Key Points
CD49d, a negative prognosticator with a key role for microenvironmental interactions in CLL, is near universally expressed in trisomy 12 CLL.
CD49d overexpression in trisomy 12 CLL is regulated by a methylation-dependent mechanism.
Abstract
CD49d is a negative prognosticator in chronic lymphocytic leukemia (CLL), expressed by ∼40% of CLL cases and associated with aggressive, accelerated clinical courses. In this study, analyzing CD49d expression in a wide CLL cohort (n = 1200) belonging to different cytogenetic groups, we report that trisomy 12 CLL almost universally expressed CD49d and were characterized by the highest CD49d expression levels among all CD49d+ CLL. Through bisulfite genomic sequencing, we demonstrated that, although CD49d+/trisomy 12 CLL almost completely lacked methylation of the CD49d gene, CD49d–/no trisomy 12 CLL were overall methylated, the methylation levels correlating inversely to CD49d expression (P = .0001). Consistently, CD49d expression was recovered in CD49d– hypermethylated CLL cells upon in vitro treatment with the hypomethylating agent 5-aza-2′-deoxycytidine. This may help explain the clinicobiological features of trisomy 12 CLL, including the high rates of cell proliferation and disease progression, lymph node involvement, and predisposition to Richter syndrome transformation.
Introduction
CD49d recently emerged as a negative prognosticator in chronic lymphocytic leukemia (CLL), marking a subset of ∼30% to 40% of CLL characterized by a more aggressive clinical course.1,2 CD49d, the α4 subunit of the α4β1 integrin heterodimer, has a role in CLL cell migration and retention in lymph node (LN) and bone marrow (BM) microenvironments, where they receive growth- and survival-supporting signals.3
Among the recurrent chromosomal abnormalities detectable in CLL,4 trisomy 12 marks a disease subset (∼15% of cases) characterized by high rates of cell proliferation and disease progression, and Richter syndrome (RS) transformation.5,6 Despite the recent demonstration of a relative enrichment in NOTCH1 mutations in CLL carrying trisomy 12,7-9 no specific genetic and/or biological features have so far been identified in trisomy 12 CLL to explain the peculiar clinical behavior of this CLL subset.10,11
Patients and methods
This study, approved by the Internal Review Boards of the Centro di Riferimento Oncologico of Aviano (Approval n. IRB-05-2010) and Salzburg Medical University (Approval n. 415-E/1287/4-2011), included peripheral blood samples from 1200 patients with typical CLL according to the current guidelines.12 Informed consent was obtained from the participants in accordance with the Declaration of Helsinki. CLL was characterized for the main cytogenetic abnormalities, flow cytometry–based prognosticators, IGHV mutations (all cases), and NOTCH1 mutations (944/1200 cases), as described.1,13 Treatment-free-survival (TFS) and RS transformation data were available for 601 and 324 cases, respectively.
Procedures for cell sorting, quantitative real-time polymerase chain reaction (qRT-PCR), bisulfite sequencing, and in vitro 5-aza-2′-deoxycytidine (DAC) experiments are reported in the supplemental Methods. All studies were performed on highly purified (>95%) CLL cells.14
Results and discussion
CD49d is expressed by a vast majority of trisomy 12 CLL
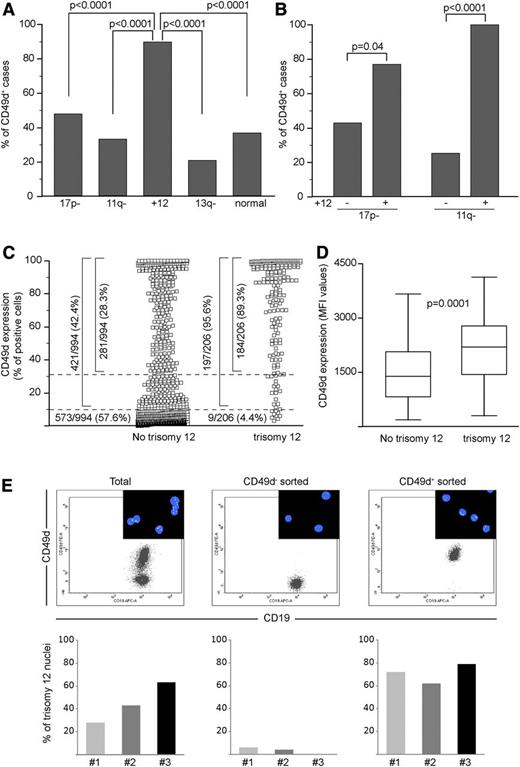
CD49d expression was investigated by flow cytometry in the neoplastic component of 1200 CLL patients. Using the 30% cutoff,1 735 cases (61%) were classified as CD49d–, whereas 465 (39%) were CD49d+. Analysis within the major cytogenetic groups4 showed a significantly higher percentage of CD49d+ cases (89.4%) in the trisomy 12 group compared with the other cytogenetic groups (P < .0001 for all pairwise comparisons, supplemental Table 1 and Figure 1A). This also held true by comparing the percentages of CD49d+ cases in CLL bearing or not bearing trisomy 12 in the context of the del17p13.1 and del11q22-q23 cytogenetic groups (Figure 1B).4 Altogether, in trisomy 12 CLL, 89% (184/206) of cases expressed CD49d in >30% of CLL cells; 6% (13/206) in 10% to 27% of cells, mostly with a bimodal pattern (not shown); and 4% (9/206) in <10% of cells (Figure 1C). In the context of trisomy 12 CLL, no differences were found between CD49d+ and CD49d– cases for the main cytogenetic abnormalities, as well as for IGHV or NOTCH1 mutations (data not shown), but, as expected,7-9 NOTCH1 mutations were significantly over-represented (P < .0001; supplemental Table 1).
CD49d is almost universally expressed by trisomy 12 CLL. The percent of CD49d+ cases among 1200 CLL split according to the cytogenetic groups defined by Döhner et al4 (A) and in the context of the 17p- and 11q- groups, split according to the presence or not of trisomy 12 (B). 17p-, 17p13.1 deletion; 11q-, 11q22-q23 deletion; +12, trisomy 12; 13q-, 13q14.3 deletion; normal, none of the above. All P values refer to the χ2 test. (C) CD49d expression in CLL cases split into 2 groups according to the presence of trisomy 12. Dotted lines were set at 30% and 10% cutoffs, and the number and percentages of cases expressing different CD49d levels are reported. (D) CD49d MFI values in CD49d+ CLL bearing or not bearing trisomy 12. Boxes represent the interquartile range (25-75%), with the middle line indicating the median and the horizontal lines indicating the minimum and maximum values. (E) FISH analysis in the total and in the CD49d– and CD49d+ sorted components from 3 CLL cases characterized by CD49d bimodal expression. The upper panels represent dot plots of CD19 vs CD49d expression from 1 representative case (case 2). The insets show representative fields of FISH analysis performed with an α satellite DNA probe CEP12, directly labeled with SpectrumGreen to detect aneuploidy of chromosome 12. Histograms represent the percent of trisomy 12 nuclei in the total CD5+CD19+ (left), CD5+CD19+CD49d– (middle), and the CD5+CD19+CD49d+ (right) sorted components from 3 CLL cases.
CD49d is almost universally expressed by trisomy 12 CLL. The percent of CD49d+ cases among 1200 CLL split according to the cytogenetic groups defined by Döhner et al4 (A) and in the context of the 17p- and 11q- groups, split according to the presence or not of trisomy 12 (B). 17p-, 17p13.1 deletion; 11q-, 11q22-q23 deletion; +12, trisomy 12; 13q-, 13q14.3 deletion; normal, none of the above. All P values refer to the χ2 test. (C) CD49d expression in CLL cases split into 2 groups according to the presence of trisomy 12. Dotted lines were set at 30% and 10% cutoffs, and the number and percentages of cases expressing different CD49d levels are reported. (D) CD49d MFI values in CD49d+ CLL bearing or not bearing trisomy 12. Boxes represent the interquartile range (25-75%), with the middle line indicating the median and the horizontal lines indicating the minimum and maximum values. (E) FISH analysis in the total and in the CD49d– and CD49d+ sorted components from 3 CLL cases characterized by CD49d bimodal expression. The upper panels represent dot plots of CD19 vs CD49d expression from 1 representative case (case 2). The insets show representative fields of FISH analysis performed with an α satellite DNA probe CEP12, directly labeled with SpectrumGreen to detect aneuploidy of chromosome 12. Histograms represent the percent of trisomy 12 nuclei in the total CD5+CD19+ (left), CD5+CD19+CD49d– (middle), and the CD5+CD19+CD49d+ (right) sorted components from 3 CLL cases.
Among CD49d+ CLL, trisomy 12 CLL expressed CD49d at higher mean fluorescence intensity (MFI) levels (median MFI = 2200; 95% confidence interval [CI], 1810-2546; n = 54) compared with no trisomy 12 CLL (median MFI = 1386; 95% CI, 1050-1673; n = 55; P = .0001; Figure 1D).
Fluorescence in situ hybridization (FISH) analysis of flow cytometry–sorted CD49d– and CD49d+ subpopulations in the context of CLL with bimodal CD49d expression (n = 3) documented that trisomy 12 was restricted to the CD49d+ fraction and was virtually absent in CD49d– cells (Figure 1E).
Overall, these data indicate the almost universal expression of CD49d in trisomy 12 CLL, corroborating the notion that this chromosomal aberration marks a CLL entity with distinct clinicobiological features.10,15
The impact of CD49d and trisomy 12 as TFS predictors and of CD49d in RS transformation are reported in the supplemental Results and supplemental Figures 1-3.
CD49d overexpression in trisomy 12 CLL is associated with ITGA4 hypomethylation
As investigated by qRT-PCR in 74 CLL (31 CD49d–/no trisomy 12 and 43 CD49d+/trisomy 12), CD49d mRNA expression positively correlated with CD49d protein levels (r = 0.6, P < .0001, supplemental Figure 4), implying a transcriptional regulation of CD49d protein expression. Therefore, we decided to study whether DNA hypomethylation, a well-known epigenetic mechanism regulating gene transcription in tumors including CLL,16 could explain the CD49d overexpression found in trisomy 12 CLL.
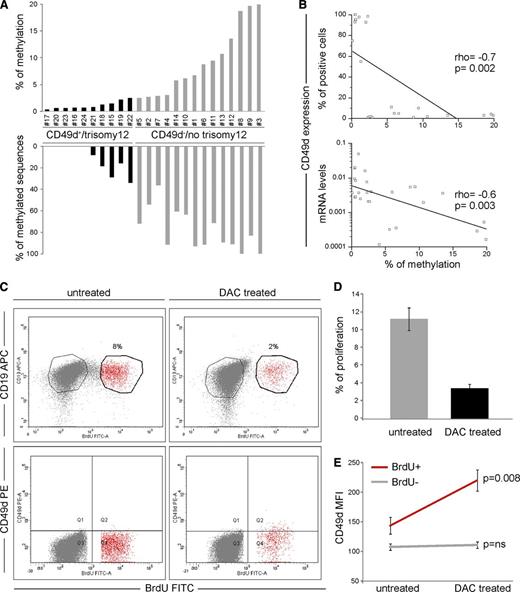
DNA methylation was studied within a 5′-UTR CpG island (77 CpGs) of the CD49d gene (ITGA4) (supplemental Figure 5 and supplemental Methods). CD49d–/no trisomy 12 CLL (n = 14) showed a higher degree of ITGA4 methylation compared with CD49d+/trisomy 12 cases (n = 10), which almost completely lacked methylated CpGs (average amount of methylated CpGs = 9.2% vs 0.9%, P < .0001, Figure 2A). Moreover, the number of methylated clones in at least 2 CpG sites was higher in CD49d–/no trisomy 12 (mean, 78%; range, 36-100%) than in CD49d+/trisomy 12 CLL cases (mean, 10%; range, 0-33%; P < .0001; Figure 2A).
CD49d expression is correlated with DNA methylation levels. (A) DNA methylation was studied within the CpG island of the ITGA4 gene (reported in supplemental Figure 5). The upper graph reports the percentage of methylation calculated as the number of methylated CpG over the total number of CpGs. The lower graph reports the percentage of sequences with at least 2 methylated CpGs. At least 10 clones for each of 10 CD49d+/trisomy 12 (black histograms) and 14 CD49d–/no trisomy 12 (gray histograms) CLL cases were analyzed. (B) Correlation between the percentage of methylation and CD49d protein expression, as detected by flow cytometry (upper panel) and mRNA as detected by qRT-PCR (lower panel). ρ and P values refer to the Spearman’s rank correlation test. (C-E) Primary CLL cells from 7 cases were cultured for 96 hours using CpG-ODN/interleukin-2 in the presence or not of 5 μmol/L DAC and analyzed by flow cytometry for the surface expression of CD49d. (C) Dot plots of CD19-APC vs BrdU-FITC (upper panels) and CD49d-PE vs BrdU-FITC (lower panels) in untreated and DAC-treated CLL cells from a representative case (case 13). The proliferative/BrdU+ and nonproliferative/BrdU– fractions are noted in red and gray, respectively. (D) Proliferation levels (mean percent ± SEM) of untreated (gray bar) and DAC-treated (black bar) CLL cells after 96 hours in culture with CpG-ODN/interleukin-2. (E) CD49d MFI (mean expression ± SEM) by proliferative/BrdU+ (red line) and nonproliferative/BrdU– (gray line) fractions in untreated and DAC-treated CLL cells; P values refer to the Student t test.
CD49d expression is correlated with DNA methylation levels. (A) DNA methylation was studied within the CpG island of the ITGA4 gene (reported in supplemental Figure 5). The upper graph reports the percentage of methylation calculated as the number of methylated CpG over the total number of CpGs. The lower graph reports the percentage of sequences with at least 2 methylated CpGs. At least 10 clones for each of 10 CD49d+/trisomy 12 (black histograms) and 14 CD49d–/no trisomy 12 (gray histograms) CLL cases were analyzed. (B) Correlation between the percentage of methylation and CD49d protein expression, as detected by flow cytometry (upper panel) and mRNA as detected by qRT-PCR (lower panel). ρ and P values refer to the Spearman’s rank correlation test. (C-E) Primary CLL cells from 7 cases were cultured for 96 hours using CpG-ODN/interleukin-2 in the presence or not of 5 μmol/L DAC and analyzed by flow cytometry for the surface expression of CD49d. (C) Dot plots of CD19-APC vs BrdU-FITC (upper panels) and CD49d-PE vs BrdU-FITC (lower panels) in untreated and DAC-treated CLL cells from a representative case (case 13). The proliferative/BrdU+ and nonproliferative/BrdU– fractions are noted in red and gray, respectively. (D) Proliferation levels (mean percent ± SEM) of untreated (gray bar) and DAC-treated (black bar) CLL cells after 96 hours in culture with CpG-ODN/interleukin-2. (E) CD49d MFI (mean expression ± SEM) by proliferative/BrdU+ (red line) and nonproliferative/BrdU– (gray line) fractions in untreated and DAC-treated CLL cells; P values refer to the Student t test.
Although ITGA4 methylation in the CD49d+/trisomy 12 CLL group was homogeneously low, 4/14 CD49d–/no trisomy 12 cases displayed <3% methylation. These cases, and 4 CD49d+/trisomy 12 cases for comparison, were therefore analyzed for methylation levels within an additional ITGA4 CpG island (44 CpGs), spanning exons 1 and 2 (supplemental Figure 5). Despite the overall lower methylation levels characterizing this CpG island, CD49d– CLL displayed higher percentages of methylated CpG than CD49d+ cases (average amount of methylated CpG = 4.0% vs 0.5%, P < .0001).
Similar levels of ITGA4 hypomethylation were found in 10 CD49d+ CLL with a normal karyotype (data not shown).
To further corroborate the direct role of DNA methylation in regulating CD49d expression, a significant inverse correlation was observed between the percentage of methylated CpGs and CD49d expression at both mRNA (r = –0.6, P = .003) and protein levels (r = –0.7, P = .002; Figure 2B).
Hypomethylating agents are known regulators of gene expression. Among them, DAC operates by inhibiting DNA methyltransferase activity, thus preventing methylation of newly replicated DNA, leading to DNA demethylation and subsequent gene activation.17 According to this notion, highly purified CLL cells from 7 CD49d– cases (supplemental Table 2) were exposed to DAC in the presence of CpG-ODN/interleukin-2 as proliferative stimulus.18,19 Although DAC-treated CLL cells displayed lower levels of proliferation compared with untreated cells (mean % proliferation levels = 11.2 ± 1.3 vs 3.4 ± 0.5, respectively; Figure 2C-D), the proliferative fraction of DAC-treated CLL cells significantly upregulated CD49d protein levels (mean MFI, 220 ± 18 vs 144 ± 14; P = .008; Figure 2E). Consistently, analysis of ITGA4 methylation in these DAC-treated proliferating cells (n = 2) highlighted lower levels of methylation (% methylated CpGs = 4.0 and 5.3) compared with proliferating cells of untreated cultures (% methylated CpGs = 12.3 and 10.0).
In CLL, the close dependency of CD49d expression on DNA methylation, as demonstrated here also outside the trisomy 12 subset, was similar to that reported for other CLL bad prognosticators and key regulators of CLL cells, such as ZAP-70, lipoprotein lipase, CLLU1, and NOTCH1.19-21
Although a functional relation between trisomy 12 and hypomethylation of the CD49d gene remains to be established, preliminary gene expression profiling data generated by us suggest that trisomy 12 CLL cells differentially express genes involved in the regulation of methyltransferase activity and chromatin modification processes (A.Z., unpublished observation).
The role of trisomy 12 in the pathogenesis of CLL has always been elusive. Previous reports showed that the proportion of trisomy 12 CLL cells is higher in LN than in peripheral blood or BM, thus reflecting a specific tropism toward LN of trisomy 12 CLL cells.22 The overexpression of CD49d may provide the molecular basis to explain the peculiar biological behavior of trisomy 12 CLL and may predict for the development of additional cytogenetic lesions, as reported.23
In light of a recent demonstration that the use of Bruton tyrosine kinase inhibitors in CLL is able to impair the integrin-mediated retention of CLL cells in the LN and BM microenvironments,24 trisomy 12 CLL may represent a CLL subset that can particularly benefit of Bruton tyrosine kinase inhibitor treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Ministero della Salute Ricerca Finalizzata I.R.C.C.S., Progetto Giovani Ricercatori (GR-2010-2317594, GR-2009-1475467, GR-2008-1138053), Rome, Italy; Fondazione Internazionale di Ricerca in Medicina Sperimentale (FIRMS); Associazione Italiana contro le Leucemie, linfomi e mielomi (AIL), Venezia Section; Pramaggiore Group, Italy; Ricerca Scientifica Applicata, Regione Friuli Venezia Giulia (“Linfonet” Project), Trieste, Italy; Associazione Italiana Ricerca Cancro (AIRC; IG-13227, MFAG-10327), Milan, Italy; and “5x1000 Intramural Program,” Centro di Riferimento Oncologico, Aviano, Italy.
Authorship
Contribution: A.Z. and V.G. designed the study, coordinated the experiments, and wrote the manuscript; C.C., D.B., E.T., F.M.R., E.H., F.P., R.B., M.D.B., and T.N.H. performed the experiments and contributed to characterize samples and to data analysis; G.D.A., F.Z., G.P., F.D.R., G.D.P, D.R., and G.G. provided well-characterized biological samples and clinical data; and all authors commented and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonella Zucchetto, PhD, Clinical and Experimental Onco-Hematology Unit, Centro di Riferimento Oncologico, I.R.C.C.S., Via Franco Gallini 2, Aviano (PN), Italy; e-mail: zucchetto.soecs@cro.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal