Key Points
Plant sterol accumulation in platelet membrane induces platelet hyperreactivity.
Internalization of the αIIbβ3 complex and filamin A degradation cause macrothrombocytopenia and bleeding phenotype.
Abstract
Sitosterolemia is a rare, autosomal recessive disease caused by mutations in the adenosine triphosphate-binding cassette transporter genes ABCG5 or ABCG8 that result in accumulation of xenosterols in the body. Clinical manifestations include tendon xanthomas, premature coronary artery disease, hemolytic anemia, macrothrombocytopenia, and bleeding. Although the effect of sterol accumulation on the predisposition for atherosclerosis is evident, how xenosterol accumulation leads to defects in platelet physiology is unknown. Sitosterolemia induced in Abcg5- and Abcg8-deficient mice fed a high plant sterol diet resulted in accumulation of free sterols in platelet plasma membranes, leading to hyperactivatable platelets characterized by constitutive binding of fibrinogen to its αIIbβ3 integrin receptor, internalization of the αIIbβ3 complex, generation of platelet-derived microparticles, and changes in the quantity and subcellular localization of filamin. The latter was associated with macrothrombocytopenia, shedding of GPIbα, impaired platelet adhesion to von Willebrand factor, and inability to form stable thrombi. Plasma levels of soluble GPIbα were strongly correlated with plasma sitosterol levels in samples from human sitosterolemic patients, implicating a similar mechanism of sterol-induced platelet passivation in the human disease. Intercalation of plant sterols into the plasma membrane therefore results in dysregulation of multiple platelet activation pathways, leading to macrothrombocytopenia and bleeding.
Introduction
Subject to cultural and geographic variation, most humans ingest approximately equal amounts of cholesterol and plant-derived (phyto) sterols—approximately 200-500 mg of each per day.1 Although 20% to 80% of dietary cholesterol is absorbed,2 dietary plant sterols, which include stigmasterol, campesterol, and sitosterol, are normally actively excreted, resulting in less than 1% retention.3 Clues that a specific molecular mechanism was involved in the selective removal of phytosterols were first provided by Bhattacharyya and Conner in a 1974 description of extensive tendon xanthomas and unusually high levels of β-sitosterol with normal cholesterol levels in 2 sisters suffering from what is now most commonly known as sitosterolemia.4
An exceedingly rare disorder affecting less than one in a million individuals, sitosterolemia (OMIM 21250; also known as Mediterranean stomatocytosis, Mediterranean macrothrombocytopenia, and phytosterolemia) is a rare, autosomal recessive disorder characterized by the accumulation of plant sterols in blood and tissues and is caused by mutations in one of the adenosine triphosphate-binding cassette (ABC) transporter ABCG5 or ABCG8 genes (sterolin-1 and sterolin-2)5-7 located on chromosome 2p21 in humans8 and syntenic chromosome 17 in mice.9 Patients with mutations in either of these sterol transport proteins, which normally form a heterodimeric sterol egress channel,10 frequently develop tendon and cutaneous xanthomas and, most importantly, are at risk of developing premature coronary artery disease.4 Paradoxically, the same individuals often exhibit clinically problematic bleeding episodes, perhaps resulting from the macrothrombocytopenia that also represents a distinctive and diagnostic hematologic feature of both human and murine sitosterolemia.11 Other diagnostic hematologic abnormalities include stomatocytic hemolysis, anemia, and loss of ristocetin-induced platelet agglutination,11 which is a measure of the ability of platelet glycoprotein (GP) Ib to function as an adhesion receptor for von Willebrand factor (VWF).12
We generated a mouse model of sitosterolemia in 200413 ; shortly thereafter, Kruit et al14 found that that mice genetically deficient in Abcg5 fully recapitulate the macrothrombocytopenia seen in human sitosterolemia, a condition that can be corrected by treatment with the sterol-absorption inhibitor ezetimibe.15 Recently, Chase et al identified a spontaneously occurring nonsense mutation in Abcg5 in trac/trac mice that also exhibits thrombocytopenia, cardiomyopathy, and decreased platelet activation responsiveness.9 Despite these observations, the mechanism by which phytosterol accumulation might negatively affect platelet structure and function is not well understood, and sitosterolemia has been the focus of a differential diagnosis of macrothrombocytopenia.16 In the present study, we sought to analyze the influence of Abcg5 and Abcg8 deficiency on platelet physiology.
Methods
Mice and diets
Abcg5- and Abcg8-deficient mice on a C57BL/6J background have been described previously13,17,18 and have been deposited with Jackson Laboratories. GPIbα-null mice19 were generously provided by Dr. Jerry Ware (University of Arkansas). Abcg5−/− and Abcg8−/− mice were fed with custom defined diets prepared by Harlan Laboratories (Madison, WI). The high plant sterol (HS) diet contains 1% w/w of plant sterols; the low plant sterol diet (LS) contains <0.01% w/w of plant sterols.18
Plant sterol analyses
Blood collection and measurement of hematologic parameters
Mice were anesthetized and blood samples were collected from the inferior vena cava and anticoagulated with sodium citrate or from the retro-orbital venous plexus using heparin-coated capillary tubes. Whole blood samples were used to determine blood cell counts, mean platelet volume, hematocrit, and hemoglobin using Scil Veterinary ABC Blood Counter (Viernheim, Germany). Blood samples from human subjects with sitosterolemia had been collected as part of an original study of the genetic and biochemical analyses of sitosterolemia7,8,20 and had been stored at −80°C since collection (∼15 years). Mouse tail bleeding times were determined by cutting a 3-mm portion of distal tail tip, which was then immersed in saline maintained at 37°C. The point at which complete cessation of visible blood flow occurred was defined as the bleeding time.
Ex vivo perfusion assay
Mouse VWF was purified from HEK293T cell culture supernatant transfected with mouse VWF expression plasmid. Platelet interaction with immobilized mouse VWF or type I collagen (Chrono-Log, Havertown, PA) was performed using VenaFlux Platform and Vena8Fluor+Biochips (Cellix, Dublin, Ireland). One unit per milliliter of mouse VWF or 50 μg/mL of type I collagen was coated on Vena8Fluor+Biochips overnight at 4°C in a humid chamber. The channels were blocked with 3% bovine serum albumin for 1 hour at room temperature. Mouse blood was drawn from vena cava using PPACK (50 μM final) and heparin (50 mU/mL final) as anticoagulants. Platelets were labeled with DyLight488-labeled anti-mGPIbβ monoclonal antibody (mAb) (Emfret Analytics) or mepacrine (quinacrine dihydrochloride; Calbiochem, La Jolla, CA). Perfusion assay was performed at shear rate of 2000 s−1. As a control experiment, platelet-reduced wild-type blood was prepared by mixing the erythrocyte fraction and diluted platelet-rich plasma with platelet-poor plasma and perfused on immobilized type I collagen.
Study approval
All animal procedures and experiments were approved by the Institutional Animal Care and Use Committee of the Clement J. Zablocki VA Medical Center. All human experiments were approved by the Institutional Review Board of Medical College of Wisconsin. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Results
Effect of Abcg8-deficiency on hematologic parameters
Previous studies have shown that Abcg5-deficient (Abcg5−/−) mice fed an HS diet develop sitosterolemia with accompanying macrothrombocytopenia.14 Targeted disruption of ABCG5/ABCG8 heterodimer subunit, Abcg8, produces phenotypically indistinguishable mice with increased plasma and tissue plant sterol levels, similar to that observed in sitosterolemia patients, but platelet abnormality had not been examined.14,18 As shown in Figure 1A-B, Abcg8−/− mice fed a defined low phytosterol diet (Abcg8−/− LS mice) had a normal platelet count and their platelets were normal size. When fed an HS diet for 6 weeks however, the mice (referred to as Abcg8−/− HS) developed profound macrothrombocytopenia, with platelet size increasing to that of mice lacking GPIbα (Bernard-Soulier mice).19 Abcg8−/− HS mice also developed hemolytic anemia (Figure 1C) typically associated with human sitosterolemia.11 These hematologic parameters were nearly identical in Abcg5-deficient mice fed an HS diet (not shown). The effect of the HS diet was specific to Abcg5- or Abcg8-deficient mice because C57BL6 wild-type mice fed an HS diet for 8 weeks did not show a significant difference either in platelet count or size compared with those fed with an LS diet (data not shown). The hemostatic capacity of Abcg8−/− mice was examined using a standard tail bleeding time assay. The bleeding time of Abcg8−/− HS mice was prolonged, and 7 of 9 mice did not stop bleeding within 5 minutes (Figure 1D). The effect of the HS diet on bleeding time was significant as determined using a log-rank test, and the percentage of tail bleeding at 5 minutes in Abcg8−/− HS mice was different from that of both Abcg8−/− LS mice and C57BL6 wild-type mice (χ2P < .01).
Hematologic parameters of Abcg8−/− and Abcg5−/− mice fed an Abcg8−/− HS or Abcg8−/− LS diet. (A) Platelet counts, (B) mean platelet volumes, and (C) hemoglobin values. Samples were analyzed using a Vet ABC Counter (n = 6 in each group); **P < .001. (D) Tail bleeding time assays of Abcg8−/− mice fed an HS or LS diet. When bleeding did not cease within 5 minutes, the tail was cauterized and bleeding time was recorded as 300 seconds.
Hematologic parameters of Abcg8−/− and Abcg5−/− mice fed an Abcg8−/− HS or Abcg8−/− LS diet. (A) Platelet counts, (B) mean platelet volumes, and (C) hemoglobin values. Samples were analyzed using a Vet ABC Counter (n = 6 in each group); **P < .001. (D) Tail bleeding time assays of Abcg8−/− mice fed an HS or LS diet. When bleeding did not cease within 5 minutes, the tail was cauterized and bleeding time was recorded as 300 seconds.
Plant sterols accumulate in platelet membranes and induce microparticle generation
Plant sterols accumulate as morphologically distinct lipid droplets inside of stomatocytic red blood cells.11,14 However, platelets from sitosterolemic humans or mice contain no abnormal inclusion bodies; they are simply large. Whole blood samples collected from Abcg8−/− mice fed an HS diet were stained with the cholesterol-binding fluorescent dye, filipin, and analyzed by flow cytometry.21 As shown in Figure 2A, filipin staining was 15-20 times greater in Abcg5−/− HS, relative to Abcg5−/− LS, platelets. This difference was not due to the increased size of Abcg5−/− HS platelets because filipin fluorescence in Abcg5−/− HS platelets was more than 10 times that of similarly enlarged GPIbα-null platelets. Similarly increased filipin staining was observed in Abcg8−/− HS platelets (not shown). Sterol composition was also measured by GC-MS using platelets derived from Abcg5−/− LS and Abcg5−/− HS and purified using anti-CD41 magnetic beads. As shown in Figure 2B, Abcg5−/− HS platelets specifically accumulated the plant sterols campesterol and sitosterol, whereas cholesterol levels were similar between Abcg5−/− LS and Abcg5−/− HS platelets. Incorporation of plant sterols into the platelet plasma membrane had disruptive effects on lipid asymmetry, as indicated by increased generation of Annexin V–positive platelet-derived microparticles in Abcg8−/− HS compared with Abcg8−/− LS samples (Figure 2C-E). Generation of platelet-derived microparticles in these mice was not due to general fragility of giant platelets, because they were also elevated in comparison with those present in the blood of GPIbα-null mice, which have similar macrothrombocytopenia. Taken together, these results demonstrate that accumulation of plant sterols in the platelet plasma membrane of sitosterolemic mice induces mild, but measurable, platelet activation and microparticle generation.
Sterol accumulation and increased platelet-derived microparticle (PMP) generation in sitosterolemic mouse platelets. (A) Whole blood samples were stained with phycoerythrin (PE)-conjugated anti-mouse αIIb mAb and platelet fraction was identified by αIIb positivity and forward-side scatter. Platelets from Abcg5−/− HS become large and are compared with GPIb-deficient (Bernard Soulier) platelets, which exhibit αIIb positivity in a larger forward-side scatter gate. Cells were incubated with filipin to detect sterol-laden membranes. The degree of filipin staining in those specific gates is shown (n = 6 in each group). (B) Comparison of cholesterol and plant sterol levels in platelets of Abcg5−/− HS and Abcg5−/− LS were determined as described in “Methods.” Whole blood samples were fixed with 2% paraformaldehyde and αIIb-positive platelets were isolated using anti-PE antibody-conjugated magnetic immunobeads. Sterol levels were analyzed by GC-MS. (C) Representative dot plot analyses of Annexin V–positive platelets and PMPs. Whole blood samples were double-stained with PE-anti-mouse αIIb mAb and fluorescein isothiocyanate-Annexin V. The αIIb-positive platelets were gated as in (A) and analyzed for Annexin V positivity. Note the increased number of Annexin V–positive PMPs in the blood of Abcg8−/− mice fed a high, but not in low plant sterol diet. (D-E) Statistical analyses of αIIb-positive, Annexin V–positive PMPs (D) and platelets (E) found in Abcg8−/− HS and Abcg8−/− LS blood samples (n = 8 for Abcg8−/− LS mice and n = 10 for Abcg8−/− HS mice). Though the increase in PMPs in the circulation of Abcg8−/− HS mice was significantly higher than in Abcg8−/− LS mice, the difference in Annexin V–positive platelets did not reach statistical significance.
Sterol accumulation and increased platelet-derived microparticle (PMP) generation in sitosterolemic mouse platelets. (A) Whole blood samples were stained with phycoerythrin (PE)-conjugated anti-mouse αIIb mAb and platelet fraction was identified by αIIb positivity and forward-side scatter. Platelets from Abcg5−/− HS become large and are compared with GPIb-deficient (Bernard Soulier) platelets, which exhibit αIIb positivity in a larger forward-side scatter gate. Cells were incubated with filipin to detect sterol-laden membranes. The degree of filipin staining in those specific gates is shown (n = 6 in each group). (B) Comparison of cholesterol and plant sterol levels in platelets of Abcg5−/− HS and Abcg5−/− LS were determined as described in “Methods.” Whole blood samples were fixed with 2% paraformaldehyde and αIIb-positive platelets were isolated using anti-PE antibody-conjugated magnetic immunobeads. Sterol levels were analyzed by GC-MS. (C) Representative dot plot analyses of Annexin V–positive platelets and PMPs. Whole blood samples were double-stained with PE-anti-mouse αIIb mAb and fluorescein isothiocyanate-Annexin V. The αIIb-positive platelets were gated as in (A) and analyzed for Annexin V positivity. Note the increased number of Annexin V–positive PMPs in the blood of Abcg8−/− mice fed a high, but not in low plant sterol diet. (D-E) Statistical analyses of αIIb-positive, Annexin V–positive PMPs (D) and platelets (E) found in Abcg8−/− HS and Abcg8−/− LS blood samples (n = 8 for Abcg8−/− LS mice and n = 10 for Abcg8−/− HS mice). Though the increase in PMPs in the circulation of Abcg8−/− HS mice was significantly higher than in Abcg8−/− LS mice, the difference in Annexin V–positive platelets did not reach statistical significance.
Constitutive binding of fibrinogen to its integrin receptor on sitosterolemic platelets induces internalization of the fibrinogen/αIIbβ3 complex
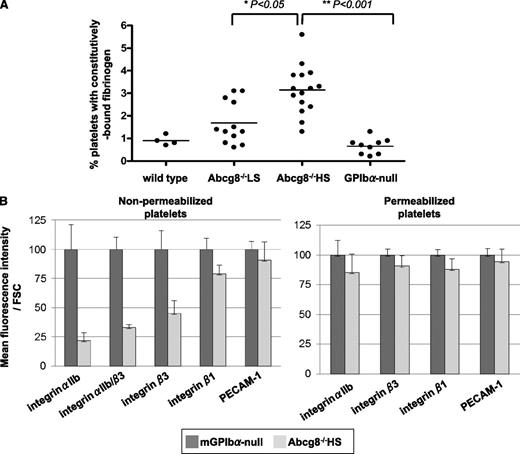
Platelets from individuals with familial hypercholesterolemia have been known for many years to exhibit signs of hyperresponsiveness because of intercalation of cholesterol into the platelet plasma membrane.22 To examine whether accumulation of plant sterols might have similar effects, we examined the constitutive association of fibrinogen with the surface of unstimulated Abcg8−/− HS platelets and compared it with that of similarly sized GPIb-deficient platelets. As shown in Figure 3A, platelets from Abcg8-deficient mice fed an HS diet contained more surface-bound fibrinogen than did similarly sized GPIb-null platelets. Such platelets showed marked reduction in the level of the αIIbβ3 fibrinogen receptor on the cell surface (Figure 3B), consistent with the well-known ability of ligand binding to induce internalization of the αIIbβ3 integrin complex.23-25
Constitutive activation and internalization of the integrin αIIbβ3 complex in Abcg8−/− HS platelets. (A) Elevated constitutively bound fibrinogen on the surface of Abcg8−/− HS platelets. Platelets in whole blood were double-stained with a PE-anti-mouse αIIb mAb and fluorescein isothiocyanate–anti-fibrinogen antibody. (B) Summary of fluorescence-activated cell sorter analysis of integrin αIIb expression on the surface (left: nonpermeabilized) or inside (right: permeabilized) of platelets from Abcg8−/− mice fed a high plant sterol diet. Mean fluorescence intensity/forward scatter were compared with those obtained from similarly sized GPIbα-null platelets, the latter of which was normalized to 100% over 5 different experiments. Surface expression of integrin αIIb and integrin β3 was decreased in Abcg8−/− HS platelets (left), whereas total cellular integrin αIIb and integrin β3 were similar to that of GPIbα-null platelets (right). FSC, forward scatter.
Constitutive activation and internalization of the integrin αIIbβ3 complex in Abcg8−/− HS platelets. (A) Elevated constitutively bound fibrinogen on the surface of Abcg8−/− HS platelets. Platelets in whole blood were double-stained with a PE-anti-mouse αIIb mAb and fluorescein isothiocyanate–anti-fibrinogen antibody. (B) Summary of fluorescence-activated cell sorter analysis of integrin αIIb expression on the surface (left: nonpermeabilized) or inside (right: permeabilized) of platelets from Abcg8−/− mice fed a high plant sterol diet. Mean fluorescence intensity/forward scatter were compared with those obtained from similarly sized GPIbα-null platelets, the latter of which was normalized to 100% over 5 different experiments. Surface expression of integrin αIIb and integrin β3 was decreased in Abcg8−/− HS platelets (left), whereas total cellular integrin αIIb and integrin β3 were similar to that of GPIbα-null platelets (right). FSC, forward scatter.
Decreased fibrinogen binding and increased proteolysis of the GPIb/filamin adhesive complex in sitosterolemic platelets
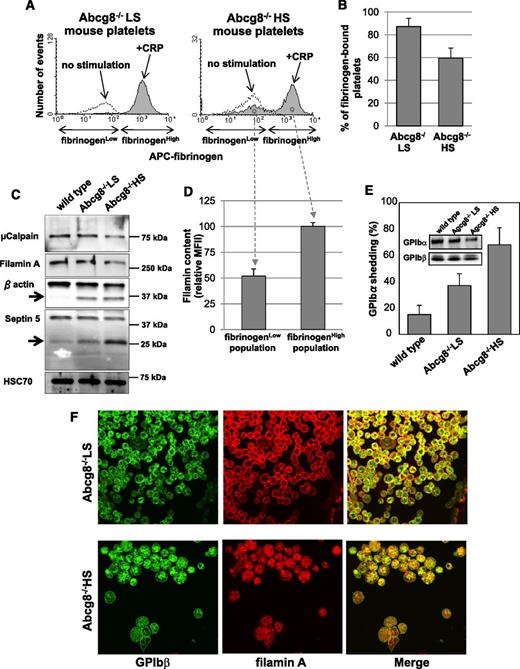
Fibrinogen binding-induced internalization of αIIbβ3 has the potential to inhibit agonist-induced fibrinogen binding, with a corresponding reduction in platelet aggregation.25 Because sitosterolemic patients sometimes experience bleeding episodes severe enough to require platelet transfusion,11 and we observed that Abcg8−/− HS mice exhibit prolonged tail-vein bleeding times, we next examined the effect of Abcg8 deficiency on agonist-induced fibrinogen binding in mice fed an HS diet. As shown in Figure 4A-B, a significant population of Abcg8−/− HS, but not Abcg8−/− LS, platelets failed to bind fibrinogen following activation by the collagen-related peptide, or by thrombin plus calcium ionophore (supplemental Figure 1), consistent with the impaired platelet responsiveness to ligand-induced platelet aggregation that has been reported in human sitosterolemia patients.11
Decreased fibrinogen binding and increased proteolysis of GPIb and filamin in sitosterolemic platelets. Blood from Abcg8−/− LS and Abcg8−/− HS mice were incubated with 10 μg/mL of collagen-related peptide in the presence of antigen-presenting cell (APC)-labeled fibrinogen for 10 minutes at room temperature. Blood samples were fixed, permeabilized, and stained with anti-FlnA antibody followed by AlexaFluor405-labeled goat anti-rabbit immunoglobulin G. Note that ∼40% of Abcg8−/− HS platelets failed to bind fibrinogen (A-B). (C) Western blot analysis of platelet lysate confirmed decrease of cellular filamin and other known µ-calpain substrates β actin and septin 5. (D) Analysis of intracellular filamin content by flow cytometry shows selective cleavage and degradation of filamins in the Abcg8−/− HS platelet population that become refractory to agonist stimulation. (E) Surface expression of GPIbα in platelets double stained with PE-labeled anti-mouse αIIb mAb and a mAb specific for the N-terminal 45 kDa domain of GPIbα and analyzed by flow cytometry. GPIbα shedding was expressed as the percentage of GPIbα-negative platelets in total platelets. Inset: western blot analysis of platelets from the same preparation, demonstrating loss of GPIbα from Abcg8−/− HS platelets. (F) Subcellular localization of FlnA in Abcg8−/− HS and Abcg8−/− LS platelets analyzed by confocal microscopy reveals marked reduction in the GPIb/filamin complex from the cell periphery.
Decreased fibrinogen binding and increased proteolysis of GPIb and filamin in sitosterolemic platelets. Blood from Abcg8−/− LS and Abcg8−/− HS mice were incubated with 10 μg/mL of collagen-related peptide in the presence of antigen-presenting cell (APC)-labeled fibrinogen for 10 minutes at room temperature. Blood samples were fixed, permeabilized, and stained with anti-FlnA antibody followed by AlexaFluor405-labeled goat anti-rabbit immunoglobulin G. Note that ∼40% of Abcg8−/− HS platelets failed to bind fibrinogen (A-B). (C) Western blot analysis of platelet lysate confirmed decrease of cellular filamin and other known µ-calpain substrates β actin and septin 5. (D) Analysis of intracellular filamin content by flow cytometry shows selective cleavage and degradation of filamins in the Abcg8−/− HS platelet population that become refractory to agonist stimulation. (E) Surface expression of GPIbα in platelets double stained with PE-labeled anti-mouse αIIb mAb and a mAb specific for the N-terminal 45 kDa domain of GPIbα and analyzed by flow cytometry. GPIbα shedding was expressed as the percentage of GPIbα-negative platelets in total platelets. Inset: western blot analysis of platelets from the same preparation, demonstrating loss of GPIbα from Abcg8−/− HS platelets. (F) Subcellular localization of FlnA in Abcg8−/− HS and Abcg8−/− LS platelets analyzed by confocal microscopy reveals marked reduction in the GPIb/filamin complex from the cell periphery.
Intercalation of phytosterols into the platelet plasma membrane, therefore, appears to have a number of disruptive, mild platelet-activating effects, including generation of Annexin V–positive microparticles (Figure 2), activation and internalization of the αIIbβ3 complex (Figure 3), and production of platelets that are partially refractory to agonist-induced activation (Figure 4A-B). Sub-threshold platelet activation also has the potential to exert other deleterious passivating effects, including the induction of activation-dependent proteolysis of transmembrane receptors and cytosolic adaptor proteins. As shown in Figure 4C, filamin A, a major substrates for µ-calpain in platelets, was markedly reduced in Abcg8−/− HS compared with Abcg8−/− LS platelets. In addition to filamin, 2 other μ-calpain substrates, β actin and septin 5,26,27 were also found to be degraded in Abcg8−/− HS platelets (arrows in Figure 4C). µ-Calpain is subject to autoproteolysis, likely accounting for its lower expression in Abcg8−/− HS platelets (Figure 4C). Interestingly, filamin appeared to be selectively degraded in the Abcg8−/− HS platelet population that had become refractory to agonist stimulation (Figure 4D). The major plasma membrane–binding partner for filamin in platelets, GPIbα,28,29 is also highly susceptible to proteolysis following platelet activation30 which results in release of a soluble extracellular domain fragment known as glycocalicin.31 Because of the role of the GPIb/filamin complex in platelet adhesion following vascular injury and the known susceptibility of both proteins to activation-dependent proteolysis,32-34 we further examined the tendency of GPIbα to become cleaved in platelets from Abcg8-deficient mice fed an HS diet. As shown in Figure 4E, a significant fraction of GPIbα was shed from the surface of Abcg8−/− HS, but not Abcg8−/− LS, platelets. Finally, confocal microscopy revealed that both the GPIb complex and filamin (Figure 4F) were redistributed from the cell periphery to the cytosol in platelets of Abcg8−/− mice fed an HS, but not LS, diet. Taken together, these data demonstrate that, in addition to proteolysis of cytoskeletal elements, the sitosterolemic condition affects 2 major GP complexes—GPIb and αIIbβ3—that function prominently in platelet adhesion and cohesion.
Consequences of αIIbβ3 internalization and GPIbα shedding for thrombus formation
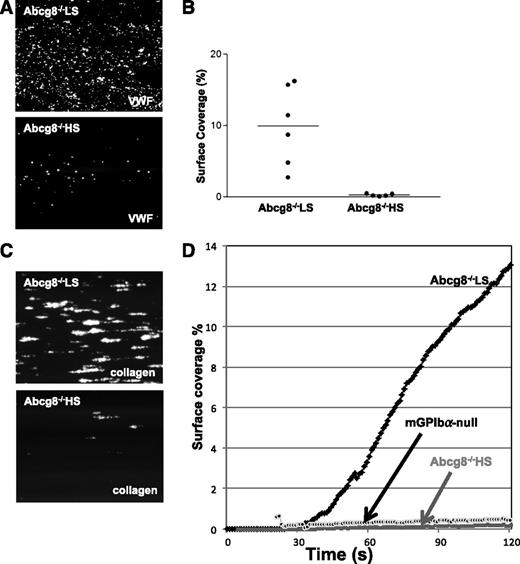
To examine the functional consequences of adhesion receptor shedding and internalization on platelet function, blood from Abcg8−/− HS and Abcg8−/− LS mice was perfused over immobilized recombinant mouse VWF or type I collagen at a shear rate of 2000 s−1. As shown in Figure 5A-B, adhesion of Abcg8−/− HS platelets (lower panel) was markedly suppressed compared with that of Abcg8−/− LS (upper panel) or wild-type (not shown) platelets. Similar results were obtained when blood was perfused over type I collagen (Figure 5C-D). Furthermore, although Abcg8−/− LS platelets formed stable thrombi on type I collagen, only unstable aggregates were observed with Abcg8−/− HS platelets, resulting in impaired thrombus formation (see supplemental Videos 1 and 2 on the Blood website). This difference could not be attributed to the low platelet count in Abcg8−/− HS mice because thrombus formation still occurred, albeit somewhat reduced, in platelet-reduced C57BL6 wild-type blood that had been prepared by diluting platelet-rich plasma with platelet-poor plasma and mixing in erythrocytes (data not shown). Taken together, these data expand upon previous laboratory findings of defective ristocetin-induced platelet agglutination in sitosterolemic individuals11 and provide a likely mechanistic explanation for their bleeding phenotype.
Impaired adhesion and thrombus formation of Abcg8−/− HS platelets. (A-B) PPACK-anticoagulated whole blood from Abcg8−/− fed a low or high sterol diet was labeled and perfused on mouse VWF immobilized surface at a shear rate of 2000 s−1. Representative images of adherent platelets (A); percent surface coverage for 11 independent experiments (n = 6 for Abcg8−/− LS and n = 5 for Abcg8−/− HS) (B). (C-D) Pooled blood samples of Abcg8−/− LS (n = 4), Abcg8−/− HS (n = 4), and mouse GPIbα-null platelets were perfused over type I collagen for 120 seconds at 2000 s−1. (C) Representative image of adhesion and thrombus formation at 120 seconds (D) quantitates the time-course of surface coverage (%) as they accumulate in the field of view. Platelet counts and hematocrit of these pooled Abcg8−/− LS, Abcg8−/− HS, and control mGPIbα-null samples were 498 × 109/L, 38.2%; 76 × 109/L, 31.3%; and 194 × 109/L, 31.3%, respectively.
Impaired adhesion and thrombus formation of Abcg8−/− HS platelets. (A-B) PPACK-anticoagulated whole blood from Abcg8−/− fed a low or high sterol diet was labeled and perfused on mouse VWF immobilized surface at a shear rate of 2000 s−1. Representative images of adherent platelets (A); percent surface coverage for 11 independent experiments (n = 6 for Abcg8−/− LS and n = 5 for Abcg8−/− HS) (B). (C-D) Pooled blood samples of Abcg8−/− LS (n = 4), Abcg8−/− HS (n = 4), and mouse GPIbα-null platelets were perfused over type I collagen for 120 seconds at 2000 s−1. (C) Representative image of adhesion and thrombus formation at 120 seconds (D) quantitates the time-course of surface coverage (%) as they accumulate in the field of view. Platelet counts and hematocrit of these pooled Abcg8−/− LS, Abcg8−/− HS, and control mGPIbα-null samples were 498 × 109/L, 38.2%; 76 × 109/L, 31.3%; and 194 × 109/L, 31.3%, respectively.
GPIbα shedding correlates with plasma phytosterol levels in human sitosterolemia patients
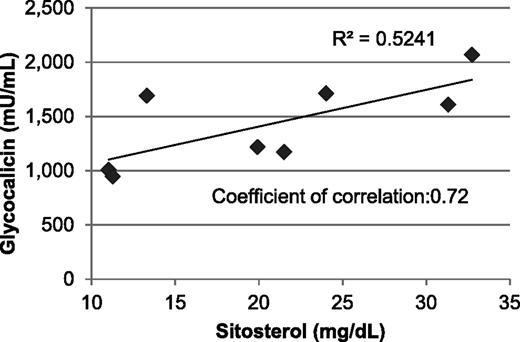
Sitosterolemia is a very rare disease, and nearly all accessible patients take ezetimibe—an inhibitor of intestinal phytosterol absorption15 —to treat their condition, making corroboration in humans of the data obtained in Abcg5- and Abcg8-deficient mice extremely difficult. Moreover, it is nearly impossible to obtain fresh blood samples from geographically disbursed patients for flow cytometric analysis of GPIbα expression on macrothrombocytopenic platelets. We were, however, able to obtain frozen plasma samples from 8 sitosterolemia patients (described in Lu et al7 ) and compare soluble glycocalicin (the shed extracellular domain of GPIbα)35 levels with that of family members who were heterozygous for the disorder. Unfortunately, the difference in glycocalicin in sitosterolemic individuals (1429 ± 399 mU/mL, n = 8), relative to that of obligate heterozygotes (1104 ± 252 mU/mL, n = 9), did not reach statistical significance, likely because of the limited number of available samples. As shown in Figure 6, however, the level of glycocalicin present in the plasma of sitosterolemia patients did show a significant positive correlation with plasma sitosterol levels (correlation coefficient 0.72), but not plasma cholesterol levels (correlation coefficient 0.45), consistent with a specific effect of elevated plant sterol levels and GPIbα shedding.
Correlation between circulating plasma glycocalicin and sitosterol levels. Plasma glycocalicin in sitosterolemia patients was analyzed using a sandwich enzyme-linked immunosorbent assay that employs 2 different mAbs against human GPIbα. The Scientific and Standardization Committee/International Society on Thrombosis and Haemostasis Secondary Coagulation Standard was assigned as 1 U/mL and used to construct a standard curve.
Correlation between circulating plasma glycocalicin and sitosterol levels. Plasma glycocalicin in sitosterolemia patients was analyzed using a sandwich enzyme-linked immunosorbent assay that employs 2 different mAbs against human GPIbα. The Scientific and Standardization Committee/International Society on Thrombosis and Haemostasis Secondary Coagulation Standard was assigned as 1 U/mL and used to construct a standard curve.
Megakaryocytopoiesis in Abcg8−/− mice
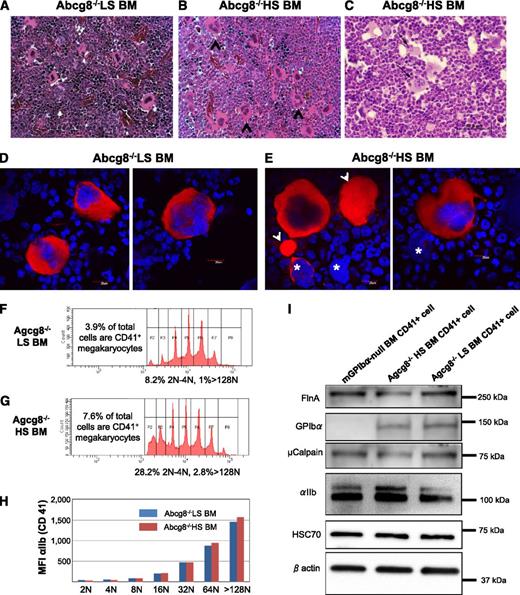
To analyze the effects of plant sterol accumulation on megakaryocytopoiesis, bone marrow (BM) from Abcg8−/− mice fed either an LS or HS diet were harvested for histologic analysis. As shown in Figure 7A-B, large mature megakaryocytes were easily recognized, and some of these megakaryocytes exhibited multifocal emperipolesis of neutrophils (arrows in Figure 7C), suggesting enhanced thrombocytogenetic activity.36 The absolute number of high ploidy megakaryocytes in the BM from Abcg8−/− mice fed an HS diet (67 ± 18/mm2, n = 4) was about 2 times higher than that from Abcg8−/− mice fed an LS diet (28 ± 10/mm2, n = 4).
Analyses of Abcg8−/− HS and Abcg8−/− LS megakaryocytes. (A-C) BM from Abcg8−/− LS (A) and Abcg8−/− HS mice (B-C) was harvested for histologic analysis. The number of multinucleated mature megakaryocytes (black arrowheads) was visually identified and counted in hematoxylin and eosin–stained sections. (C, black arrows) Emperipolesis of neutrophils into megakaryocytes. (D-E) BM megakaryocytes isolated by discontinuous gradient centrifugation over bovine serum albumin were stained with anti-αIIb mAb (red) and 4,6 diamidino-2-phenylindole (blue). Large αIIb-positive cytoplasmic extrusions (arrowhead) and “bare” megakaryocyte nuclei (asterisks) were noted in the BM samples from Abcg8−/− HS (E) but not in Abcg8−/− LS mice. (F-H) DNA ploidy profiles and integrin αIIb expression in BM megakaryocytes isolated using anti-CD41 magnetic beads and cultured in the presence of thrombopoietin for 60 hours. An HS diet resulted in a greater total number of CD41+ cells (7.6% vs 3.9%), with a corresponding proportional increase in both the high (>128N) and low (2N-4N) ploidy cells (F-G). Expression of integrin αIIb was similar in Abcg8−/− HS and Abcg8−/− LS cells regardless of ploidy (H). (I) Western blot analysis of purified, CD41-positive megakaryocytes. Expression of filamin A (FlnA), GPIbα, and µ-calpain in megakaryocytes derived from Abcg8−/− HS mice was slightly decreased compared with that of Abcg8−/− LS mice. No significant differences were observed in the expression of HSC70 and β actin.
Analyses of Abcg8−/− HS and Abcg8−/− LS megakaryocytes. (A-C) BM from Abcg8−/− LS (A) and Abcg8−/− HS mice (B-C) was harvested for histologic analysis. The number of multinucleated mature megakaryocytes (black arrowheads) was visually identified and counted in hematoxylin and eosin–stained sections. (C, black arrows) Emperipolesis of neutrophils into megakaryocytes. (D-E) BM megakaryocytes isolated by discontinuous gradient centrifugation over bovine serum albumin were stained with anti-αIIb mAb (red) and 4,6 diamidino-2-phenylindole (blue). Large αIIb-positive cytoplasmic extrusions (arrowhead) and “bare” megakaryocyte nuclei (asterisks) were noted in the BM samples from Abcg8−/− HS (E) but not in Abcg8−/− LS mice. (F-H) DNA ploidy profiles and integrin αIIb expression in BM megakaryocytes isolated using anti-CD41 magnetic beads and cultured in the presence of thrombopoietin for 60 hours. An HS diet resulted in a greater total number of CD41+ cells (7.6% vs 3.9%), with a corresponding proportional increase in both the high (>128N) and low (2N-4N) ploidy cells (F-G). Expression of integrin αIIb was similar in Abcg8−/− HS and Abcg8−/− LS cells regardless of ploidy (H). (I) Western blot analysis of purified, CD41-positive megakaryocytes. Expression of filamin A (FlnA), GPIbα, and µ-calpain in megakaryocytes derived from Abcg8−/− HS mice was slightly decreased compared with that of Abcg8−/− LS mice. No significant differences were observed in the expression of HSC70 and β actin.
Because mature megakaryocytes derived from Abcg8−/− HS are fragile, they were isolated using anti-CD41 magnetic beads and cultured in vitro for 60 hours in the presence of mouse thrombopoietin before performing ploidy analysis. As shown in Figure 7F-G, the percentage of high ploidy megakaryocytes (ploidy > 128N) as well as immature megakaryocytes (ploidy 2N and 4N) were increased in Abcg8−/− mice fed an HS diet. This result was compatible with the results obtained from visual scanning of megakaryocytes in the BM tissues. Surface expression of integrin αIIb in each ploidy population was similar between Abcg8−/− mice fed an HS and LS diet (Figure 7H). These purified, CD41-positive, BM megakaryocytes were also analyzed by western blotting. Slightly decreased expression of filamin A, GPIbα, and µ-calpain was observed in megakaryoctes derived from Abcg8−/− mice fed an HS diet compared with those fed an LS diet, suggestive of mild activation. Collectively, these data suggest that megakaryocytes of Abcg8−/− mice fed an HS diet differentiate normally, but are prone to be activated in the process of maturation and platelet production.
Dose-dependent effect of plant sterol accumulation on hematopoiesis
Abcg5−/− and Abcg8−/− mice fed an HS diet exhibit progressive weight loss and shortened life span (not shown). trac/trac mice also have a shortened life span that is at least partially attributable to fibrotic cardiomyopathy.9 The effect of plant sterol accumulation on organ histology in Abcg8−/− female mice having moderate (34 mg/dL) vs high (64 mg/dL) phytosterol plasma levels was also determined. As shown in supplemental Figure 2A-B, the number of mature megakaryocytes was increased in the BM of Abcg8−/− HS-fed mice. Interestingly, extramedullary hematopoiesis was observed in the spleen, liver, and lungs of HS-fed mice (supplemental Figure 2D,F,H), suggesting a dose-dependent effect of plant sterol accumulation on hematopoiesis. BM embolism in the pulmonary vessels (supplemental Figure 2H) and myocardial degeneration with multifocal histiocytic infiltration (supplemental Figure 2J) was also observed in Abcg8−/− mice fed an HS diet.
Discussion
The involvement of the ABC transporter genes ABCG5 and ABCG8 in removing plant sterols from the body originated from mapping studies of genes responsible for the rare genetic disorder, sitosterolemia,5-8 and mice missing Abcg5 or Abcg8, either by design14 or through a mistake in nature9 have existed for a number of years. In the present study, we exploited murine knockout models of sitosterolemia to develop a mechanistic understanding of how excessive plasma levels of plant sterols might exert their effects on platelet structure and function. By carefully controlling exposure to dietary plant sterols in mice genetically deficient in Abcg5 or Abcg8, we were able to induce dramatic, reversible differences in platelet physiology in mice exposed to low vs high levels of dietary phytosterols. Abcg5−/− or Abcg8−/− mice fed an HS diet developed macrothrombocytopenia and hemolysis that closely mimicked human sitosterolemia. Previous studies have documented the accumulation of phytosterols in red blood cells and its effects on membrane rigidity and flexibility that may lead to hemolytic anemia.37 However, enrichment of phytosterols in platelets and the mechanism how phytosterols affect platelet size, number, and function have not been described. In this study, we demonstrate accumulation of plant sterols in the membranes of Abcg5−/− HS and Abcg8−/− HS mouse platelets, the resulting hyperactivatable status of which may explain the mechanism of the platelet phenotype in sitosterolemia.
The increased fibrinogen binding observed in Abcg8−/− HS platelets (Figure 3), perhaps as a result of phytosterol-induced receptor clustering, induces internalization of this major integrin receptor, with obvious platelet passivating effects. Ligand binding to αIIbβ3, in turn, is known to initiate a variety of signal transduction pathways that induce calcium flux and shedding of platelet-derived microparticles.38 Microparticles are pro-inflammatory and may contribute to low-grade inflammation involved in atheroma development,39 whereas cytosolic calcium activates, among other things, the calcium-activated protease calpain. Calpain has numerous targets in the cell, but relevant to this discussion, acts on metalloproteinases to initiate ectodomain cleavage of GPIbα40 and degradation of filamin A34 —both characteristics found in Abcg8−/− HS platelets (Figure 4). Reduction of filamin A content is particularly informative because this abundant cytosolic scaffolding protein has been implicated in stabilizing trafficking of GPIbα to the platelet surface.41 We have previously shown that the cytoplasmic domain of GPIbα, and coordinated expression of GPIbα with filamins that enables their trafficking to the plasma membrane, are required for normal size platelet production.29 Filamin A also plays an important role in positioning the tyrosine kinase, Syk, near the plasma membrane, where it acts to propagate signals downstream of platelet activation by collagen.42 Thus, activation-induced filamin A degradation and cytosolic redistribution likely contribute to production of giant platelets in murine sitosterolemia, although it remains possible that accumulation of plant sterols somehow inhibits synthesis or stability of filamin A. Taken together, these effects explain the macrothrombocytopenia (Figure 1), loss of ristocetin-induced agglutination,11 and impaired thrombus formation (Figure 5) that characterize sitosterolemia. A scheme summarizing the likely events leading to the sitosterolemic phenotype is shown in supplemental Figure 4.
Previous studies observed an increased number of low, but not high, ploidy megakaryocytes in Abcg5−/− and trac/trac mice.9,14 In our study, the percentage of both immature (2N and 4N) and high ploidy megakaryocytes (ploidy > 128N) was increased (Figure 7G-H). One explanation for this discrepancy may be differences in the severity of sitosterolemia achieved. In the previous 2 studies, Abcg5−/− mice or trac/trac mice were fed a standard diet, whereas our Abcg5−/− and Abcg8−/− mice were fed a custom-defined diet enriched with plant sterols (1% w/w) for 6-12 weeks before analysis. Thus, differences in ploidy distribution observed might be attributable to the degree of plant sterol accumulation in BM megakaryocytes, thereby affecting the severity of the sitosterolemic condition. Differences in the isolation efficiency of relatively fragile high-ploidy megakaryocytes derived from Abcg8−/− HS mice may have also contributed to the discrepancy between the 2 studies.
The bleeding abnormality that some sitosterolemic humans11 and mice9 experience, other than the obvious consequence of being thrombocytopenic, is likely from a number of factors. First, xenosterols, including phytosterols, inhibit cholesterol uptake in the intestine,40 and the effects of relative cholesterol deficiency on platelet reactivity are incompletely understood. Second, although phytosterols, which differ from cholesterol in only a single additional alkyl or alkenyl side chain at position 2443 are incorporated less efficiently than is cholesterol into membranes,44 the downstream consequences having plasma phytosterols absorbed into megakaryocyte and platelet membranes (Figure 2A) are not known. Disturbance of sterol trafficking in other mouse models have been demonstrated to affect platelet phenotypes; mice deficient in scavenger receptor class B type I, a cell-surface receptor for high-density lipoprotein, exhibit macrothrombocytopenia, anemia, infertility, and an increased susceptibility to atherosclerosis.45 In contrast, a high level of cholesterol accumulation, present in apolipoprotein E or low-density lipoprotein receptor-deficient mice do not result in macrothrombocytopenia.45 In vitro loading of cholesterol increases platelet sensitivity to activation by weak agonists such as adenosine 5′-diphosphate and epinephrine22 —a potentiating property that is nearly the opposite of that exhibited by sitosterolemic platelets.11 It is also possible that phytosterols accumulate and depolarize the mitochondrial inner membrane, resulting in phosphatidylserine exposure and microparticle generation. Using MitoTracker Red CMXRos as an indicator dye,46 however, we found that the mitochondrial membrane potential (ΔΨm) simply correlated with platelet size and was not significantly reduced in sitosterolemic versus GPIb-deficient platelets (supplemental Figure 3). Clues into the cell biological basis for the differing effects of intercalating various sterols into cell membranes might be found in an classic study by Clejan and colleagues, who found that phytosterols become preferentially translocated into the outer leaflet of the lipid bilayer, whereas cholesterol becomes rapidly distributed into both leaflets.47 Whether and how the properties of these closely related sterols affect the architecture of ion channels, adhesion receptors, and underlying signaling molecules is an unsolved and potentially fruitful area of future investigation.
The observation of phytosterol-induced activation leading to platelet passivation and bleeding in sitosterolemia is just the latest piece of evidence that, contrary to common belief, platelet hyperreactivity, rather than leading to thrombosis often manifests itself as a bleeding phenotype. Thus, a constitutively active form of the GPIb VWF receptor is responsible for platelet-type von Willebrand disease,48 whereas an activating mutation in the αIIbβ3 receptor that supports binding to platelets of fibrinogen, fibronectin, and VWF leads to a Glanzmann thrombasthenia–like bleeding abnormality.49 Future studies aimed at identifying the mechanism by which accumulation of free sterols within membranes of platelets elicits platelet passivating effects while also accelerating the development of atherosclerosis may provide important clues into the diverse effects that dietary lipids exert on thrombosis, hemostasis, and vascular biology.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cai Yanhong and Brian Stogsdill (Medical College of Wisconsin) for technical support, Dr David Wilcox (Medical College of Wisconsin) and Dr Susan Maroney (Blood Research Institute) for helpful advice for the experiments, and Dr Raymond G. Hoffmann (Medical College of Wisconsin) for help with statistical analysis.
This work was supported by a grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL-44612) (P.J.N.), a Postdoctoral Fellowship Award from the Midwest Affiliate of the American Heart Association (10POST261016) (S.K.), and by the Biomedical Laboratory Research and Development Program, Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (S.B.P.).
Authorship
Contribution: T.K. and S.K. designed and performed experiments, interpreted data, and wrote the manuscript; R.R.M. contributed vital reagents and reviewed the manuscript; S.B.P. helped in the design of the project, provided the knockout mice, contributed patient samples and data, and reviewed the manuscript; and P.J.N. supervised experiments, provided research support, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Taisuke Kanaji, Blood Research Institute, BloodCenter of Wisconsin, 8733 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: taisuke.kanaji@bcw.edu; and Shailendra B. Patel, Medical College of Wisconsin, 9200 West Wisconsin Ave, Milwaukee, WI 53226; e-mail: sbpatel@mcw.edu.
References
Author notes
T.K. and S.K. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal