Key Points
Administration of anti-mouse CD1d blocking mAb prior to A-RBC immunization abolished IL-5 production and anti-A Ab production in mice.
In human peripheral blood mononuclear cell–NOD/SCID mice, administration of anti-human CD1d mAb prior to A-RBC immunization completely inhibited anti-A Ab production.
Abstract
Previously, we detected B cells expressing receptors for blood group A carbohydrates in the CD11b+CD5+ B-1a subpopulation in mice, similar to that in blood group O or B in humans. In the present study, we demonstrate that CD1d-restricted natural killer T (NKT) cells are required to produce anti-A antibodies (Abs), probably through collaboration with B-1a cells. After immunization of wild-type (WT) mice with human blood group A red blood cells (A-RBCs), interleukin (IL)-5 exclusively and transiently increased and the anti-A Abs were elevated in sera. However, these reactions were not observed in CD1d−/− mice, which lack NKT cells. Administration of anti-mouse CD1d blocking monoclonal Abs (mAb) prior to immunization abolished IL-5 production by NKT cells and anti-A Ab production in WT mice. Administration of anti-IL-5 neutralizing mAb also diminished anti-A Ab production in WT mice, suggesting that IL-5 secreted from NKT cells critically regulates anti-A Ab production by B-1a cells. In nonobese diabetic/severe combined immunodeficient (NOD/SCID/γcnull) mice, into which peripheral blood mononuclear cells from type O human volunteers were engrafted, administration of anti-human CD1d mAb prior to A-RBC immunization completely inhibited anti-A Ab production. Thus, anti-CD1d treatment might constitute a novel approach that could help in evading Ab-mediated rejection in ABO-incompatible transplant recipients.
Introduction
Invariant natural killer T (iNKT) cells are CD1d (nonmajor histocompatibility complex [MHC]-encoded class I-like molecule)–restricted, lipid antigen (Ag)–reactive T cells that express invariant Vα14-Jα18 T-cell Ag receptors (iTCRs) in mice and Vα24-Jα28 iTCRs in humans.1,2 Upon activation by glycolipid Ags, including α-galactosylceramide (αGalCer),3 these cells transactivate a variety of other cells, including NK, T, B, and dendritic cells.4-7 Recent studies have shown that activated iNKT cells enhance antibody (Ab) responses against T-dependent and T-independent Ags and pathogens.8-12 These observations prompted us to investigate the possible role of iNKT cells in Ab production against transplant-related Ags such as ABO blood group carbohydrates, xenogeneic carbohydrates, and histocompatibility complex allopeptides.
Previously, we performed surface staining of B cells using fluorescein-labeled synthetic human blood group A carbohydrates and demonstrated that B cells with surface immunoglobulin M (sIgM) receptors for group A carbohydrate determinants are present exclusively in a small, significant B-cell subpopulation—sIgM+ CD11b+ CD5+ B-1a cells—in mice, similar to the case of humans with blood group O or B.13,14 The serum antigroup A Ab levels in the mice significantly increased through activation of these B-1a cells, which bear receptors for A determinants following their immunization with human group A red blood cells (RBCs). Further, we used a similar technique and demonstrated that sIgM+ CD11b+ CD5– B-1b cells with receptors for Galα1-3Galβ1-4GlcNAc (Gal) epitopes, which are major xenogeneic Ags, were present in α1,3-galactosyltransferase–deficient (GalT−/−) mice, similar to the case in humans deficient in this enzyme.15 When these mice were immunized with Gal-bearing rat thymocytes, the serum anti-Gal Ab levels significantly increased following the activation of the above-mentioned B-1b cells bearing receptors for Gal determinants. Also, we have shown that cytidine monophospho-N-acetylneuraminic acid hydroxylase–deficient (CMAH−/−) mice, which are completely deficient in N-glycolylneuraminic acid (NeuGc), non-Gal antigenic epitopes, produce anti-NeuGc Abs.16,17 In this study, using GalT−/−, CMAH−/−, Jα18−/−, and CD1d−/− mice, we investigated whether iNKT cells function to produce anti-A, anti-Gal, anti-NeuGc, or anti-allopeptide Abs.
Methods
Mice
C57BL/6J (B6) (H-2b), BALB/c (H-2d), and nude mice (Balb/c) and F344 rats were purchased from CLEA Japan (Tokyo, Japan). Jα18−/− mice on a B6 genetic background and CD1d−/− mice on a B6 and Balb/c background, which are established by specific deletion of the Jα18 and CD1d gene segments, respectively, were used (kindly provided by Dr K. Seino, Laboratory for Immune Regulation, RIKEN Research Center for Allergy and Immunology, Yokohama, Japan).18 MHC class II-deficient C2tatm1Ccum (C2D) mice on the B6 background were purchased from Jackson Laboratory. GalT−/− mice on the B6 background, which completely lacked Gal expression, were used (kindly provided by Dr M. Sykes, Massachusetts General Hospital, Boston).19 CMAH−/− mice on the B6 background, which are completely deficient in NeuGc and completely lacked NeuGc expression, were used (kindly provided by Dr Y. Kozutsumi, Kyoto University, Japan).17 Both GalT−/− and CMAH−/− mice were crossed with CD1d−/− mice to produce double-knockout mice. To generate double-knockout mice, F2 mice (produced by intercrossing F1 mice) were typed for each gene, and the appropriate mice were intercrossed and typed until double-gene knockouts were established (typically 4 generations). Finally, the genotypes were confirmed by fluorescence-activated cell sorting analysis (FACS), genomic Southern blotting, and polymerase chain reaction (PCR). All the mice were housed in the animal facility of Hiroshima University, Japan, in a pathogen-free, micro-isolated environment and used when they were aged 8–16 weeks.
Anti-NeuGc and anti-Gal Ab production was elicited by intraperitoneal immunization of CMAH−/− and GalT−/− mice with NeuGc- and Gal-expressing thymocytes obtained from F344 rats 2 times during a 1-week interval (10 × 106 cells/mouse at each immunization). As indicated, anti-A Ab production was similarly elicited by intraperitoneal immunization of mice with human A-RBCs from blood group A volunteers 2 times during a 1-week interval (5 × 108 cells/mouse at each immunization). Informed consent was obtained from all human volunteers in accordance with the Declaration of Helsinki.
All experiments were approved by the institutional review board of Hiroshima University and conducted according to the guidelines of the National Institutes of Health (publication no. 86–23, revised 1996).
Conditioning regimen for experimental mice
As indicated, each mouse was intraperitoneally injected with 500 μg anti-mouse CD1d monoclonal Abs (mAb; 1B1) or with 100 μg anti-mouse interleukin (IL)-5 mAb (TRFK5; BD PharMingen, San Diego, CA) diluted in phosphate-buffered saline (PBS) 2 times at 1-week intervals. Mice that received injections of isotype-matched Abs served as the controls.
To determine whether iNKT cells enhance Ab responses to specific Ag, we immunized mice with human A-RBCs together with intraperitoneal injection of either αGalCer (KRN7000; 4 μg/mouse) or PBS (control).
Human peripheral blood mononuclear cell-chimeric mouse study
Nonobese diabetic/severe combined immunodeficient (NOD/SCID)/γcnull mice were purchased from the Central Institute of Experimental Animals (Kawasaki, Japan). Human peripheral blood mononuclear cells (PBMCs; 20 × 106 cells/mouse) from type O volunteers were engrafted in NOD/SCID/γcnull mice by intraperitoneal injection after 1 Gy of whole body irradiation. The human PBMC-chimeric mice received intraperitoneal injection of anti-human CD1d mAb (CD1d42) diluted in PBS at a dose of 500 μg/mouse on days 7 and 10 following the engrafting. Mice that received injections of isotype-matched Ab served as the controls. The CD1d42 clone cell line was kindly provided by Dr S. Porcelli (Albert Einstein College of Medicine, Bronx, NY).20,21
Cell preparation and flow cytometry analyses
Anti-NeuGc and anti-Gal Abs were detected by indirect immunofluorescence staining of rat thymocytes. A total of 106 thymocytes were incubated with 100 μL of serially diluted mouse serum, washed, and then incubated with biotin-conjugated rat anti-mouse IgM mAb (R6-60.2; BD PharMingen) or rat anti-mouse IgG Ab (eBioscience, San Diego, CA). The biotinylated mAbs were visualized using allophycocyanin-streptavidin (BD PharMingen). Median fluorescence intensity values were used to follow Ab levels.
B cells with receptors for human blood group A trisaccharide were detected using fluorescein isothiocyanate (FITC)-conjugated GalNAca1–3Fuca1–2Gal–BSA (bovine serum albumin) (A-BSA; Dextra, Reading, United Kingdom) and control FITC-conjugated BSA (Roche, Indianapolis, IN). FITC conjugation of A-BSA and BSA was performed using a SureLINK fluorescein labeling kit (KPL, Gaithersburg, MD). We incubated 106 spleen cells/100 μL from human PBMC-chimeric mice with 0.5 μg/100 μL FITC–A-BSA or control FITC-BSA in medium for 1 hour at 4°C. Nonspecific Fcγ receptor binding of labeled Abs was blocked by anti-mouse CD16/32 (2.4G2; BD PharMingen). The cells were further stained with phycoerythrin (PE)-conjugated anti-human CD19 mAb (HIB19; BD PharMingen). Isotype-matched irrelevant mAb was used as the control. Dead cells detected using light scatter and staining with propidium iodide were excluded from the analysis.
All flow cytometry (FCM) analyses were performed on a flow cytometer (FACSCalibur; Becton Dickinson, Mountain View, CA).
Cell sorting
Liver mononuclear cells (LMNCs) were stained with allophycocyanin (APC)-conjugated anti-mouse CD1d-tetramer (Proimmune, Bradenton, FL) and PE-Cy7 conjugated anti-mouse TCRβ (H57-597; eBioscience). NKT cells (CD1d-tetramer+, TCRβ+), T cells (CD1d-tetramer–, TCRβ+), and the others (CD1d-tetramer–, TCRβ–) were isolated by sorting with FACS Aria II (BD Biosciences).
Enzyme-linked immunosorbent assay
Total mouse immunoglobulin and the serum anti-A- and anti-Gal–specific Ab levels were determined by enzyme-linked immunosorbent assay (ELISA) as described previously.22,23 Briefly, ELISA plates were coated with 5 μg/mL of goat anti-mouse Ig (IgG + IgM + IgA, heavy chain + light chain; Southern Biotechnology, Birmingham, AL), 5 μg/mL synthetic A-BSA (Dextra), Gal-BSA (Dextra), or control BSA (Roche). The diluted serum samples were added to the plates and incubated for 2 hours, and the bound Abs were detected using horseradish peroxidase–conjugated goat anti-mouse IgG (Jackson ImmunoResearch)/IgM-specific Abs (KPL, Guilford, United Kingdom). Color development was achieved using 0.1 mg/mL O-phenylenediamine (Sigma, St. Louis, MO) in a substrate buffer. The reaction was discontinued by adding 3M H2SO4, and absorbance was measured at 492 nm. Anti-A– and anti-Gal–specific Ab levels were determined by subtracting the absorbance of the wells coated with control BSA from that of the wells coated with A-BSA. Similarly, the serum anti-A IgM and IgG levels in the humanized mice were determined. The diluted serum samples were added to the ELISA plates precoated with either A-BSA or BSA and incubated. The bound Abs were detected using horseradish peroxidase–conjugated goat anti-human IgG (Jackson ImmunoResearch)/IgM-specific Abs (KPL).
Cytometric bead array
Cytokine levels in sera were analyzed by BD Cytometric Bead Array using a mouse Flex Set (BD Bioscience) according to the manufacturer’s instructions for the production of IL-4, IL-5, IL-9, IL-17, IL-21, and interferon-gamma (INF-γ).
ELISPOT
Enzyme-linked immunospot (ELISPOT) assay to detect IL-5–producing cells was performed using ELISPOT kits (R & D Systems, Minneapolis, MN). An mAb specific for mouse IL-5 was precoated onto a polyvinylidene defluoride (PVDF)-backed microplate. Serial dilutions of cell suspension were prepared in RPMI 1640 medium, supplemented with 2 mM l-glutamine, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 0.2% sodium carbonate, 100 U/mL penicillin, and 10% fetal bovine serum. The cell suspension was incubated in PVDF-backed microplates at 37°C for 24 hours. After the membranes were dried, the wells of 96-well filtration plates were observed using a microscope (Leica MZ6; Wetsler, Germany; magnification 10×/0.63), and the spots in each well were counted.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). The results were statistically analyzed using the unpaired Student t test of means or analysis of variance. A P value of <.05 was considered statistically significant.
Results
Ab production against blood group A determinants is dependent on iNKT cells but independent of T cells
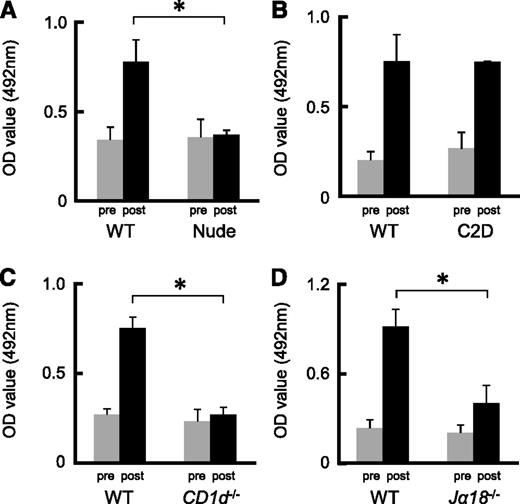
Previously, we detected naturally occurring Abs against anti-human blood group A carbohydrate determinants in the sera obtained from mice.13,14 Extensive anti-A IgM and IgG production occurred when the mice were immunized with human group A-RBCs. To determine whether or not anti-A Ab responses were T-cell dependent, we immunized Balb/c nude mice and B6 C2D mice, both of which lack CD4+ T cells, using human group A-RBCs 2 times per week. Even after immunization, there was no increase in the anti-A Ab titer in nude mice, whereas the serum anti-A Ab titer in C2D mice significantly increased (Figure 1A-B). Unlike C2D mice, nude mice completely lacked NKT cells.24 Consequently, we could not rule out the possibility that NKT cells play a role in the production of anti-A Abs. The predominant NKT subset is represented by type I NKT cells, which express Vα14-Jα18 iTCRs in mice. Type II NKT cells have variable Vα usage and, although they are CD1d restricted, they are thought to be stimulated by many glycolipids but not αGalCer.1,2 In CD1d−/− mice deficient in types I and II NKT cells, the anti-A Ab response was completely impaired, even in the presence of αGalCer (Figure 1C). In addition, the anti-A Ab response was impaired in Jα18−/− mice expressing CD1d but lacking type I αGalCer-reactive NKT cells (Figure 1D). We also observed that αGalCer significantly enhanced blood group A–specific Ab titers in Balb/c wild-type (WT) mice, but this was not observed in CD1d−/− mice (Figure 2A-D). Therefore, these results suggest that induction of anti-A Ab production requires iNKT cells, which are not required for naturally produced anti-A Abs.
Ab production against blood group A determinants was dependent on iNKT cells but independent of T cells. (A-B) Balb/c nude mice and B6 MHC C2D mice were immunized with human group A-RBCs 2 times per week. Balb/c and B6 WT mice were used as the respective controls. The serum anti-A Ab (IgM) concentrations at 2 weeks after the second immunization were measured by ELISA. Even after immunization, the serum anti-A Ab titers were not elevated in nude mice, but Ab concentrations significantly increased in C2D mice. Balb/c nude mice, n = 3; Balb/c WT mice, n = 3; B6 C2D mice, n = 4; B6 WT mice, n = 3. (C-D) Balb/c CD1d−/− mice and B6 Jα18−/− mice were immunized with human group A-RBCs 2 times per week. Balb/c and B6 WT mice served as the respective controls. The serum anti-A Ab levels were measured 2 weeks after the second immunization. The response of anti-A Abs was completely impaired in CD1d−/− mice and partially impaired in Jα18−/− mice. Average values ± SEM for the individual groups are shown. P values were shown to compare pre- and post-levels at the bottom of each figure. Balb/c CD1d−/− mice, n = 4; Balb/c WT mice, n = 3; B6 Jα18−/− mice, n = 5; B6 WT mice, n = 4. *P < .05 compared with the respective control mice.
Ab production against blood group A determinants was dependent on iNKT cells but independent of T cells. (A-B) Balb/c nude mice and B6 MHC C2D mice were immunized with human group A-RBCs 2 times per week. Balb/c and B6 WT mice were used as the respective controls. The serum anti-A Ab (IgM) concentrations at 2 weeks after the second immunization were measured by ELISA. Even after immunization, the serum anti-A Ab titers were not elevated in nude mice, but Ab concentrations significantly increased in C2D mice. Balb/c nude mice, n = 3; Balb/c WT mice, n = 3; B6 C2D mice, n = 4; B6 WT mice, n = 3. (C-D) Balb/c CD1d−/− mice and B6 Jα18−/− mice were immunized with human group A-RBCs 2 times per week. Balb/c and B6 WT mice served as the respective controls. The serum anti-A Ab levels were measured 2 weeks after the second immunization. The response of anti-A Abs was completely impaired in CD1d−/− mice and partially impaired in Jα18−/− mice. Average values ± SEM for the individual groups are shown. P values were shown to compare pre- and post-levels at the bottom of each figure. Balb/c CD1d−/− mice, n = 4; Balb/c WT mice, n = 3; B6 Jα18−/− mice, n = 5; B6 WT mice, n = 4. *P < .05 compared with the respective control mice.
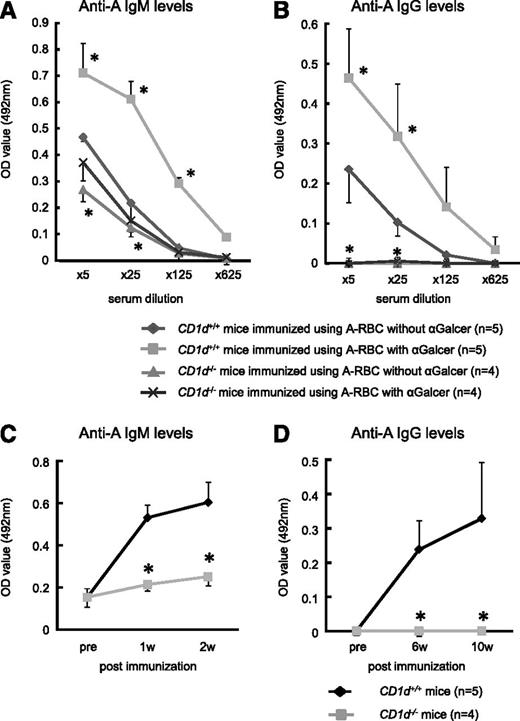
Effect of αGalCer administration on anti-blood group A responses in CD1d−/− mice. Balb/c CD1d−/− mice and Balb/c WT (CD1d+/+) mice were immunized using blood group A-RBCs together with intraperitoneal injection of either αGalCer (4 μg/mouse) or PBS (control) 2 times per week. (A-B) The serum anti-A–specific IgM and IgG levels were determined using ELISA at 2 and 10 weeks after the last immunization, respectively. αGalCer significantly increased the blood group A-specific Ab levels in CD1d+/+ mice, but the Ab levels did not increase in CD1d−/− mice. The average values ± SEM for the individual groups are shown. *P < .05 compared with the data from CD1d+/+ mice without αGalCer. (C-D) The kinetics of the serum IgM and IgG titers against blood group A determinants in Balb/c CD1d+/+ mice and Balb/c CD1d−/− mice (25× diluted serum was used). Anti-A Ab production was elicited by intraperitoneal immunization of mice with A-RBC 2 times per week. The average values ± SEM for the individual groups are shown. Balb/c CD1d−/− mice, n = 4; WT Balb/c mice, n = 5. *P < .05 compared with the respective CD1d+/+ mice.
Effect of αGalCer administration on anti-blood group A responses in CD1d−/− mice. Balb/c CD1d−/− mice and Balb/c WT (CD1d+/+) mice were immunized using blood group A-RBCs together with intraperitoneal injection of either αGalCer (4 μg/mouse) or PBS (control) 2 times per week. (A-B) The serum anti-A–specific IgM and IgG levels were determined using ELISA at 2 and 10 weeks after the last immunization, respectively. αGalCer significantly increased the blood group A-specific Ab levels in CD1d+/+ mice, but the Ab levels did not increase in CD1d−/− mice. The average values ± SEM for the individual groups are shown. *P < .05 compared with the data from CD1d+/+ mice without αGalCer. (C-D) The kinetics of the serum IgM and IgG titers against blood group A determinants in Balb/c CD1d+/+ mice and Balb/c CD1d−/− mice (25× diluted serum was used). Anti-A Ab production was elicited by intraperitoneal immunization of mice with A-RBC 2 times per week. The average values ± SEM for the individual groups are shown. Balb/c CD1d−/− mice, n = 4; WT Balb/c mice, n = 5. *P < .05 compared with the respective CD1d+/+ mice.
Ab production against Gal and NeuGc epitopes is independent of iNKT cells
Next, we investigated the possible role of iNKT cells in Ab production against xenogeneic carbohydrates, such as Gal and NeuGc epitopes, to which B-1b cells respond.15,19 To this end, we generated CD1d−/−GalT−/− mice and CD1d−/−CMAH−/− mice, both of which lacked iNKT cells. These mice were immunized with Gal-bearing and NeuGc-bearing rat thymocytes, and anti-Gal and anti-NeuGc Abs were determined in their sera, respectively. The serum anti-Gal Ab titers (both IgM and IgG subclasses) of CD1d−/−GalT−/− mice were elevated to levels similar to those in CD1d+/+GalT−/− mice (Figure 3A-B). Likewise, CD1d−/−CMAH−/− mice also exhibited increased anti-Gal Ab levels similar to those in CD1d+/+CMAH−/− mice (Figure 3C-D). In addition, αGalCer administration at immunization with xenogeneic cells did not accelerate anti-Gal Ab and anti-NeuGc Ab production in the CD1d+/+GalT−/− and CD1d+/+CMAH−/− mice, respectively (supplemental Figure 1). Thus, unlike anti-A Ab production, the production of anti-Gal and anti-NeuGc Abs does not require iNKT cells.
Correlation of iNKT cells with Ab production against Gal and NeuGc epitopes. B6 CD1d−/−GalT−/− and CD1d−/−CMAH−/− mice were immunized with Gal- and NeuGc-bearing rat thymocytes, and the levels of anti-Gal and anti-NeuGc Abs were then determined in their respective sera using FCM. (A-B) CD1d−/−GalT−/− mice showed increased anti-Gal Ab titer (both IgM and IgG subclasses) similar to that in CD1d+/+GalT−/− mice (n = 4 per group). (C-D) CD1d−/−CMAH−/− mice also showed increased anti-NeuGc Ab titer similar to that in CD1d+/+CMAH−/− mice (n = 4 per group). Median fluorescence intensity values were used to follow Ab levels. The average values ± SEM for the individual groups are shown.
Correlation of iNKT cells with Ab production against Gal and NeuGc epitopes. B6 CD1d−/−GalT−/− and CD1d−/−CMAH−/− mice were immunized with Gal- and NeuGc-bearing rat thymocytes, and the levels of anti-Gal and anti-NeuGc Abs were then determined in their respective sera using FCM. (A-B) CD1d−/−GalT−/− mice showed increased anti-Gal Ab titer (both IgM and IgG subclasses) similar to that in CD1d+/+GalT−/− mice (n = 4 per group). (C-D) CD1d−/−CMAH−/− mice also showed increased anti-NeuGc Ab titer similar to that in CD1d+/+CMAH−/− mice (n = 4 per group). Median fluorescence intensity values were used to follow Ab levels. The average values ± SEM for the individual groups are shown.
Ab production against allopeptides does not require iNKT cells
To investigate the possible role of iNKT cells in Ab production against allopeptides, to which it is believed that conventional B cells (B-2 cells) respond, Balb/c WT (CD1d+/+) mice and Balb/c CD1d−/− mice were immunized with thymocytes obtained from B6 mice. In the sera of the CD1d−/− mice, the levels of anti-B6 IgM and IgG1 subclass Abs were rather lower, but the levels of IgG2 and IgG3 subclass Abs were higher when compared with levels in CD1d+/+ mice; however, the difference was not statistically significant. Thus, Abs against histocompatibility complex allopeptides were produced in response to allostimulation in CD1d−/− mice, although the class switching of the Abs might be somewhat influenced (supplemental Figure 2).
CD1d−/− mice display a slightly reduced proportion of B-1a cells
We investigated the possibility of a difference in the proportion of B-cell subclasses between CD1d+/+ and CD1d−/− mice. The proportion of B-1a cells in the peritoneal cavity and the liver was slightly lower, but the proportion of B-1b cells was relatively higher in Balb/c CD1d−/− mice as compared with that in Balb/c CD1d+/+ mice (supplemental Figure 3). In contrast, the proportion of B-2 cells in those anatomical sites did not differ between CD1d−/− and CD1d+/+ mice. This suggests that CD1d-restricted NKT cells play a partial role in the differentiation of B-1a cells but not of B-1b cells and B-2 cells.
Administration of anti-mouse CD1d mAb abolishes anti-A Ab production in mice
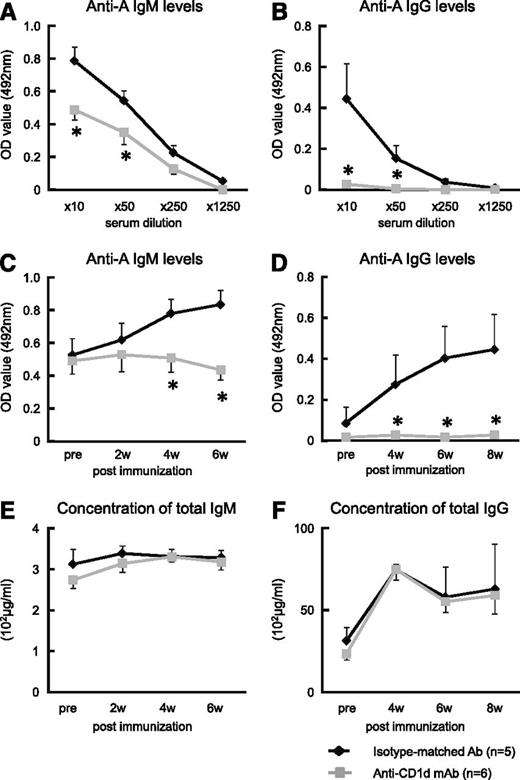
We tested the hypothesis that the collaboration between iNKT and B-1a cells that proceeds via iTCR-CD1d interactions for anti-A Ab production is inhibited by anti-mouse CD1d-blocking mAb. A single injection of anti-mouse CD1d mAb (500 μg/mouse) adequately blocks CD1d molecules on B cells for at least 7 days (data not shown). When Balb/c mice were treated with anti-CD1d mAb 1 day before and 1 day after they were immunized with human blood group A-RBCs, they completely lost the ability to produce anti-A Abs, although their total immunoglobulin levels remained normal (Figure 4A-F). In contrast, when Balb/c mice were injected with isotype-matched irrelevant control Abs, there was a significant elevation in the anti-A IgM and IgG class switching in the serum after the immunization.
Effect of administration of anti-mouse CD1d mAb on anti-blood group A titers in mice. Balb/c WT mice received intraperitoneal injection of anti-mouse CD1d mAb (n = 6). Mice that received injections of isotype-matched Ab served as the controls (n = 5). The mice were immunized with human blood group A-RBCs (5 × 108/mouse) on days 1 and 8 after mAb administration. After immunization, blood samples were obtained and the total IgM/IgG and anti-A IgM/IgG concentrations were measured using ELISA. (A-B) Treatment with anti-CD1d mAb significantly inhibited Ab production against blood group A epitopes in the mice. Anti-A IgM levels were detected 6 weeks after mAb administration, and anti-A IgG levels were detected at 8 weeks. (C-D) The kinetics of anti-A Abs in Balb/c WT mice that were injected with either anti-mouse CD1d mAb or isotype-matched Ab are shown (10× diluted serum was used). (E-F) The kinetics of the total serum immunoglobulin (IgM and IgG) levels of the Balb/c WT mice treated with anti-CD1d mAb are presented. The average values ± SEM for the individual groups are shown. *P < .05 compared with the data from WT mice treated with isotype-matched Ab.
Effect of administration of anti-mouse CD1d mAb on anti-blood group A titers in mice. Balb/c WT mice received intraperitoneal injection of anti-mouse CD1d mAb (n = 6). Mice that received injections of isotype-matched Ab served as the controls (n = 5). The mice were immunized with human blood group A-RBCs (5 × 108/mouse) on days 1 and 8 after mAb administration. After immunization, blood samples were obtained and the total IgM/IgG and anti-A IgM/IgG concentrations were measured using ELISA. (A-B) Treatment with anti-CD1d mAb significantly inhibited Ab production against blood group A epitopes in the mice. Anti-A IgM levels were detected 6 weeks after mAb administration, and anti-A IgG levels were detected at 8 weeks. (C-D) The kinetics of anti-A Abs in Balb/c WT mice that were injected with either anti-mouse CD1d mAb or isotype-matched Ab are shown (10× diluted serum was used). (E-F) The kinetics of the total serum immunoglobulin (IgM and IgG) levels of the Balb/c WT mice treated with anti-CD1d mAb are presented. The average values ± SEM for the individual groups are shown. *P < .05 compared with the data from WT mice treated with isotype-matched Ab.
IL-5 critically regulates anti-A Ab production via iTCR-CD1d interactions
To investigate the mechanism through which anti-CD1d mAb abolishes anti-A Ab production in mice, we analyzed the kinetics of serum levels of various cytokines (IL-4, IL-5, IL-9, IL-17, IL-21, and IFN-γ) after immunization with human blood group A-RBCs in CD1d+/+ and CD1d−/− Balb/c mice. The stimulation with blood group A-RBCs rapidly and transiently induced IL-5 production in CD1d+/+ mice (Figure 5A), while other cytokines were not detectable during the observation period (data not shown). In contrast, such IL-5 production was completely abolished in CD1d−/− mice. In addition, administration of anti-CD1d mAb also abrogated IL-5 production in CD1d+/+ mice even after immunization with blood group A-RBCs (Figure 5B). To evaluate the impact of IL-5 on anti-A Ab production, anti-IL-5–neutralizing mAb was administered prior to each immunization with human blood group A-RBCs in CD1d+/+ mice, which led to a constantly undetectable level of IL-5 in those mice (data not shown). The mice treated with anti-IL-5 mAb displayed a significantly lower level of anti-A Ab than the mice treated with the isotype-matched control Ab (Figure 5C-E). Consistent with an increase in the serum levels of IL-5 in WT CD1d+/+ mice immunized with A-RBCs, IL-5–producing cells were detectable in the LMNCs by ELISPOT assay, whereas these cells were not in the spleen cells (Figure 6A-B). The IL-5–producing cells were not observed in the LMNCs after administration of anti-CD1d mAb. Among the LMNCs, NKT cells (CD1d-tetramer+ TCRβ+), T cells (CD1d-tetramer– TCRβ+), and others (CD1d-tetramer– TCRβ–) were isolated by multiparameter FCM sorting and were subjected to ELISPOT assay for determining the frequency of total IL-5–producing cells in each sorted cell fraction. The NKT cell fraction was greatly enriched for IL-5–producing cells, whereas the other fractions were markedly depleted for those cells (Figure 6C-E), indicating that NKT cells were the predominant source of IL-5 secreted after immunization with group A-RBCs. Thus, IL-5 critically regulates anti-A Ab production via iTCR-CD1d interactions.
Impact of IL-5 on anti-A Ab production after stimulation with blood group A-RBCs. (A) Balb/c CD1d−/− mice and WT CD1d+/+ mice (n = 5 in each group) were immunized with human blood group A-RBCs (5 × 108/mouse). The levels of cytokines in serum were analyzed at the indicated time points using cytometric bead array flex sets (CBA). Blood group A determinants significantly increased the level of IL-5 in CD1d+/+ mice, but this cytokine level did not increase in CD1d−/− mice. In contrast, blood group A determinants did not increase the levels of IL-4, IL-9, IL-17, IL-21, and IFN-γ in either CD1d−/− or CD1d+/+ mice. (B) Balb/c WT mice received intraperitoneal injection of anti-mouse CD1d mAb (n = 5). Mice that received injections of isotype-matched Ab served as controls (n = 5). The mice were immunized with human blood group A-RBCs (5 × 108/mouse) on day 1 after mAb administration. The level of IL-5 in serum was analyzed at the indicated time points using CBA. Treatment with anti-CD1d mAb significantly inhibited IL-5 production against blood group A epitopes in the mice. (C-E) Balb/c WT mice received intraperitoneal injection of anti-mouse IL-5 mAb (n = 5) 30 minutes prior to immunization with human blood group A-RBCs (5 × 108/mouse). Mice that received injections of isotype-matched Ab served as controls (n = 5). The mice were immunized with human blood group A-RBCs 2 times at 1-week interval after mAb administration. (C) Anti-A IgM concentrations were measured using ELISA before immunization. (D) Anti-A IgM concentrations were measured at 2 weeks after the first immunization. (E) Anti-A IgM concentrations were measured at 3 weeks after the first immunization. Treatment with anti-IL-5 mAb significantly inhibited Ab production against blood group A epitopes in the mice. The average values ± SEM for the individual groups are shown. *P < .05 compared with the data from CD1d−/− mice and data from WT mice treated with isotype-matched Ab.
Impact of IL-5 on anti-A Ab production after stimulation with blood group A-RBCs. (A) Balb/c CD1d−/− mice and WT CD1d+/+ mice (n = 5 in each group) were immunized with human blood group A-RBCs (5 × 108/mouse). The levels of cytokines in serum were analyzed at the indicated time points using cytometric bead array flex sets (CBA). Blood group A determinants significantly increased the level of IL-5 in CD1d+/+ mice, but this cytokine level did not increase in CD1d−/− mice. In contrast, blood group A determinants did not increase the levels of IL-4, IL-9, IL-17, IL-21, and IFN-γ in either CD1d−/− or CD1d+/+ mice. (B) Balb/c WT mice received intraperitoneal injection of anti-mouse CD1d mAb (n = 5). Mice that received injections of isotype-matched Ab served as controls (n = 5). The mice were immunized with human blood group A-RBCs (5 × 108/mouse) on day 1 after mAb administration. The level of IL-5 in serum was analyzed at the indicated time points using CBA. Treatment with anti-CD1d mAb significantly inhibited IL-5 production against blood group A epitopes in the mice. (C-E) Balb/c WT mice received intraperitoneal injection of anti-mouse IL-5 mAb (n = 5) 30 minutes prior to immunization with human blood group A-RBCs (5 × 108/mouse). Mice that received injections of isotype-matched Ab served as controls (n = 5). The mice were immunized with human blood group A-RBCs 2 times at 1-week interval after mAb administration. (C) Anti-A IgM concentrations were measured using ELISA before immunization. (D) Anti-A IgM concentrations were measured at 2 weeks after the first immunization. (E) Anti-A IgM concentrations were measured at 3 weeks after the first immunization. Treatment with anti-IL-5 mAb significantly inhibited Ab production against blood group A epitopes in the mice. The average values ± SEM for the individual groups are shown. *P < .05 compared with the data from CD1d−/− mice and data from WT mice treated with isotype-matched Ab.
NKT cells were predominant sources of IL-5 secreted after immunization with group A-RBCs. WT CD1d+/+ Balb/c mice received intraperitoneal injection of anti-mouse CD1d mAb (n = 3). Mice that received injections of isotype-matched Ab served as controls (n = 3). The mice were immunized with human blood group A-RBCs (5 × 108/mouse) on day 1 after mAb administration. The mice were sacrificed to determine the IL-5–producing cells 6 hours after immunization. (A-B) LMNCs and spleen cells were seeded. The representative pictures of ELISPOT wells are shown (A) and the frequency of IL-5 producing cells is (B). Number in each picture refers to the total cells seeded per well (×103). (C-E) Six hours after immunization with A-RBCs, the LMNCs were isolated from CD1d+/+ Balb/c mice (n = 12). The pooled cells were used in ELISPOT assay to determine the frequency of IL-5–producing cells. The LMNCs were stained with APC-conjugated anti-mouse CD1d-tetramer and PE-Cy7–conjugated anti-mouse TCRβ. NKT cells (CD1d-tetramer+, TCRβ+), T cells (CD1d-tetramer–, TCRβ+), and the others (CD1d-tetramer–, TCRβ–) were isolated by sorting with FACS Aria. After sorting, the purities of NKT, T, and other cells were reanalyzed by FCM. (D-E) The representative pictures of ELISPOT wells are shown (D) and the frequency of IL-5–producing cells is shown (E). Number in each picture refers to the total cells seeded per well (×103). The results shown are the average ± SEM calculated from red spot number in quadruplicate wells. The results are representative of 2 similar experiments. *P < .05.
NKT cells were predominant sources of IL-5 secreted after immunization with group A-RBCs. WT CD1d+/+ Balb/c mice received intraperitoneal injection of anti-mouse CD1d mAb (n = 3). Mice that received injections of isotype-matched Ab served as controls (n = 3). The mice were immunized with human blood group A-RBCs (5 × 108/mouse) on day 1 after mAb administration. The mice were sacrificed to determine the IL-5–producing cells 6 hours after immunization. (A-B) LMNCs and spleen cells were seeded. The representative pictures of ELISPOT wells are shown (A) and the frequency of IL-5 producing cells is (B). Number in each picture refers to the total cells seeded per well (×103). (C-E) Six hours after immunization with A-RBCs, the LMNCs were isolated from CD1d+/+ Balb/c mice (n = 12). The pooled cells were used in ELISPOT assay to determine the frequency of IL-5–producing cells. The LMNCs were stained with APC-conjugated anti-mouse CD1d-tetramer and PE-Cy7–conjugated anti-mouse TCRβ. NKT cells (CD1d-tetramer+, TCRβ+), T cells (CD1d-tetramer–, TCRβ+), and the others (CD1d-tetramer–, TCRβ–) were isolated by sorting with FACS Aria. After sorting, the purities of NKT, T, and other cells were reanalyzed by FCM. (D-E) The representative pictures of ELISPOT wells are shown (D) and the frequency of IL-5–producing cells is shown (E). Number in each picture refers to the total cells seeded per well (×103). The results shown are the average ± SEM calculated from red spot number in quadruplicate wells. The results are representative of 2 similar experiments. *P < .05.
Administration of anti-human CD1d mAb significantly inhibited anti-A Ab production in humanized mice
We further hypothesized that blocking the iTCR-CD1d interactions using anti-CD1d mAb could prevent Ab-mediated rejection in ABO-incompatible transplant recipients. To address this possibility, we examined the inhibitory effects of anti-human CD1d mAb on anti-group A Ab production in a humanized mouse model where PBMCs from type O human volunteers had been engrafted into NOD/SCID/γcnull mice. The same dose of PBMCs from each human volunteer was then injected into 2 mice (20 × 106 cells/mouse) of which 1 subsequently received anti-human CD1d mAb and the other received the isotype-matched irrelevant control Ab 7 10 days after the engrafting. These PBMC-chimeric mice were then immunized with human blood group A-RBCs 8 days after the PBMC injection. Anti-CD1d mAb completely inhibited anti-A IgM/IgG production in the humanized mice, whereas the mice treated with control Abs showed a significant increase in serum anti-A IgM/IgG levels (Figure 7A). Three weeks after the human PBMC engrafting, the recipients were sacrificed and the proportion of B cells with receptors for group A carbohydrates was assayed. We then used synthetic A carbohydrate determinants conjugated with FITC-labeled A-BSA and found CD19+ B-cell receptors for A carbohydrates in the spleen of humanized mice treated with the control Abs. In contrast, there were significantly fewer B-cell receptors for A carbohydrates in the spleen of humanized mice treated with anti-CD1d mAb (Figure 7B-C). Thus, blocking the iTCR-CD1d interactions by CD1d mAb could be a novel approach for preventing Ab-mediated rejection in ABO-incompatible transplant recipients.
Effect of administration of anti-human CD1d mAb on anti-A Ab production in humanized mice. The same dose of PBMCs from each type O human volunteer was intraperitoneally injected into 2 NOD/SCID/γcnull mice (20 × 106 cells/mouse). Of these mice, 1 subsequently received anti-human CD1d mAb and the other received isotype-matched irrelevant control Ab at days 7 and 10 after PBMC engrafting. The humanized mice were immunized with human blood group A-RBCs 8 days after PBMC injection. (A) The serum anti-A IgM and IgG levels in the humanized mice were determined using ELISA at 14 and 21 days after engraftment. Each point represents an individual mouse. Each group contained 5 animals. (B) Three weeks after human PBMC engrafting, the humanized mice were sacrificed to determine the proportion of B cells with receptors for group A carbohydrates. Spleen cells were prepared from the humanized mice (n = 4 in each group). The pooled cells were stained with FITC-labeled A-BSA or control FITC-labeled BSA together with PE-conjugated anti-human CD19 mAb. Representative FCM results of group A-BSA–binding spleen cells. We analyzed 50 000 cells per contour plot. The percentages in the figure represent percentages of total CD19+ B cells. (C) The frequencies of A-BSA–binding B cells among the total B cell population in mice treated with either anti-human CD1d mAb or isotype-matched control Ab are shown. *P < .05 compared with the data from humanized mice treated with isotype-matched Ab.
Effect of administration of anti-human CD1d mAb on anti-A Ab production in humanized mice. The same dose of PBMCs from each type O human volunteer was intraperitoneally injected into 2 NOD/SCID/γcnull mice (20 × 106 cells/mouse). Of these mice, 1 subsequently received anti-human CD1d mAb and the other received isotype-matched irrelevant control Ab at days 7 and 10 after PBMC engrafting. The humanized mice were immunized with human blood group A-RBCs 8 days after PBMC injection. (A) The serum anti-A IgM and IgG levels in the humanized mice were determined using ELISA at 14 and 21 days after engraftment. Each point represents an individual mouse. Each group contained 5 animals. (B) Three weeks after human PBMC engrafting, the humanized mice were sacrificed to determine the proportion of B cells with receptors for group A carbohydrates. Spleen cells were prepared from the humanized mice (n = 4 in each group). The pooled cells were stained with FITC-labeled A-BSA or control FITC-labeled BSA together with PE-conjugated anti-human CD19 mAb. Representative FCM results of group A-BSA–binding spleen cells. We analyzed 50 000 cells per contour plot. The percentages in the figure represent percentages of total CD19+ B cells. (C) The frequencies of A-BSA–binding B cells among the total B cell population in mice treated with either anti-human CD1d mAb or isotype-matched control Ab are shown. *P < .05 compared with the data from humanized mice treated with isotype-matched Ab.
Discussion
Unlike allopeptide Ags, which are presented to conventional T cells via MHC molecules, glycolipid Ags are presented to T cells by the MHC-like molecule CD1. Humans express several nonpolymorphic CD1 molecules, including CD1d, which presents lipids to NKT cells. NKT cells are innate-like lymphocytes defined by their characteristic semi-invariant T-cell receptor that recognizes the potent glycolipid Ag αGalCer.3,25 In addition to this nonphysiological Ag, NKT cells have been shown to respond to exogenous bacterial lipid Ags as well as endogenous glycolipids presented by APCs responding to innate stimuli.26-32 Glycosphingolipid isoglobotrihexosylceramide (iGb3) has been identified as an endogenous glycolipid Ag species recognized by healthy, noninfected NKT cells.33 In addition, recently it has been demonstrated that a ubiquitous endogenous lipid, β-d-glucopyranosylceramide (β-GlcCer), accumulates during infection and in response to Toll-like receptor agonists; it also potently activates iNKT cells in both mice and humans through a cognate TCR interaction.32,34 Despite the similarity in the molecular structure of histo-blood group Ags, Gal and NeuGc epitopes, with either the iGb3 or β-GlcCer glycolipid, it is not yet known whether these glycolipids are also recognized by NKT cells. The use of Jα18−/−, CD1d−/−, CD1d−/−GalT−/−, and CD1d−/−CMAH−/− mice in this study allowed us to focus on the specific role of CD1d molecules in the Ab response to those glycolipids. We demonstrated that Ab production against blood group A carbohydrates, but not against Gal and NeuGc epitopes, was dependent on CD1d and NKT cells. Recently, another study used a similar mouse model to demonstrate that Ab responses to Gal do not require CD1 molecules or NKT cells.35 Taking these findings into consideration, our results indicate that anti-A Ab production could be specifically inhibited by blocking iTCR-CD1d interactions using anti-CD1d mAb while maintaining Ab responses to Gal and NeuGc epitopes. Consistently, our results demonstrated that anti-CD1d mAb specifically inhibited the production of Abs against blood group A Ags in both mice and humans. Since Gal and NeuGc epitopes are expressed in environmental bacteria and neoplastic cells and Abs against those determinants have been implicated in antibacterial and antitumor immunity,36-40 this novel concept of using CD1d mAb is a preferable strategy for preventing Ab-mediated rejection in ABO-incompatible transplant recipients while preserving their immunity to infection and cancer.
B-1a and B-1b cells are essentially identical in their phenotype and are distinguishable by the presence or absence of the CD5 marker alone. To date, no functional differences between the 2 cell populations have been clearly identified. However, differing activities of IL-5 and IL-9 have been reported. IL-5 transgenic mice have an expanded B-1a population associated with high levels of auto-Abs, whereas IL-9 transgenic mice have an expanded B-1b population without the production of auto-Abs; however, both mice exhibit enhanced IgM production.41 Also, it has been shown that IL-5 receptor α-chain–deficient (IL-5Rα−/−) mice show decreased numbers of CD5+ B-1a cells and sustained numbers of CD5- B-1b cells.42 Those mice showed low serum IgG3 and IgM and no IL-5–induced enhancement of B-cell proliferation. These results suggest that IL-5 contributes to early development of B-1a cells but not of B-1b cells. In addition, it has been reported that injection of IL-5 or IL-10, but not IL-4, increases serum anti-RBC auto-Ab and induction of hemolytic anemia in transgenic mice bearing Ig heavy- and light-chain genes that encode an Ab against the mouse RBCs. This raises the speculation that IL-5 or IL-10 might play an important role in the terminal differentiation of B-1a cells into Ab-producing cells in vivo.43 Taken together with the difference in the B-1a/B-1b proportion between CD1d−/− and CD1d+/+ mice in our study (Figure 4), the cytokines derived from NKT cells may have some impact on the differentiation of B-1a cells responding to blood group carbohydrates but may not affect that of B-1b cells responding to Gal or NeuGc. As a more striking clue, we found that IL-5 exclusively increased after immunization with A-RBCs in WT CD1d+/+ mice but remained undetectable in CD1d−/− mice. The combined FCM sorting and ELISPOT assay revealed that NKT cells predominantly secreted IL-5. Anti-mouse CD1d blocking mAb completely abolished such IL-5 production in the WT mice. In addition, anti-IL-5 neutralizing mAb significantly diminished anti-A Ab production in the WT mice, indicating that IL-5 secreted from NKT cells critically regulates anti-A Ab production by B-1a cells. In addition, a recent demonstration that iNKT cells direct B-cell responses to cognate lipid Ag in an IL-21–dependent manner would also pave the way for addressing how NKT cells stimulate B-1a cell responses to blood group carbohydrates.44 This particular experiment, in which IL-21R–deficient mice were used, revealed that IL-21 derived from iNKT cells was required for Ab class switching, not merely for Ab production in responses to lipid Ags. Further studies are needed to determine whether a similar mechanism is responsible for Ab production/class switching from B-1a cells in response to blood group carbohydrate Ags, although IL-21 remained undetectable in sera of mice immunized with A-RBCs in this study.
To demonstrate a different pattern of VH family usage in B-1b cells as compared with B-1a or conventional B cells in mice, a previous study used FCM sorting and single-cell PCR. It was found that the VH1 (J558) and VH2 (Q52) families were underutilized and that the VH10 (DNA4) and VH3 (3660) families were overrepresented among B-1b cells, suggesting differences in the repertoires between the B-1a and B-1b populations.45 Currently, the question of which B-cell subset can recognize glycolipids to which iNKT cells respond can be answered only speculatively. Since Gal and NeuGc epitopes are independent of CD1d in response to B-1b cells, this B-cell subset might not share the recognition of the same Ag with NKT cells. In contrast, B-1a cells, some of which recognize blood group A epitopes in a CD1d-dependent manner, might share recognition of the corresponding Ag with NKT cells.
In conclusion, we found that iTCR-CD1d interactions were required for the production of anti-A Abs, whereas these interactions were not required for the production of anti-Gal and anti-NeuGc Abs. Anti-CD1d mAb significantly inhibited the development of B cells with receptors for blood group A carbohydrates and completely inhibited anti-A Ab production. This suggests that they could be used in a novel approach to prevent Ab-mediated rejection in ABO-incompatible transplant recipients.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Exploratory Research (19659323) from the Japan Society for the Promotion of Science. We thank Drs Kentaro Ide and Hiroyuki Tahara for their advice and encouragement, Yuko Ishida for expert technical assistance, and Dr Steve Porcelli (Albert Einstein College of Medicine, Bronx, NY) for providing CD1d42 clone cells. This work was carried out in part at the Analysis Center of Life Science, Hiroshima University.
Authorship
Contribution: H.O., H. Tazawa, and T.I. designed the research; H. Tazawa, T.I., Y.T., Y.I., M.Y., and H.S. performed the research; H.O., H. Tazawa, T.I., and H. Tashiro analyzed the data; and H.O. and H. Tazawa wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hideki Ohdan, Department of Surgery, Division of Frontier Medical Science, Programs for Biomedical Research, Graduate School of Biomedical Sciences, Hiroshima University, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8551, Japan; hohdan@hiroshima-u.ac.jp.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal