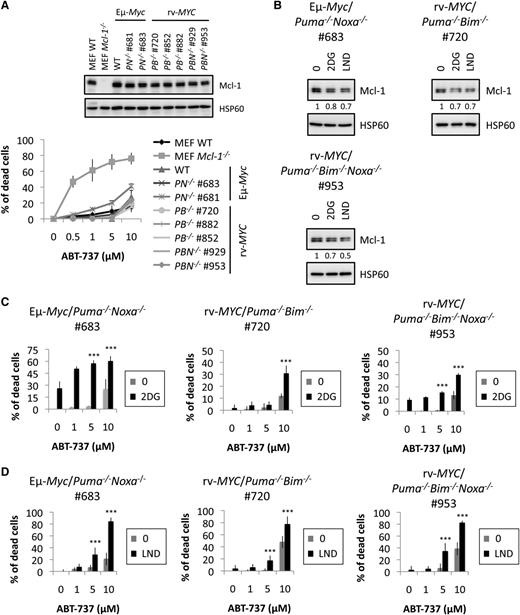
Because Bim S was upregulated upon CR (Figure 1C-D), we next examined the roles of key BH3-only proteins, which are Mcl-1 partners (Bim, Puma, and Noxa; Figure 4C). We isolated lymphoma cells from mice bearing Eµ-Myc/Bim−/− lymphomas (supplemental Figure 5A) and measured their response to ABT-737–induced death. No significant cell death could be measured with exposure to ABT-737, regardless of Bim status, whereas Mcl-1−/− MEF cells were sensitive to ABT-737–induced death, indicating that the drug was active (Figure 4D). We established that CR mimetics decreased Mcl-1 protein expression in Eµ-Myc/Bim−/− lymphoma cells (Figure 4E). Interestingly, Bim deficiency could not prevent sensitization by CR mimetics to ABT-737–induced apoptosis (Figure 4F-G). Results were reproduced in independent Eµ-Myc/Bim−/− lymphoma cells (supplemental Figure 6B). Thus, Bim is not required for sensitization to ABT-737 by CR mimetics.
To explore more thoroughly how CR sensitizes mice to ABT-737, we evaluated the roles of Noxa and Puma. Therefore, we isolated lymphoma cells from mice bearing either Eµ-Myc/Puma−/− or Eµ-Myc/Noxa−/− cells (supplemental Figure 5B-C).
As expected, we verified that the presence or absence of Puma or Noxa did not affect the response of Eµ-Myc cells to ABT-737–induced cell death (Figure 5A). As seen with Eµ-Myc lymphoma cells lacking Bim, neither the loss of Puma nor the loss of Noxa prevented the ability of CR mimetics to reduce Mcl-1 expression (Figure 5B) or to sensitize cells to ABT-737 death (Figure 5C-D).
Sensitization of primary Eµ-Myc cells to ABT-737–induced death by CR mimetics is independent of Puma or Noxa. (A) MEF and indicated Eµ-Myc cells were incubated with varied doses of ABT-737 for 20 hours. Dead cells were counted by PI staining and analyzed by FACS. The insert shows Mcl-1 expression. (B) Eµ-Myc/Noxa−/− and Eµ-Myc/Puma−/− cells were incubated with 2DG 100 µg/mL or LND 100 µM for 20 hours, and the levels of Mcl-1 were analyzed by immunoblot. HSP60 was used as a loading control. Quantifications of Mcl-1 over HSP60 levels are indicated. (C-D) Eµ-Myc/Puma−/− and Eµ-Myc/Noxa−/− cells were treated with the indicated doses of ABT-737 with or without 2DG 100 µg/mL (C) or LND 100 µM (D) for 20 hours. Cell death was determined as in Figure 3A. The results represent the mean ± SD from 3 independent experiments. *P < .05, **P < .01, ***P < .001.
Sensitization of primary Eµ-Myc cells to ABT-737–induced death by CR mimetics is independent of Puma or Noxa. (A) MEF and indicated Eµ-Myc cells were incubated with varied doses of ABT-737 for 20 hours. Dead cells were counted by PI staining and analyzed by FACS. The insert shows Mcl-1 expression. (B) Eµ-Myc/Noxa−/− and Eµ-Myc/Puma−/− cells were incubated with 2DG 100 µg/mL or LND 100 µM for 20 hours, and the levels of Mcl-1 were analyzed by immunoblot. HSP60 was used as a loading control. Quantifications of Mcl-1 over HSP60 levels are indicated. (C-D) Eµ-Myc/Puma−/− and Eµ-Myc/Noxa−/− cells were treated with the indicated doses of ABT-737 with or without 2DG 100 µg/mL (C) or LND 100 µM (D) for 20 hours. Cell death was determined as in Figure 3A. The results represent the mean ± SD from 3 independent experiments. *P < .05, **P < .01, ***P < .001.
Next, we reasoned that the removal of individual proapoptotic binding partners of Mcl-1 might not be sufficient to prevent sensitization to ABT-737 induced by CR mimetics, since the other BH3-only proteins are still present and could be sufficient to elicit apoptosis. We therefore examined the combinatorial roles of Bim, Puma, and Noxa in our models. We used Eµ-Myc/Puma−/−Noxa−/− lymphoma cells. Because it was not feasible to generate Eµ-Myc lymphomas lacking Puma and Bim or lacking Puma, Noxa, and Bim by conventional breeding due to breeding complications and early lethality of intermediate genotypes, fetal liver cells from Puma−/−Bim−/− double-knockout mice and from Puma−/−Bim−/−Noxa−/− triple-knockout mice were infected with a c-MYC retrovirus, injected into lethally irradiated recipient mice, and lymphomas were harvested for subsequent analysis.26
Lymphoma genotypes were confirmed (supplemental Figure 5D-E). We verified that sensitization to ABT-737 was not affected in those cells (Figure 6A) and that CR mimetics led to a decrease in Mcl-1 protein expression regardless of the genotype (Figure 6B). Perhaps surprisingly, neither Eµ-Myc/Puma−/−Noxa−/− lymphoma cells nor rv-MYC/Puma−/−Bim−/− nor rv-MYC/Puma−/−Bim−/−Noxa−/− knockout lymphoma cell lines (TKO cells) were protected from sensitization to 10 μM ABT-737–induced death observed in the presence of CR mimetics, although some protection was observed at 5 μM compared with death seen in Eµ-Myc/WT lymphoma cells (Figure 6C-D and supplemental Figure 6C-E). We then verified that TKO cells were dying through apoptosis, possibly through tBid activation upon CR mimetics–mediated caspase-8 activation,35 as a caspase-inhibitor reduced cell death (supplemental Figure 7A). However, a complete caspase inhibition did not allow clonogenic outgrowth of the cells (supplemental Figure 7B), suggesting that TKOs were finally eliminated by a caspase-independent cell death mechanism.3
There is no compensatory effect of Bim, Puma, or Noxa on the CR mimetics–mediated sensitization to ABT-737–induced death. (A) The indicated cells were treated for 20 hours with increasing doses of ABT-737. Cell death (percentage of PI-positive cells) was determined by FACS. The insert shows Mcl-1 expression in the indicated cell types. (B) The indicated cells were incubated with 2DG 100 µg/mL or LND 100 µM for 20 hours, and the levels of Mcl-1 were analyzed by immunoblot. HSP60 was used as a loading control. Quantifications of Mcl-1 over HSP60 levels are indicated. (C-D) The indicated cells were treated for 20 hours with 1, 5, or 10 µM ABT-737 with or without 2DG 100 µg/mL or LND 100 µM. Dead cells were counted as in Figure 3A. The results represent the mean ± SD of 3 independent experiments. Each experiment was performed on cells from 2 independently derived lymphomas for each genotype. ***P < .001.
There is no compensatory effect of Bim, Puma, or Noxa on the CR mimetics–mediated sensitization to ABT-737–induced death. (A) The indicated cells were treated for 20 hours with increasing doses of ABT-737. Cell death (percentage of PI-positive cells) was determined by FACS. The insert shows Mcl-1 expression in the indicated cell types. (B) The indicated cells were incubated with 2DG 100 µg/mL or LND 100 µM for 20 hours, and the levels of Mcl-1 were analyzed by immunoblot. HSP60 was used as a loading control. Quantifications of Mcl-1 over HSP60 levels are indicated. (C-D) The indicated cells were treated for 20 hours with 1, 5, or 10 µM ABT-737 with or without 2DG 100 µg/mL or LND 100 µM. Dead cells were counted as in Figure 3A. The results represent the mean ± SD of 3 independent experiments. Each experiment was performed on cells from 2 independently derived lymphomas for each genotype. ***P < .001.
Taken together, these results demonstrate that CR can sensitize myc-driven lymphomas to ABT-737–induced apoptosis in vivo associated with Mcl-1 downregulation independently of p53 or of the Mcl-1–binding BH3-only proapoptotic proteins Bim, Noxa, and Puma. Thus, the relative reduction in availability of Mcl-1, together with the neutralization of Bcl-2, Bcl-xL and possibly Bcl-w by ABT-737, prevents sequestration of Bax and Bak, freeing them to initiate the apoptotic cascade required for therapeutic efficacy.
Discussion
Over the last few years, many attempts have been made to develop more specific cancer therapies that target specific properties of tumor cells, such as their avidity for glucose (Warburg effect) or their dependence on the Bcl-2 protein family (using BH3 mimetics). By using the Eµ-Myc model, we have shown that CR can modulate the expression of the important prosurvival member of the Bcl-2 family, Mcl-1, and can promote the sensitization of lymphomas to ABT-737 treatment in mice (Figures 1C and 2A). We have extended this observation by using lymphoma cells that were originally isolated from knockout mice to show that this sensitization was independent of Bim, Puma, or Noxa, the three BH3-only proteins known to bind to Mcl-1 (Figures 4-6). Notably, this sensitization was also independent of p53 status (Figure 3), thereby introducing an innovative strategy with the potential to enhance the efficiency of killing tumor cells.
To address whether CR could modulate the expression of Bcl-2 family members, we restricted the food intake of mice by 25% for 24 days (Figure 1C) because this approach has been shown to have no significant impact on the weights of the animals27 but will lead to a significant decrease in glycemia (Figure 1A-B). We used a lymphoma transplantation model because our goal was to analyze the impact of ABT-737 on established tumors and not to study the impact of CR during lymphoma development (Figure 2A).36 It is worth noting that the number of WBCs that were measured in circulating blood and the overall survival rates of the mice in control (fed ad libitum), ABT-737, or CR groups were not significantly different (Figure 2A,C), underscoring the fact that lymphoma development was minimally altered by CR in our settings.
We established that CR led to a significant reduction of Mcl-1 expression in vivo (Figure 1C). This reduction was partial (39% ± 10%; n = 5; P < .05; Figure 1D) but was sufficient to significantly increase the sensitivity of the lymphomas to ABT-737 in vivo. Indeed, mice treated with CR + ABT-737 showed a median survival of 41 days compared with that of the CR group (30 days; n = 10; P < .01; Figure 2A), which is consistent with our recent work showing that in the Eµ-Myc model, the CR mimetic 2DG decreased Mcl-1 levels in vivo and sensitized lymphoma-bearing mice to ABT-737.12 Here, we establish that the impact of CR on Mcl-1 levels was also observed ex vivo by using CR mimetics (Figures 3B, 4E, 5B, and 6B), regardless of the status of p53, Puma, Noxa, or Bim. Our observation is in agreement with several reports that suggest that the level of Mcl-1 is directly correlated with sensitivity to ABT-737–induced death.8,37
It has been demonstrated extensively that CR can modulate messenger RNA translation regulation (mainly via the mTOR pathway) and that the reduction of glucose metabolism (using CR mimetics) affects the translation of Mcl-1 protein.12,15-17 Accordingly, we observed in vivo that CR led to AMPK activation and inhibition of protein translation (Figure 1E). We also verified ex vivo that 2DG and LND could inhibit protein translation in primary Eµ-Myc cells (supplemental Figure 4). Since the Mcl-1 protein has a short half-life, a reduction in its translation leads to decreased expression.
Mcl-1 was recently described as an important regulator of mitochondrial functions.38 Using Bax/Bak−/− MEF cells, we observed that CR mimetics–mediated decrease of Mcl-1 was minimally impairing the long-term survival of those cells (Figure 4B), suggesting that the partial decrease in Mcl-1 induced by CR mimetics is sufficient to sensitize the cells to ABT-737–induced death via MOMP but not sufficient for impairing the function of these organelles (probably because enough Mcl-1 remains).
Unfortunately, we were unable to obtained freshly isolated biopsies from patients suffering from Burkitt or diffuse large B-cell lymphoma to test the efficiency of our cotreatment. However, preliminary data from freshly isolated human lymphoma cells from patients with follicular B-cell lymphoma (n = 1) or Hodgkin lymphoma (n = 1) showed sensitization to killing with ABT-737 by the addition of either 2DG or LND (P < .05; supplemental Figure 8), underlying the potential benefit of such cotreatment in patients. Primary tumor cells were isolated from the remaining part of patients’ lymph node biopsies collected for diagnosis after patients’ agreement and directly analyzed in our laboratory. Informed consent was obtained in accordance with the Declaration of Helsinki.
Several reports have suggested that DNA-damaging agents31,39 could sensitize tumor cells to ABT-737–induced death. However, since p53 is mutated or deleted in approximately 50% of human tumors, this approach may reduce the potential impact of such observations. In striking comparison, we have demonstrated that CR could sensitize lymphomas to ABT-737–induced apoptosis independently of p53 (Figure 3), as suggested in other models,13 thereby introducing new strategies to kill such cancer cells.
A question remains concerning our work: Which proapoptotic member(s) are involved in sensitization to ABT-737 induced by CR mimetics? The Bcl-2 family comprises at least 11 proapoptotic members that share some redundant functions.4 It is now generally accepted that the BH3-only members play an important role in Bax and Bak activation, even though the mechanisms for this process are still not well understood.4
We investigated the roles of some of the preferential binding partners of Mcl-1 (Bim, Puma, Noxa) by using single, double, or triple gene deletion models, since these were recently found to be required for the induction of maximal killing of lymphoma cells in response to DNA-damaging agents.26 In addition, Noxa has been described to play a role in glucose metabolism by promoting glucose uptake.40 It has further been suggested that the CPT-11 or bortezomib-dependent expression of Noxa may increase the sensitivity of Mcl-1–expressing cancer cells to ABT-737.41 Importantly, Noxa was shown to play a critical role in conjunction with 2DG treatment in other models.42 However, neither Noxa nor Bim seems to be involved in the CR mimetics sensitization to ABT-induced death in our models (Figures 5 and 6). This discrepancy may be context-dependent: perhaps in myc-driven lymphomas, the relative reduction in Mcl-1 levels achieved with CR is sufficient to initiate Bax/Bak-dependent apoptosis, whereas in other contexts, such as in rhabdomyosarcoma, metabolic effects on Mcl-1 may differ, resulting in insufficient neutralization of Mcl-1 and thus a dependence on Noxa-induced killing.
In fact, we repeatedly established that our combination therapy was efficient, regardless of the genotype of the myc-driven lymphoma cells (Figures 4-6 and supplemental Figure 6). Therefore, it is possible that one (or several) other proapoptotic members are activated or de-repressed upon the CR-dependent decrease in Mcl-1 expression. Among these proteins, Bad has been linked extensively to glucose metabolism and glucose deprivation–induced cell death.43 Recent studies report that upon glycolysis inhibition, Bak dissociation from Mcl-1 or BMF was induced, thereby sensitizing the cells to BH3 mimetic–induced death.13,14 According to the roles of these proteins in cell death and the impact of metabolism on their activation, it is possible that Bad, BMF, and/or Bak are playing roles in myc-driven lymphoma. Nevertheless, we cannot rule out the possibility that Bid, Bax, HRK, or Bik could also play central roles in CR-mediated sensitization to ABT-737–induced death.
It has been shown recently that specific oncogenes, such as activated PI3K, render tumors unresponsive to CR, suggesting that the efficacy of reduced food intake may be limited to certain molecular subsets of cancers.44 Additionally, the side effects of long-term CR may impose significant risks to cancer patients who are receiving chemotherapy.45 However, our work establishes that short-term CR may represent a safe and efficient way to sensitize tumor cells to BH3 mimetics, independently of the status of p53. This study suggests that one challenge for future investigations is the identification of the therapeutic window and the necessary conditions to sensitize the patient to this innovative treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the Centre Méditerranéen de Médecine Moléculaire animal room facility and Drs. Cory, Strasser, and Muñoz-Pinedo for their valuable comments.
This work was supported by “la Fondation Association pour la Recherche sur le Cancer,” “la Fondation de France,” le Centre Scientifique de Monaco, “l’Agence Nationale de la Recherche” (ANR-09-JCJC-0003), la Ville de Nice, and “la Fondation pour la Recherche Medicale.” L.H. is the recipient of a scholarship from the Leukemia Foundation Australia. C.L.S. received fellowships from the Australian National Health and Medical Research Council (Australia RD Wright Biomedical [CDA 406675]), the Victorian Cancer Agency, and the Cancer Council Victoria (Sir Edward Dunlop Research Fellow). J.-E.R. and M.C. are recipients of a contrat d’interface from Institut national de la santé et de la recherche médicale and Centre Hospitalier Universitaire de Nice.
Authorship
Contribution: O.M. and B.Z. performed most experiments; B.Z. and M.C. performed statistical analysis; J.C., M.A.J., and L.M. performed experiments; L.A.P., L.H., J.-F.T., C.L.S., and E.M.M. contributed reagents and scientific input; B.T., G.G., J.R.-M., N.M., and J.-F.M. collected patient samples; and J.-E.R. designed research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Ehrland Ricci, Inserm U1065, équipe 3, 151 route de St Antoine de Ginestière, BP 23194, 06204 Nice Cedex 03, France; e-mail: ricci@unice.fr.
References
Author notes
O.M. and B.Z. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal