Key Points
Ibrutinib is the first clinically viable irreversible ITK inhibitor.
Ibrutinib inhibits the formation of Th2 but not Th1 immunity.
Abstract
Given its critical role in T-cell signaling, interleukin-2–inducible kinase (ITK) is an appealing therapeutic target that can contribute to the pathogenesis of certain infectious, autoimmune, and neoplastic diseases. Ablation of ITK subverts Th2 immunity, thereby potentiating Th1-based immune responses. While small-molecule ITK inhibitors have been identified, none have demonstrated clinical utility. Ibrutinib is a confirmed irreversible inhibitor of Bruton tyrosine kinase (BTK) with outstanding clinical activity and tolerability in B-cell malignancies. Significant homology between BTK and ITK alongside in silico docking studies support ibrutinib as an immunomodulatory inhibitor of both ITK and BTK. Our comprehensive molecular and phenotypic analysis confirms ITK as an irreversible T-cell target of ibrutinib. Using ibrutinib clinical trial samples along with well-characterized neoplastic (chronic lymphocytic leukemia), parasitic infection (Leishmania major), and infectious disease (Listeria monocytogenes) models, we establish ibrutinib as a clinically relevant and physiologically potent ITK inhibitor with broad therapeutic utility. This trial was registered at www.clinicaltrials.gov as #NCT01105247 and #NCT01217749.
Introduction
The interplay between antigen-presenting cells and T lymphocytes forms an indispensable component of adaptive immunity, yet certain neoplastic, autoimmune, parasitic, and infectious diseases subvert adaptive immunity by specifically misdirecting helper T-cell polarity.1,2 A common mechanism of immune subversion is the aberrant recruitment of a Th2-dominant response that promotes B-cell antibody production and interferes with direct effector cell cytotoxicity. In contrast, a Th1-dominant response evokes cytotoxic effects with the production of interferon γ (IFN-γ) and interleukin 2 (IL-2), which contribute to effector-cell–based immune surveillance. Clearance of certain intracellular bacterial pathogens such as Listeria and parasites such as Leishmania, as well as tumor immune surveillance, hinge upon the capacity to elicit robust Th1 and CD8 T-cell responses.
IL-2–inducible kinase (ITK) is a T-cell–dominant member of the TEC-kinase family that drives proximal T-cell receptor (TCR) signaling.3 Upon TCR ligation in Th1 and CD8 T cells, ITK and redundant resting lymphocyte kinase (RLK or TXK) activate phospholipase Cγ (PLCγ), launching a signaling cascade that includes the nuclear factor of activated T cells (NFAT), nuclear factor κB, and mitogen-activated protein kinase pathways, resulting in cellular activation, cytokine release, and rapid proliferation.4 In cancer, ITK is a critical signaling motif important to acute lymphoblastic T-cell leukemia and Sézary syndrome/cutaneous T-cell lymphoma due to aberrant activation and heightened expression.5 In healthy Th1-polarized and CD8 effector cells, ITK plays a supportive yet dispensable role to RLK. However, the epigenetic evolution of Th2 cells conserves a singular dominant role for ITK, pinning it as the Achilles’ heel of Th2 T cells.6-9
Clinically applicable ITK inhibitors are sought by the medical community given their potential to inhibit a number of Th2-dominant autoimmune, inflammatory, and infectious diseases ranging from cancer immunosuppression and atopic dermatitis to inflammatory bowel disease and even HIV/AIDS.10,11 Moreover, a viable ITK inhibitor would be a promising therapeutic advancement for many T-cell malignancies that are currently difficult to manage.12,13 Although multiple chemical analogs have been reported, no ITK inhibitors have successfully transitioned into clinical trials.14
Ibrutinib is an irreversible inhibitor of Bruton tyrosine kinase (BTK) that blocks downstream B-cell receptor activation.15,16 Numerous in vitro and in vivo studies confirm the specific activity of ibrutinib against BTK-restricted targets.17,18 Ibrutinib has demonstrated clinical activity in phase 1 and 2 clinical trials, with durable remissions against a variety of B-cell malignancies including mantle cell lymphoma, follicular lymphoma, and chronic lymphocytic leukemia (CLL).19-22 Intriguingly, ITK shares significant sequence and functional homology with BTK and both contain an ibrutinib inhibition motif consisting of an SH3 autophosphorylatable tyrosine (Tyr) and a covalent binding cysteine (Cys) residue within the hinge region connecting the C and N lobes of the active site.23 ITK had previously been discounted as a relevant target of ibrutinib given a lack of sufficient in vitro evidence; not long after, however, Herman et al noted effects on T-cell cytokine production, reigniting the inquiry.16,18
The striking homology between BTK and ITK combined with intriguing in silico docking led to the hypothesis that ibrutinib is the first clinically viable ITK inhibitor. This was explored using healthy human T cells and human and murine CLL as a model system of dysregulated Th2-biased immunosuppression. In CLL, an increasingly defective immune synapse enables malignant B cells to evade immune detection by inducing T-cell anergy as well as improper Th2 polarization.24,25 In addition to being incapable of responding to environmental pathogens, these improperly polarized T cells contribute both cytokine and direct signaling support to malignant B cells.26,27 The end result of this immunosuppression is a high incidence of severe infections, which is the leading cause of patient mortality.28,29
Our molecular analysis confirms that ibrutinib irreversibly binds ITK and inhibits activation of Th2 cells after TCR stimulation. This inhibition is specific to Th2-polarized CD4 T cells, because RLK remains functional, thus providing a compensatory platform for activation of Th1 and CD8 T cells. These data demonstrate that CD4 T-cell populations isolated from CLL patients are skewed toward a Th1 profile after exposure to ibrutinib. Findings were validated using mouse models of leukemia, cutaneous leishmaniasis, and Listeria monocytogenes infection. Ibrutinib’s immunomodulatory activity and ITK inhibition in humans were confirmed using irreversible ITK binding, cytokine, and T-cell signaling analysis from CLL patients treated with ibrutinib in 2 separate clinical trials. Together, these results confirm that ibrutinib is the first potent and irreversible inhibitor of ITK to achieve clinical viability, potentially repurposing the drug for a multitude of novel therapeutic applications.
Methods
Subject populations
Sera and peripheral blood mononuclear cells (PBMCs) were obtained from normal donors or patients with CLL in accordance with the Declaration of Helsinki. All subjects gave written informed consent for their blood products to be used for research under an institutional review board–approved protocol. Blood was collected at The Ohio State University Wexner Medical Center (Columbus, OH). For additional information, see supplemental Methods.
Cell culture, drug treatments, and T-cell polarization
Primary T cells were isolated using RosetteSep or EasySep T-cell enrichment kits (STEMCELL Technologies, Vancouver, BC, Canada). Cells were pretreated for 30 minutes with ibrutinib, washed 2 times, then stimulated with plate-bound anti-CD3 and soluble anti-CD28 (eBiosciences, San Diego, CA). For additional details, see supplemental Methods.
Calcium flux analysis
Jurkat cells were stained with Fluo4-AM (Invitrogen), washed twice, and resuspended in phenol-red free RPMI. Fluo4 fluorescence was measured using a plate reader at 535 nm. For additional information, see supplemental Methods.
Flow cytometry and cytokine bead array
Flow-cytometric analysis was performed using fluorochrome-labeled antibodies using conventional methods. For specific antibodies and experimental design, see supplemental Methods.
Ibrutinib probe assay
Protein lysates were labeled with a biotinylated derivative of ibrutinib and added to a Streptavidin-coated plate, washed 3 times, and incubated with mouse anti-ITK. After washing with SULFO-TAG–conjugated anti-mouse antibody (MSD, catalog #R32AC-5), lysates were read on an SI2400. For additional details, see supplemental Methods.
Immunoblot analysis
Experiments were conducted using conventional sodium dodecyl sulfate polyacrylamide gel electrophoresis methodology. For specific antibodies and densitometry, see supplemental Methods.
Confocal immunofluorescence microscopy
Cells were centrifugally concentrated on microscope slides and stained with monoclonal antibodies. Images were taken using a ×60 objective and ×4 digital zoom with Olympus Fluoview 1000 laser scanning confocal microscope at The Ohio State University Microscopy and Imaging Facility. See supplemental Methods for details.
Mouse models
All animal procedures were performed in accordance with Federal and Institutional Animal Care and Use Committee requirements. Detailed methods for mouse models are provided in supplemental Methods.
ELISA
An enzyme-linked immunoabsorbent assay (ELISA) assay was performed for each immunoglobulin G (IgG) subisotype using a clonotyping system (B6/C57J-AP-5300-04B; Southern Biotech, Birmingham, AL) according to the manufacturer’s instructions. For additional details, see supplemental Methods.
Statistics
Unless otherwise noted, a 2-tailed Student t test was used for normal data at equal variance. Significance was considered for P < .05. For detailed statistics, see supplemental Methods.
Results
Ibrutinib is an irreversible inhibitor of ITK
As a Th2-critical TEC family kinase, ITK retains significant structural and functional homology to ibrutinib’s known irreversible target BTK, including a Cys442 putative covalent binding moiety located within the hinge region of the active site and an autophosphorylatable Tyr180 in the SH3 domain (Figure 1A). In silico docking studies showed potential covalent binding of ITK at Cys442 and occupancy of the active site similar to that achieved when ibrutinib irreversibly binds BTK (Figure 1B). In vitro probe binding assays confirmed that ibrutinib was capable of irreversibly binding a significant percentage of endogenous ITK in the Jurkat T-cell leukemia cell line (Figure 1C).
Ibrutinib is an irreversible molecular inhibitor of ITK, displaying BTK-independent antileukemic potential. (A) A graphical depiction of the sequence and domain homology between ITK and BTK. The relevant phosphorylation sites as well as ibrutinib irreversible covalent binding sites are labeled. (B) In silico representation of docked ibrutinib within the active site of crystallized ITK (top panel) (Protein Data Bank code 3QGW) or BTK (bottom panel) showing close approximation of Cys442 or Cys481 to reactive moiety of ibrutinib. Shape and chemical complementarity of ibrutinib are shown in surface representation. (C) A molecular probe assay was used to calculate the percent irreversible occupancy of total ITK in Jurkat whole-cell lysates irreversibly bound by ibrutinib. Error bars represent standard error of the mean (SEM). (D) A molecular probe assay was used to calculate the percent irreversible occupancy of ITK by ibrutinib in cryopreserved PBMCs obtained from patients immediately prior to (predose) and 8 days into (ibrutinib) daily oral ibrutinib therapy for CLL (n = 8). Error bars represent SEM. (E) Primary CD4 T cells isolated from healthy donors were pretreated with ibrutinib (1 µM) or vehicle and subjected to stimulation with anti-CD3, anti-CD28, or anti-CD3/anti-CD28 for 6 hours and analyzed via fluorescence-activated cell sorter for CD69 surface expression. Baseline (unstimulated) CD69 percentage was subtracted and data are represented in log percent CD69+CD4+ T cells. A 2-tailed paired Student t test was used for statistical analysis (nonsignificant [ns] = P > .05). Error bars represent SEM.
Ibrutinib is an irreversible molecular inhibitor of ITK, displaying BTK-independent antileukemic potential. (A) A graphical depiction of the sequence and domain homology between ITK and BTK. The relevant phosphorylation sites as well as ibrutinib irreversible covalent binding sites are labeled. (B) In silico representation of docked ibrutinib within the active site of crystallized ITK (top panel) (Protein Data Bank code 3QGW) or BTK (bottom panel) showing close approximation of Cys442 or Cys481 to reactive moiety of ibrutinib. Shape and chemical complementarity of ibrutinib are shown in surface representation. (C) A molecular probe assay was used to calculate the percent irreversible occupancy of total ITK in Jurkat whole-cell lysates irreversibly bound by ibrutinib. Error bars represent standard error of the mean (SEM). (D) A molecular probe assay was used to calculate the percent irreversible occupancy of ITK by ibrutinib in cryopreserved PBMCs obtained from patients immediately prior to (predose) and 8 days into (ibrutinib) daily oral ibrutinib therapy for CLL (n = 8). Error bars represent SEM. (E) Primary CD4 T cells isolated from healthy donors were pretreated with ibrutinib (1 µM) or vehicle and subjected to stimulation with anti-CD3, anti-CD28, or anti-CD3/anti-CD28 for 6 hours and analyzed via fluorescence-activated cell sorter for CD69 surface expression. Baseline (unstimulated) CD69 percentage was subtracted and data are represented in log percent CD69+CD4+ T cells. A 2-tailed paired Student t test was used for statistical analysis (nonsignificant [ns] = P > .05). Error bars represent SEM.
To confirm that ibrutinib irreversibly binds ITK in vivo, we conducted an ITK probe assay on PBMC samples obtained from CLL patients in a phase 2 clinical trial of ibrutinib. Samples were tested immediately prior to receiving ibrutinib and after 8 days of daily oral administration (420 mg/day). The data revealed between 40% and 80% of ITK is covalently bound by ibrutinib, similar to that achieved in vitro (Figure 1D). Multimerization is an established ITK regulatory mechanism that sequesters inactive ITK within the cytoplasm, potentially blocking complete ibrutinib occupancy of ITK. To explore this, we disrupted the cytoplasmic architecture and observed near-complete occupancy of the ITK active site at drug concentrations nearing 0.3 µM (supplemental Figure 1).
In their initial description of ibrutinib, Honigberg et al did not find inhibition of the T-cell activation marker CD69 after stimulation with anti-CD3 and anti-CD28.18 Based upon this evidence, a T-cell–specific target had been ruled out. One reason for these divergent results could be the fact that CD28 costimulation alone can induce CD69 surface expression in a TCR- and ITK-independent fashion. To explore this possibility, we examined the CD69 activation marker in CD4 T-cells isolated from healthy donors and stimulated with anti-CD3, anti-CD28, or anti-CD3/anti-CD28 (Figure 1E).30 Ibrutinib significantly attenuated anti-CD3–induced surface expression of CD69. However, we did not observe any significant alteration to CD69 in CD4 T cells stimulated via CD28 or via CD3/CD28, indicating that CD28 costimulation provides an additional noninhibited pathway that elicits surface presentation of CD69.
Ibrutinib inhibits ITK signaling and molecular characteristics of TCR-induced activation in primary CD4 T cells and Jurkat cells
T-cell activation is predicated upon robust downstream nuclear factor κB, mitogen-activated protein kinase, and NFAT signaling; therefore, components of each pathway were examined to determine the T-cell–specific effects of ibrutinib. Ibrutinib treatment yielded a dose-dependent inhibition of ITK autophosphorylation at Y180 resulting in downstream inactivation of IkBα, JunB, and NFAT signaling in both primary CD4 and Jurkat T cells (Figure 2A-B; supplemental Figure 2). Notably, inhibition of both JunB and STAT6 was observed, both of which are critical components of the IL-4 pathway.31,32 Although JAK3 inhibition could explain some of our in vitro data, our target validation studies demonstrate that ibrutinib does not directly influence this kinase in cell-based assays (supplemental Figure 3).
In T cells, ibrutinib specifically targets ITK, inhibiting TCR-induced cellular signaling and activation. (A) Immunoblot analysis of freshly isolated ibrutinib pretreated primary CD4+ cells from a healthy donor, anti-CD3/anti-CD28 stimulated (or unstimulated), whole-cell lysates. Blot probed for pITK-Y180, total ITK, pSTAT6-Y641, total STAT6, pIkBα-S32/36, total IkBα, JunB, and actin. Densitometry analysis normalized to dimethylsulfoxide (DMSO)-treated (0 µM) sample. (B) Immunoblot analysis of freshly isolated ibrutinib pretreated primary CD4+ cells from a healthy donor, anti-CD3/anti-CD28–stimulated (or unstimulated), cytoplasmic, and nuclear lysates. Blots probed for NFAT (and activated hyperdephosphorylated NFAT), Brg1, and actin. Densitometry analyses are normalized to the DMSO-treated (0 µM) sample. (C) Immunoblot analysis of freshly isolated ibrutinib-pretreated primary CD4+ cells from a healthy donor, anti-CD3/anti-CD28–stimulated (or unstimulated), whole-cell lysates. Blots were probed for pZAP70-Y319, total ZAP70, pLAT-Y191, total LAT, pLCK-Y505, total LCK, pIkBα-S32/36, total IkBα, and actin. Densitometry analyses are normalized to the DMSO-treated (0 µM) sample. (D) Nuclear or whole-cell lysate immunoblot analysis of Jurkat cells pretreated with ibrutinib and stimulated with either anti-CD3/anti-CD28 or phorbol 12-myristate 13-acetate/ionomycin for 45 minutes. Blots were probed with Brg1, NFAT1, and actin (nuclear lysates) or pIkBα-S32/36, total IkBα, and actin (cellular lysates). (E) Immunofluorescent microscopy of ibrutinib-pretreated, freshly isolated, primary CD4+ cells from healthy donors (panels A and B) were stimulated for 45 minutes with anti-CD3/anti-CD28 (or unstimulated), fixed, and stained for NFAT (green) and nuclei (4,6 diamidino-2-phenylindole [DAPI], blue). Activated cells are characterized by influx of NFAT into nuclear region (green overlay with blue = cyan) and are denoted by white arrows. (F) Percent relative NFAT1/DAPI colocalization derived from Pearson correlation analysis of 10 independent immunofluorescent microscopy fields (different donors than pictured in panel E and normalized to the average unstimulated value. Cyclosporin A (CSA) treatment was used as an additional negative control. Error bars represent SEM. (G) Phosphoflow analysis of pPLCγ1-Tyr783 in 1hr anti-CD3/anti-CD28–stimulated cryopreserved PBMCs obtained immediately predose or after 8 days of receiving ibrutinib therapy for CLL (n = 11). A minimum of 400 000 events were collected. Graph displays the overall percent of live CD3+CD4+pPLCγ1−Tyr783+ events in each sample. Error bars represent SEM. (H) Calcium flux analysis of ibrutinib (n = 8), vehicle (n = 24), or BAPTA-AM (n = 8) pretreated Jurkat cells after TCR stimulation by anti-CD3. Area under the curve (AUC) is presented for each dataset in the center. All data were normalized to baseline and BAPTA-treated fluorescent averages. Time points depicted on horizontal axis are relative to stimulation with anti-CD3. (I) AUC for calcium flux of various concentrations of ibrutinib. Each symbol indicates a single replicate experiment. Statistical analysis represented on graph is relative to DMSO treatment. PMA/Iono., phorbol 12-myristate 13-acetate and ionomycin; Unstim, unstimulated.
In T cells, ibrutinib specifically targets ITK, inhibiting TCR-induced cellular signaling and activation. (A) Immunoblot analysis of freshly isolated ibrutinib pretreated primary CD4+ cells from a healthy donor, anti-CD3/anti-CD28 stimulated (or unstimulated), whole-cell lysates. Blot probed for pITK-Y180, total ITK, pSTAT6-Y641, total STAT6, pIkBα-S32/36, total IkBα, JunB, and actin. Densitometry analysis normalized to dimethylsulfoxide (DMSO)-treated (0 µM) sample. (B) Immunoblot analysis of freshly isolated ibrutinib pretreated primary CD4+ cells from a healthy donor, anti-CD3/anti-CD28–stimulated (or unstimulated), cytoplasmic, and nuclear lysates. Blots probed for NFAT (and activated hyperdephosphorylated NFAT), Brg1, and actin. Densitometry analyses are normalized to the DMSO-treated (0 µM) sample. (C) Immunoblot analysis of freshly isolated ibrutinib-pretreated primary CD4+ cells from a healthy donor, anti-CD3/anti-CD28–stimulated (or unstimulated), whole-cell lysates. Blots were probed for pZAP70-Y319, total ZAP70, pLAT-Y191, total LAT, pLCK-Y505, total LCK, pIkBα-S32/36, total IkBα, and actin. Densitometry analyses are normalized to the DMSO-treated (0 µM) sample. (D) Nuclear or whole-cell lysate immunoblot analysis of Jurkat cells pretreated with ibrutinib and stimulated with either anti-CD3/anti-CD28 or phorbol 12-myristate 13-acetate/ionomycin for 45 minutes. Blots were probed with Brg1, NFAT1, and actin (nuclear lysates) or pIkBα-S32/36, total IkBα, and actin (cellular lysates). (E) Immunofluorescent microscopy of ibrutinib-pretreated, freshly isolated, primary CD4+ cells from healthy donors (panels A and B) were stimulated for 45 minutes with anti-CD3/anti-CD28 (or unstimulated), fixed, and stained for NFAT (green) and nuclei (4,6 diamidino-2-phenylindole [DAPI], blue). Activated cells are characterized by influx of NFAT into nuclear region (green overlay with blue = cyan) and are denoted by white arrows. (F) Percent relative NFAT1/DAPI colocalization derived from Pearson correlation analysis of 10 independent immunofluorescent microscopy fields (different donors than pictured in panel E and normalized to the average unstimulated value. Cyclosporin A (CSA) treatment was used as an additional negative control. Error bars represent SEM. (G) Phosphoflow analysis of pPLCγ1-Tyr783 in 1hr anti-CD3/anti-CD28–stimulated cryopreserved PBMCs obtained immediately predose or after 8 days of receiving ibrutinib therapy for CLL (n = 11). A minimum of 400 000 events were collected. Graph displays the overall percent of live CD3+CD4+pPLCγ1−Tyr783+ events in each sample. Error bars represent SEM. (H) Calcium flux analysis of ibrutinib (n = 8), vehicle (n = 24), or BAPTA-AM (n = 8) pretreated Jurkat cells after TCR stimulation by anti-CD3. Area under the curve (AUC) is presented for each dataset in the center. All data were normalized to baseline and BAPTA-treated fluorescent averages. Time points depicted on horizontal axis are relative to stimulation with anti-CD3. (I) AUC for calcium flux of various concentrations of ibrutinib. Each symbol indicates a single replicate experiment. Statistical analysis represented on graph is relative to DMSO treatment. PMA/Iono., phorbol 12-myristate 13-acetate and ionomycin; Unstim, unstimulated.
To confirm that TCR-induced activation events preceding ITK autophosphorylation were not altered, we examined the proximal pathway components using both primary CD4 and Jurkat T cells. Immunoblot data revealed that upstream phosphorylation of LCK, ZAP70, and LAT remain unchanged (Figure 2C; supplemental Figure 4). Furthermore, we used the PKC activator, phorbol 12-myristate 13-acetate, and the calcium ionophore, ionomycin, to confirm that distal elements of TCR activation, including NFAT activity and IkBα phosphorylation, were engaged regardless of ibrutinib treatment in Jurkat cells (Figure 2D).
To functionally confirm our molecular data set, we examined NFAT nuclear translocation via confocal microscopy. As expected, NFAT nuclear translocation was inhibited by ibrutinib (Figure 2E-F). Remnant populations of activated CD4 T cells were observed, indicating that not all CD4 T cells were inhibited. We also confirmed that TCR-induced proliferation as well as naïve, central, effector, and terminal memory subsets were unaffected by in vitro ibrutinib treatment (supplemental Figures 5 and 6).
We sought to confirm functional ITK inhibition in patients receiving oral ibrutinib. Because PLCγ1 is directly phosphorylated at Tyr783 by active ITK, we conducted pPLCγ1-Tyr783 phosphoflow analysis on CD3/CD28-stimulated CD4 T cells collected from CLL patients receiving ibrutinib as part of a phase 2 clinical trial. Results reveal a significant decrease in TCR-induced pPLCγ1 activation, confirming inhibition of CD4 T-cell ITK signaling in these patients (Figure 2G; supplemental Figure 7).
It has been demonstrated in mice that loss of ITK attenuates, yet does not ablate, intracellular calcium flux in response to TCR signaling.33,34 Ibrutinib treatment of Jurkat cells yielded similar results (Figure 2H-I), demonstrating that ibrutinib-based ITK inhibition significantly reduces intracellular calcium flux in response to TCR stimulation.
Ibrutinib-induced ITK-C442–irreversible inhibition provides a selective advantage to RLK-expressing Th1 and CD8 T cells
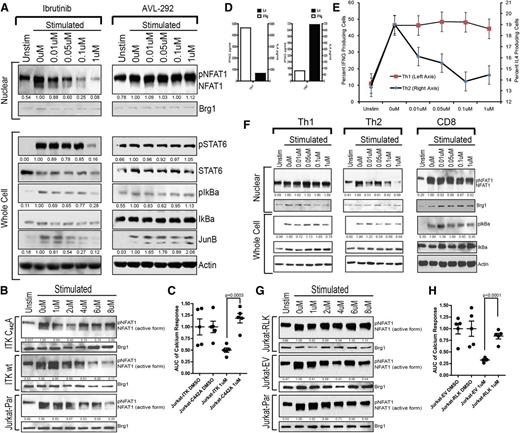
To confirm that the primary molecular target of ibrutinib in CD4 T cells was ITK, TCR-induced activation of NFAT, pSTAT6, pIkBα, and JunB was evaluated in primary CD4 T cells pretreated with ibrutinib or 1 of 2 alternative BTK inhibitors, AVL-292 and PCI-45292, which do not target ITK (50% inhibition/inhibitory concentration > 22.5 nM) (Figure 3A; supplemental Figures 8-13). Only ibrutinib (ITK 50% inhibition/inhibitory concentration = 2.2 nM) was capable of inhibiting TCR downstream molecular activation.
Ibrutinib irreversibly binds to ITK-C442 and RLK expression provides compensatory kinase activity, which protects Th1 and CD8 T cells. (A) Immunoblot analysis of 45-minute nuclear and 2-hour whole-cell extracts from ibrutinib or alternate BTK inhibitor–pretreated, freshly purified healthy donor primary CD4+ cells stimulated with anti-CD3/anti-CD28. Nuclear extracts were probed for NFAT1 and Brg1; whole-cell extracts were probed for pSTAT6-Y641, total STAT6, pIkBα-S32/36, total IkBα, JunB, and actin. (B) Immunoblot analysis of Jurkat parental, Jurkat-ITKwt, and Jurkat-ITKC442A nuclear lysates after ibrutinib pretreatment and anti-CD3/anti-CD28 stimulation. Blots were probed for NFAT1 and Brg1. (C) AUC for Fluo4-AM calcium release analysis of Jurkat-ITK and Jurkat-ITKC442A cell lines after pretreatment with ibrutinib or DMSO and stimulation with anti-CD3. Each symbol represents a single replicate experiment. Error bars represent SEM. (D) Cytokine analysis of IL-4 (black bars and right y-axis) and IFN-γ (open bars and left y-axis) media levels in anti-CD3/anti-CD28–stimulated Th1- and Th2-polarized cell cultures. These are the same cell cultures used in panels E and F. (E) Intracellular cytokine analysis of Th1(IFN-γ)– and Th2(IL-4)–polarized T-cell cultures pretreated with the indicated concentration of ibrutinib or DMSO and stimulated for 6 hours via anti-CD3/anti-CD28. Cytokine measurements were taken on separately cultured subsets of cells after 3 weeks of polarizing cell culture with weekly anti-CD3/anti-CD28 stimulation. Error bars represent SEM. (F) Th1-, Th2-, and CD8-purified primary cells were stimulated with anti-CD3/anti-CD28 after pretreatment with ibrutinib. Immunoblot analysis was conducted probing for NFAT and Brg1 as well as pIkBα-S32/36, total IkBα, and actin. (G) Immunoblot analysis of Jurkat parental, Jurkat-RLK, and Jurkat-EV (empty vector) nuclear lysates after ibrutinib pretreatment and anti-CD3/anti-CD28 stimulation. Blots were probed for NFAT1 and Brg1. (H) AUC for Fluo4-AM calcium release analysis of Jurkat-EV (empty vector) and Jurkat-RLK cell lines after pretreatment with ibrutinib or DMSO and stimulation with anti-CD3. Each symbol represents a single replicate experiment. Error bars represent SEM. Unstim, unstimulated.
Ibrutinib irreversibly binds to ITK-C442 and RLK expression provides compensatory kinase activity, which protects Th1 and CD8 T cells. (A) Immunoblot analysis of 45-minute nuclear and 2-hour whole-cell extracts from ibrutinib or alternate BTK inhibitor–pretreated, freshly purified healthy donor primary CD4+ cells stimulated with anti-CD3/anti-CD28. Nuclear extracts were probed for NFAT1 and Brg1; whole-cell extracts were probed for pSTAT6-Y641, total STAT6, pIkBα-S32/36, total IkBα, JunB, and actin. (B) Immunoblot analysis of Jurkat parental, Jurkat-ITKwt, and Jurkat-ITKC442A nuclear lysates after ibrutinib pretreatment and anti-CD3/anti-CD28 stimulation. Blots were probed for NFAT1 and Brg1. (C) AUC for Fluo4-AM calcium release analysis of Jurkat-ITK and Jurkat-ITKC442A cell lines after pretreatment with ibrutinib or DMSO and stimulation with anti-CD3. Each symbol represents a single replicate experiment. Error bars represent SEM. (D) Cytokine analysis of IL-4 (black bars and right y-axis) and IFN-γ (open bars and left y-axis) media levels in anti-CD3/anti-CD28–stimulated Th1- and Th2-polarized cell cultures. These are the same cell cultures used in panels E and F. (E) Intracellular cytokine analysis of Th1(IFN-γ)– and Th2(IL-4)–polarized T-cell cultures pretreated with the indicated concentration of ibrutinib or DMSO and stimulated for 6 hours via anti-CD3/anti-CD28. Cytokine measurements were taken on separately cultured subsets of cells after 3 weeks of polarizing cell culture with weekly anti-CD3/anti-CD28 stimulation. Error bars represent SEM. (F) Th1-, Th2-, and CD8-purified primary cells were stimulated with anti-CD3/anti-CD28 after pretreatment with ibrutinib. Immunoblot analysis was conducted probing for NFAT and Brg1 as well as pIkBα-S32/36, total IkBα, and actin. (G) Immunoblot analysis of Jurkat parental, Jurkat-RLK, and Jurkat-EV (empty vector) nuclear lysates after ibrutinib pretreatment and anti-CD3/anti-CD28 stimulation. Blots were probed for NFAT1 and Brg1. (H) AUC for Fluo4-AM calcium release analysis of Jurkat-EV (empty vector) and Jurkat-RLK cell lines after pretreatment with ibrutinib or DMSO and stimulation with anti-CD3. Each symbol represents a single replicate experiment. Error bars represent SEM. Unstim, unstimulated.
Ibrutinib presumably requires a cysteine residue within the hinge region to form an irreversible covalent bond and inhibit a kinase target. In ITK, that cysteine moiety resides at amino acid 442. Therefore, as molecular confirmation that ITK is the sole irreversible target in CD4 T cells, a stably transduced Jurkat line was generated with ITK-C442A, a version of ITK that lacks the putative covalent binding site for ibrutinib (supplemental Figure 14). The ITK-C442A Jurkat line maintained NFAT activation to drug concentrations exceeding 8 µM, whereas the parental line and Jurkat cells transfected with wild-type ITK were inhibited at 2 to 4 µM (Figure 3B). These data were confirmed by intracellular calcium flux showing that the ibrutinib-resistant ITK-C442A Jurkat line significantly and completely restored calcium response (Figure 3C; supplemental Figure 15).
A key reason why ITK inhibitors retain clinical interest is the potential to selectively inhibit Th2 T cells, which do not contain the compensatory RLK kinase. To elucidate the differential inhibition of Th2-polarized T cells in relation to Th1, naïve CD4 T cells were polarized in vitro to obtain enriched cultures of IFN-γ–producing Th1 cells and IL-4–producing Th2 cells (Figure 3D). In contrast to Th1, Th2 cultures were sensitive to pharmacologically relevant levels of ibrutinib as demonstrated by reduced IL-4 production (Figure 3E). Additionally, ibrutinib inhibited NFAT and IkBα activation in Th2 T cells, whereas Th1-polarized CD4 and CD8 T cells were resistant (Figure 3F).
In Th1 CD4 and CD8 T cells, RLK plays a redundant role to ITK; however, neither Th2 polarized CD4 T cells nor Jurkat cells express RLK.35 To test the hypothesis that RLK expression protects Th1 T cells from ibrutinib inhibition, Jurkat cells stably transduced to express RLK were tested (supplemental Figure 16). TCR downstream activation of NFAT was protected in the Jurkat-RLK cell line, whereas both the parental and empty vector stable transfectants were susceptible to ibrutinib inhibition (Figure 3G). Confirmatory intracellular calcium release experiments demonstrate a significant restoration of calcium flux in Jurkat cells stably expressing RLK (Figure 3H; supplemental Figure 17). This result demonstrates that RLK can compensate for ibrutinib-inhibited ITK, thereby providing an alternate activation platform for specific RLK-expressing T-cell subsets.
Ibrutinib selectively limits Th2 activation, thereby initiating a Th1-selective pressure in a mixed population of CD4 T cells in vitro, in vivo, and in human CLL patients receiving ibrutinib
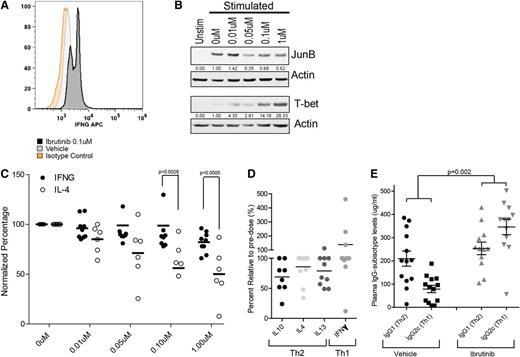
To evaluate the effects of ibrutinib on the Th1/Th2 polarization of a CD4 T-cell population over time, CD4 T cells isolated from healthy donors were cultured for 3 days following ibrutinib pretreatment. Outgrowth of IFN-γ–positive T cells was confirmed by intracellular staining analysis (Figure 4A). This outgrowth correlated with a decrease in the Th2-dominant transcription factor JunB and an increase in the Th1-specific transcription factor T-bet (Figure 4B).
Ibrutinib can limit Th2 activation, thereby selectively promoting Th1 expansion and skewing Th1/Th2 cytokines in human CLL patients and IgG subisotypes in TCL1 leukemic mice. (A) Intracellular staining analysis of IFN-γ in 5-day anti-CD3/anti-CD28–stimulated CD4+ T-cell cultures pretreated with ibrutinib or vehicle. Experiment was repeated 5 times; isotype staining control is provided. (B) Immunoblot analysis of JunB (top) and T-bet (bottom) levels in bulk CD4+ cultures pretreated with ibrutinib and anti-CD3/anti-CD28 stimulated (or unstimulated) for 3 days in vitro. Actin is used as loading control. Densitometry analyses are normalized to the DMSO-treated (0 µM) sample. (C) Normalized intracellular staining analysis of IL-4 (open bars n = 6) and IFN-γ (closed bars n = 9) CD4+ cells derived from CLL patients pretreated with ibrutinib and stimulated with anti-CD3/anti-CD28. Error bars represent SEM. (D) Percent relative alteration in plasma cytokine levels from pretreatment to day 28 of therapy in relapsed refractory CLL patients enrolled in a phase 1 trial of oral ibrutinib. (E) Plasma IgG1 (Th2) and IgG2c (Th1) subisotype analysis of C57BL/6 EµTCL1 mice at 8 months of age after 7 consecutive months of ibrutinib (25 mg/kg/day) (n = 12) or vehicle (n = 13) administration via drinking water. IFNG, interferon γ; Unstim, unstimulated.
Ibrutinib can limit Th2 activation, thereby selectively promoting Th1 expansion and skewing Th1/Th2 cytokines in human CLL patients and IgG subisotypes in TCL1 leukemic mice. (A) Intracellular staining analysis of IFN-γ in 5-day anti-CD3/anti-CD28–stimulated CD4+ T-cell cultures pretreated with ibrutinib or vehicle. Experiment was repeated 5 times; isotype staining control is provided. (B) Immunoblot analysis of JunB (top) and T-bet (bottom) levels in bulk CD4+ cultures pretreated with ibrutinib and anti-CD3/anti-CD28 stimulated (or unstimulated) for 3 days in vitro. Actin is used as loading control. Densitometry analyses are normalized to the DMSO-treated (0 µM) sample. (C) Normalized intracellular staining analysis of IL-4 (open bars n = 6) and IFN-γ (closed bars n = 9) CD4+ cells derived from CLL patients pretreated with ibrutinib and stimulated with anti-CD3/anti-CD28. Error bars represent SEM. (D) Percent relative alteration in plasma cytokine levels from pretreatment to day 28 of therapy in relapsed refractory CLL patients enrolled in a phase 1 trial of oral ibrutinib. (E) Plasma IgG1 (Th2) and IgG2c (Th1) subisotype analysis of C57BL/6 EµTCL1 mice at 8 months of age after 7 consecutive months of ibrutinib (25 mg/kg/day) (n = 12) or vehicle (n = 13) administration via drinking water. IFNG, interferon γ; Unstim, unstimulated.
To confirm the functional relevance of these results in the setting of CLL, intracellular staining was performed for IFN-γ and IL-4 in ibrutinib-treated, TCR-stimulated CD4 T-cell cultures purified from CLL patients not previously treated with ibrutinib. Following stimulation, a significant decrease was identified in the IL-4–producing Th2 population of CD4 T cells, whereas IFN-γ–producing Th1 cells were largely unaffected (Figure 4C). These results very closely matched data obtained on primary CD4 T cells from healthy donors (supplemental Figure 18). These data confirm that a significant divergence of the 2 populations can be achieved in a purified T-cell culture at ibrutinib doses ranging from 0.1 to 1 µM. This dose range is consistent with serum concentrations observed in vivo during pharmacokinetic studies of ibrutinib in both mouse and human trials.18,36
To validate these findings in human patients, serial serum cytokine levels were investigated in relapsed or refractory CLL patients receiving ibrutinib as part of a phase 1 study. The data demonstrated a decrease in serum Th2-type cytokines, including IL-10, IL-4, and IL-13, from pretreatment to day 28 of ibrutinib therapy (Figure 4D). This was in sharp contrast to an increase in the Th1 cytokine IFN-γ. To rule out the potential contribution of B cells to the observed Th1 cytokines, we analyzed peripheral CD19+ B-cell and CLL messenger RNA levels and found no such alteration in B-cell cytokine expression (supplemental Figure 19).
To assess the long-term implications of ibrutinib-induced Th1 cytokine skewing, IgG subisotype analyses were conducted in a cohort of 8-month-old C57BL/6 EµTCL1 mice. These mice were treated continuously for 7 months with ibrutinib (25 mg/kg/day) or vehicle. Results revealed a significant (P = .002) inversion of the Th1/Th2 ratio as measured by the relative levels of IgG1 (Th2) and IgG2c (Th1), confirming an in vivo ibrutinib-related Th1 skewing (Figure 4E). As confirmation that in vivo effects were related to ITK inhibition we examined TCR-induced CD4 activation via CD69 in wild-type and Itk−/− mice after 7 days of in vivo treatment with ibrutinib or vehicle. As expected, wild-type mice treated with ibrutinib phenocopy Itk−/− mice, which display attenuated TCR-induced stimulation that is not altered by ibrutinib (supplemental Figure 20).
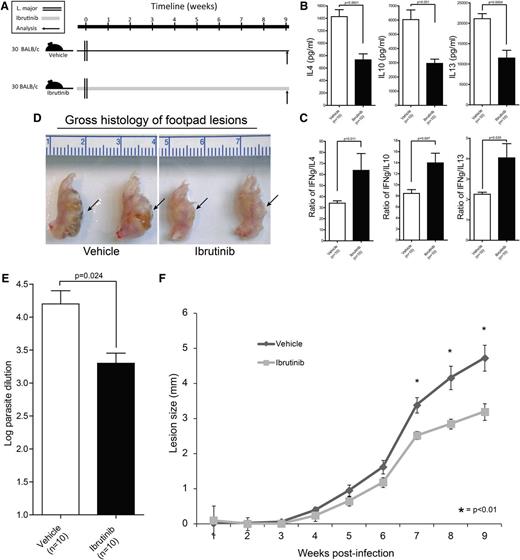
Ibrutinib drives Th1-mediated L major immunity in an in vivo model of Th2-dominant cutaneous leishmaniasis
Studies conducted in ITK-knockout mice revealed Th1-biased immunity that was capable of mounting effective Th1-adaptive immunity against cutaneous L major parasitic infection.8 Thus far, no clinically relevant ITK inhibitor has proven capable of recapitulating this pharmacologic response in vivo. Using this archetypal model of Th1/Th2 immunity, we sought to demonstrate comparable results using ibrutinib (Figure 5A). T-cell cytokine analysis demonstrated a significant decrease in Th2 cytokines IL-10, IL-4, and IL-13 relative to the Th1 cytokine IFN-γ, which was not significantly altered (Figure 5B-C). The suppression of Th2 immunity and stabilization of Th1 immunity correlated with improved parasite clearance in ibrutinib-treated mice as evidenced by smaller cutaneous lesions and lower parasitic burden (Figure 5D-F). Notably, direct treatment of L major parasites with ibrutinib did not alter growth or development, removing the possibility of direct effects on the parasite itself (supplemental Figure 21).
Ibrutinib drives Th1-mediated L major immunity in an in vivo model of Th2-dominant cutaneous leishmaniasis. (A) Schematic representation of the L major mouse experiment time course. Mice were initiated on ibrutinib (25 mg/kg/day) or vehicle 2 days prior to being infected with 2 × 106 stationary-phase L major promastigotes. Lesion size was tracked for 9 weeks and immune correlates were collected upon sacrifice at week 9. (B) Lymphocytes isolated from draining lymph nodes were stimulated with L major antigens for 72 hours and culture supernatant was analyzed by ELISA for IL-4 and IL-10. Error bars represent SEM. (C) Lymphocytes isolated from draining lymph nodes were stimulated with L major antigens for 72hr and culture supernatant was analyzed by ELISA for IFN-γ. IFN-γ responses are displayed as a ratio with IL-4 (left panel) or IL-10 (right panel) to compare relative Th1 and Th2 immunity in ibrutinib- or vehicle-treated groups. (D) Whole-mount gross histologic preparations of vehicle- and ibrutinib-treated L major–infected footpads are depicted along with a centimeter ruler for size comparison. Cutaneous lesions are visible on the underside of the footpad as indicated by arrows. (E) Log dilution of parasites obtained from footpad lesions are displayed. Error bars represent SEM. (F) Time course analysis of cutaneous lesion size over the 9-week period of L major infection. Measurements were taken at weekly intervals. Error bars represent SEM.
Ibrutinib drives Th1-mediated L major immunity in an in vivo model of Th2-dominant cutaneous leishmaniasis. (A) Schematic representation of the L major mouse experiment time course. Mice were initiated on ibrutinib (25 mg/kg/day) or vehicle 2 days prior to being infected with 2 × 106 stationary-phase L major promastigotes. Lesion size was tracked for 9 weeks and immune correlates were collected upon sacrifice at week 9. (B) Lymphocytes isolated from draining lymph nodes were stimulated with L major antigens for 72 hours and culture supernatant was analyzed by ELISA for IL-4 and IL-10. Error bars represent SEM. (C) Lymphocytes isolated from draining lymph nodes were stimulated with L major antigens for 72hr and culture supernatant was analyzed by ELISA for IFN-γ. IFN-γ responses are displayed as a ratio with IL-4 (left panel) or IL-10 (right panel) to compare relative Th1 and Th2 immunity in ibrutinib- or vehicle-treated groups. (D) Whole-mount gross histologic preparations of vehicle- and ibrutinib-treated L major–infected footpads are depicted along with a centimeter ruler for size comparison. Cutaneous lesions are visible on the underside of the footpad as indicated by arrows. (E) Log dilution of parasites obtained from footpad lesions are displayed. Error bars represent SEM. (F) Time course analysis of cutaneous lesion size over the 9-week period of L major infection. Measurements were taken at weekly intervals. Error bars represent SEM.
The EµTCL1 leukemia model confirms immunomodulatory Th1/Th2 skewing and a direct functional relevance in the setting of infection
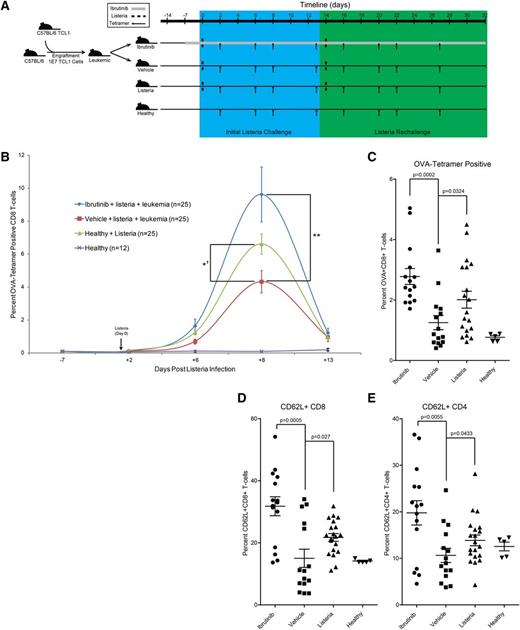
Infection linked to immunosuppression is the primary cause of death in CLL patients. Listeria infections occur in CLL, and because this is an intracellular pathogen, a robust Th1 and CD8 T-cell response is required to achieve sterilizing immunity.29 Therefore, the therapeutic relevance of ibrutinib immunomodulation in a concurrent leukemia/listeriosis mouse model was investigated. In this model, EµTCL1 leukemia-engrafted mice were treated with ibrutinib or vehicle. After 7 days of therapy, mice (along with nonleukemic control cohorts) were challenged with a sublethal intravenous dose (5000 colony-forming units [CFU]) of L monocytogenes (Listeria) expressing the immunodominant chicken ovalbumin (OVA) protein (Figure 6A). Tetramer analysis revealed a significant depression in the OVA-specific CD8 T-cell response in the vehicle-treated leukemic group; however, this immunosuppression was reversed by ibrutinib therapy (Figure 6B; supplemental Figure 22). Longitudinal analysis confirmed that the overall magnitude of Listeria response was significantly lower in leukemic mice. Surprisingly, ibrutinib not only restored the veracity and magnitude of immune response but also enhanced it beyond what was observed in healthy animals (Figure 6B). To perform interim analyses, we sacrificed 2 mice per group and quantified Listeria load in the liver. This revealed that healthy and ibrutinib-treated leukemic mice cleared the infection by day 8, yet 1 of 2 vehicle-treated mice analyzed at day 8 displayed uncontrolled Listeria infections within the liver (supplemental Figure 23). Notably, direct ibrutinib treatment of cultured L monocytogenes showed no inhibition of growth or infectious capacity (supplemental Figure 24).
Ibrutinib functionally restores immunity in a leukemia/listeriosis mouse model. (A) Schematic representation of the leukemia/listeriosis mouse experiment time course. Mice were engrafted via intravenous injection with leukemic cells purified from the spleen of a EµTCL1 transgenic animal. Engrafted mice were randomly divided between vehicle and ibrutinib (25 mg/kg/day) groups on day 7. IV L monocytogenes-OVA inoculation (5000 CFU) was conducted 14 days after engraftment. Rechallenge began 14 days after initial inoculation and consisted of a single 5000 CFU L monocytogenes OVA intravenous injection. Mice were sacrificed at day 32 and tissues were collected for memory cell analysis. (B) Time course analysis of OVA major histocompatibility complex I tetramer-positive peripheral CD8 T cells from leukemia/listeriosis mouse study. A total of 5000 CFU of OVA-expressing L monocytogenes was injected at day 0. Statistical analysis for day 8 mean is presented (**P = .0052; *′P = .0438 for repeat experiment). Error bars represent SEM. (C) Analysis of OVA-tetramer+ CD8+ T cells within the spleen of animals killed on day 32 of the leukemia/listeria infection experiment. Data are displayed as percentage of CD8+ T cells. Error bars represent SEM. (D) Analysis of CD8+CD62L+ central memory cells within the spleen of mice killed at day 32 of the leukemia/listeria infection experiment. Data are presented as the average of the total CD8+ population. Error bars represent SEM. (E) Analysis of CD4+CD62L+ central memory cells within the spleen of mice killed at day 32 of the leukemia/listeria infection experiment. Data are presented as the average of the total CD4+ population. Error bars represent SEM.
Ibrutinib functionally restores immunity in a leukemia/listeriosis mouse model. (A) Schematic representation of the leukemia/listeriosis mouse experiment time course. Mice were engrafted via intravenous injection with leukemic cells purified from the spleen of a EµTCL1 transgenic animal. Engrafted mice were randomly divided between vehicle and ibrutinib (25 mg/kg/day) groups on day 7. IV L monocytogenes-OVA inoculation (5000 CFU) was conducted 14 days after engraftment. Rechallenge began 14 days after initial inoculation and consisted of a single 5000 CFU L monocytogenes OVA intravenous injection. Mice were sacrificed at day 32 and tissues were collected for memory cell analysis. (B) Time course analysis of OVA major histocompatibility complex I tetramer-positive peripheral CD8 T cells from leukemia/listeriosis mouse study. A total of 5000 CFU of OVA-expressing L monocytogenes was injected at day 0. Statistical analysis for day 8 mean is presented (**P = .0052; *′P = .0438 for repeat experiment). Error bars represent SEM. (C) Analysis of OVA-tetramer+ CD8+ T cells within the spleen of animals killed on day 32 of the leukemia/listeria infection experiment. Data are displayed as percentage of CD8+ T cells. Error bars represent SEM. (D) Analysis of CD8+CD62L+ central memory cells within the spleen of mice killed at day 32 of the leukemia/listeria infection experiment. Data are presented as the average of the total CD8+ population. Error bars represent SEM. (E) Analysis of CD4+CD62L+ central memory cells within the spleen of mice killed at day 32 of the leukemia/listeria infection experiment. Data are presented as the average of the total CD4+ population. Error bars represent SEM.
To ensure that ibrutinib does not negatively impact immune memory following clearance of their primary infection, we rechallenged Listeria-infected cohorts with a second infection (5000 CFU) of Listeria-OVA. Over the next 2 weeks, mice in all groups responded rapidly to the rechallenge and we observed no loss of functional immunologic memory (supplemental Figure 25). On day 32, splenocytes from all mice were examined for OVA-tetramer–positive CD8 T cells as well as the development of CD62L+ CD4 and CD8 populations, which are presumably central memory in this multiple challenge system (supplemental Figure 26). Vehicle-treated leukemic mice were significantly deficient in the total percentage of OVA-tetramer–positive CD8 T cells and developed fewer CD62L+ CD4 and CD8 T cells. Meanwhile, ibrutinib-treated leukemic mice showed a significantly enhanced potential for developing CD62L+ CD4 and CD8 subsets and had the highest overall percentage of OVA-tetramer–positive CD8 T cells (Figure 6C-E).
Discussion
Our results confirm that ibrutinib, in addition to blocking BTK in B cells as previously reported, irreversibly binds ITK and inhibits downstream activation of Th2 cells after TCR stimulation both in vitro and in vivo. This inhibition is specific to Th2-polarized CD4 T cells, because RLK remains uninhibited providing a potential compensatory platform for activation and proliferation of Th1 and CD8 T cells. Furthermore, CD4 T-cell populations isolated from CLL patients are skewed at a molecular and phenotypic level toward a Th1 profile after a brief in vitro dose of ibrutinib, confirming a T-cell–specific effect of ibrutinib. Irreversible ITK binding, ITK signal inhibition, and Th1-skewed cytokine patterns were also identified in CLL patients treated with ibrutinib in 2 clinical trials.37,38 These findings were validated using a well-established mouse model of cutaneous leishmaniasis, and further clinical relevance was demonstrated using a leukemia and listeriosis infectious disease model. Together, these results demonstrate that ibrutinib is a potent and clinically relevant immunomodulatory drug, thus showing the potential to repurpose this therapeutic in other diseases that result from or cause a disproportionate polarization of Th2 immunity. Notably, clinical treatment of CLL with ibrutinib results in a dramatic decrease of infectious complications between the first 6 months of treatment and thereafter.22
In many ways, our studies recapitulate the phenotype of Itk−/− mice; however, unlike studies conducted in Itk−/− mice, we observed no loss in CD4 or CD8 T-cell effector potential.39,40 This is likely a result of short-term inhibition as compared with broad genetic ablation that disrupts the ontogeny and thymic development of T cells. In addition, our study focused on the T-cell–specific effects of ibrutinib; however, the effects of ibrutinib on NKT cells and mast cells, both of which express ITK, remain unclear.
The full extent to which inhibition of BTK and ITK contributes to the immunomodulatory potential of ibrutinib remains unclear. In our listeriosis model, we cannot exclude the possibility that direct antileukemic activity disrupted an immunosuppressive system; however, the fact that ibrutinib boosts T-cell responses to levels that exceeded those of nonleukemic animals leads us to the conclusion that BTK inhibition alone cannot account for the entirety of immunomodulatory activity. A single leishmaniasis study implied that BTK signaling could inhibit Th1 immunity against parasitic infection, though this effect was considerably more prominent in ITK-knockout mice.8,41 A separate study implicated murine dendritic cell BTK expression in a broadly immunosuppressive phenotype; however, this effect was not reproducible in humans.42,43
This report establishes a previously unidentified immunomodulatory mechanism of action for ibrutinib that has the potential to expand its antitumor applications. Aside from T-cell malignancies for which ITK acts as a driver, it is well appreciated that tumors develop a prosurvival niche enhanced by Th2-polarized CD4 T cells.1,2 In leukemia, these cells provide constitutive IL-4, IL-6, and CD40L stimulation, driving the expansion of disease.44 In solid tumors, subversion of Th1 in lieu of Th2 promotes tumor immune evasion.45 In such settings, a specific advantage given to Th1 cells may allow the effective generation of antitumor immunity. The combination of ibrutinib with T-cell–based immunotherapy may prove synergistic.
We demonstrate that ibrutinib has potential for the treatment of leishmaniasis. Our current study design required the sacrifice of both vehicle and ibrutinib treatment groups at week 9 for critical immune correlative data; however, ongoing studies will investigate the potential of an extended therapeutic course.
Our studies reveal that ibrutinib can restructure host resistance toward a Th2-mediated listeriosis in an immunocompromised mouse model. This raises the possibility that ibrutinib may provide benefit in other inflammatory disorders, ranging from allergic asthma and atopic dermatitis to inflammatory bowel disease.11 Moreover, it was recently demonstrated that ITK activation plays a vital role in the replication of influenza A virus in T cells46 and is critical for HIV replication and infection cycles, making ITK a valuable target in the development of antiviral therapeutics.10 Given the global threat of HIV and pandemic influenza, ibrutinib’s potential utility as an irreversible ITK inhibitor should be explored further.
Presented in part at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 9, 2012.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the NIH Tetramer Facility, the Bevan laboratory, and Dr Leslie J. Berg for providing critical reagents as well as Jessica MacMurray, Stella Chang and Ashley Smith for experimental assistance.
This work was supported by the National Cancer Institute (grants 5 T32 CA009338-33-03, P01 CA095426, K12 CA133250-05, and P50 CA140158), the American Cancer Society (grant 125039-PF-13-246-01-LIB), the Leukemia & Lymphoma Society, the American Society of Hematology, Mr and Mrs Michael Thomas, the Harry Mangurian Foundation, and the D. Warren Brown Family Foundation.
Authorship
Contribution: J.A.D. planned, organized, and performed the research; K.A.B., G.N., J.A.W., Y.Z., J.D.H., T.-M.L., B.Y.C., K.M.L., M.R.S., D.L.C., A.M.L., A.R.S., J.J.B., L.L.S., K.A.S., A.M., and F.W.F. performed experiments; S.J., J.M.F., J.A.J., L.A.A., K.M., R.F., and J.S. provided clinical samples; M.A.C., J.C.B., A.J.J., and N.M. supervised the research, reviewed the manuscript, and obtained funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John C. Byrd, 455 OSUCCC, 410 W 12th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu.
References
Author notes
N.M., A.J.J., and J.C.B. contributed equally to this study.

![Figure 1. Ibrutinib is an irreversible molecular inhibitor of ITK, displaying BTK-independent antileukemic potential. (A) A graphical depiction of the sequence and domain homology between ITK and BTK. The relevant phosphorylation sites as well as ibrutinib irreversible covalent binding sites are labeled. (B) In silico representation of docked ibrutinib within the active site of crystallized ITK (top panel) (Protein Data Bank code 3QGW) or BTK (bottom panel) showing close approximation of Cys442 or Cys481 to reactive moiety of ibrutinib. Shape and chemical complementarity of ibrutinib are shown in surface representation. (C) A molecular probe assay was used to calculate the percent irreversible occupancy of total ITK in Jurkat whole-cell lysates irreversibly bound by ibrutinib. Error bars represent standard error of the mean (SEM). (D) A molecular probe assay was used to calculate the percent irreversible occupancy of ITK by ibrutinib in cryopreserved PBMCs obtained from patients immediately prior to (predose) and 8 days into (ibrutinib) daily oral ibrutinib therapy for CLL (n = 8). Error bars represent SEM. (E) Primary CD4 T cells isolated from healthy donors were pretreated with ibrutinib (1 µM) or vehicle and subjected to stimulation with anti-CD3, anti-CD28, or anti-CD3/anti-CD28 for 6 hours and analyzed via fluorescence-activated cell sorter for CD69 surface expression. Baseline (unstimulated) CD69 percentage was subtracted and data are represented in log percent CD69+CD4+ T cells. A 2-tailed paired Student t test was used for statistical analysis (nonsignificant [ns] = P > .05). Error bars represent SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/15/10.1182_blood-2013-06-507947/4/m_2539f1.jpeg?Expires=1767702068&Signature=ZC1x25VrXErtezHabZeRdCW~fbxZZPcA2X5wi5yTCAvnc4R-Hn89-qT0H9a9uQbNsP5XmNo1J6GQ5tn5LY0SYKO7WMV9HO8cb~4GBaYJmXBvHtwAPjRWOrzfQuHSGTtCXcx0Tk0CPCIBikVUtnkobFYDRMtgU43zfayf4mtPxwSLK1vLr6Ey2B-UGWA~gyp8Xm3M1lH7hRuUk49s9ZVv11MDGVQDnvnuV8ghgxvgCqPygh-MSulkQ6It65MosfHaz222bH5tvk5gA~JsCaUmxYiImeGZDYr7EfCUVuKOtMHl4JnGu8K4D-LnPEbihU2L203~k-Vk-NwbW3ywPw9-TQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. In T cells, ibrutinib specifically targets ITK, inhibiting TCR-induced cellular signaling and activation. (A) Immunoblot analysis of freshly isolated ibrutinib pretreated primary CD4+ cells from a healthy donor, anti-CD3/anti-CD28 stimulated (or unstimulated), whole-cell lysates. Blot probed for pITK-Y180, total ITK, pSTAT6-Y641, total STAT6, pIkBα-S32/36, total IkBα, JunB, and actin. Densitometry analysis normalized to dimethylsulfoxide (DMSO)-treated (0 µM) sample. (B) Immunoblot analysis of freshly isolated ibrutinib pretreated primary CD4+ cells from a healthy donor, anti-CD3/anti-CD28–stimulated (or unstimulated), cytoplasmic, and nuclear lysates. Blots probed for NFAT (and activated hyperdephosphorylated NFAT), Brg1, and actin. Densitometry analyses are normalized to the DMSO-treated (0 µM) sample. (C) Immunoblot analysis of freshly isolated ibrutinib-pretreated primary CD4+ cells from a healthy donor, anti-CD3/anti-CD28–stimulated (or unstimulated), whole-cell lysates. Blots were probed for pZAP70-Y319, total ZAP70, pLAT-Y191, total LAT, pLCK-Y505, total LCK, pIkBα-S32/36, total IkBα, and actin. Densitometry analyses are normalized to the DMSO-treated (0 µM) sample. (D) Nuclear or whole-cell lysate immunoblot analysis of Jurkat cells pretreated with ibrutinib and stimulated with either anti-CD3/anti-CD28 or phorbol 12-myristate 13-acetate/ionomycin for 45 minutes. Blots were probed with Brg1, NFAT1, and actin (nuclear lysates) or pIkBα-S32/36, total IkBα, and actin (cellular lysates). (E) Immunofluorescent microscopy of ibrutinib-pretreated, freshly isolated, primary CD4+ cells from healthy donors (panels A and B) were stimulated for 45 minutes with anti-CD3/anti-CD28 (or unstimulated), fixed, and stained for NFAT (green) and nuclei (4,6 diamidino-2-phenylindole [DAPI], blue). Activated cells are characterized by influx of NFAT into nuclear region (green overlay with blue = cyan) and are denoted by white arrows. (F) Percent relative NFAT1/DAPI colocalization derived from Pearson correlation analysis of 10 independent immunofluorescent microscopy fields (different donors than pictured in panel E and normalized to the average unstimulated value. Cyclosporin A (CSA) treatment was used as an additional negative control. Error bars represent SEM. (G) Phosphoflow analysis of pPLCγ1-Tyr783 in 1hr anti-CD3/anti-CD28–stimulated cryopreserved PBMCs obtained immediately predose or after 8 days of receiving ibrutinib therapy for CLL (n = 11). A minimum of 400 000 events were collected. Graph displays the overall percent of live CD3+CD4+pPLCγ1−Tyr783+ events in each sample. Error bars represent SEM. (H) Calcium flux analysis of ibrutinib (n = 8), vehicle (n = 24), or BAPTA-AM (n = 8) pretreated Jurkat cells after TCR stimulation by anti-CD3. Area under the curve (AUC) is presented for each dataset in the center. All data were normalized to baseline and BAPTA-treated fluorescent averages. Time points depicted on horizontal axis are relative to stimulation with anti-CD3. (I) AUC for calcium flux of various concentrations of ibrutinib. Each symbol indicates a single replicate experiment. Statistical analysis represented on graph is relative to DMSO treatment. PMA/Iono., phorbol 12-myristate 13-acetate and ionomycin; Unstim, unstimulated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/15/10.1182_blood-2013-06-507947/4/m_2539f2.jpeg?Expires=1767702068&Signature=crNt781AvAqrtINgtLa4R9Nx0pFQA0wGPzAkiOxWf65J481r~z9z3RN-lseF5IrFXTe-j~aDBtU7z8x2hNjc4N~25gYqqyQd782QWEEuyJJKRbIBogDYTs6MI9qHkerP92ObOkuy7hdnbQHCCRFYU3do8wgu2E21ZVmIVJGzisMzzP-~dffSznYTOvKTkAfoX9hiiLC8o56Src4B7xwPiG09e-bZm~MZwRSeG3PM7lsAj3xtn7kI3u7w7yPo0szaeTRmFH1pYIAsre4St1375QfMcc3Nq-en4udWVC-IJAPPLC8dvWwsYaNdtitoMFCpulaD3VdItAZPyDS1ftlNdA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)