Key Points
Nbeal2−/− mice are a model of human GPS, characterized by macrothrombocytopenia and α-granule-deficient platelets.
NBEAL2 is required for normal platelet function and megakaryocyte development.
Abstract
Gray platelet syndrome (GPS) is an inherited bleeding disorder associated with macrothrombocytopenia and α-granule-deficient platelets. GPS has been linked to loss of function mutations in NEABL2 (neurobeachin-like 2), and we describe here a murine GPS model, the Nbeal2−/− mouse. As in GPS, Nbeal2−/− mice exhibit splenomegaly, macrothrombocytopenia, and a deficiency of platelet α-granules and their cargo, including von Willebrand factor (VWF), thrombospondin-1, and platelet factor 4. The platelet α-granule membrane protein P-selectin is expressed at 48% of wild-type levels and externalized upon platelet activation. The presence of P-selectin and normal levels of VPS33B and VPS16B in Nbeal2−/− platelets suggests that NBEAL2 acts independently of VPS33B/VPS16B at a later stage of α-granule biogenesis. Impaired Nbeal2−/− platelet function was shown by flow cytometry, platelet aggregometry, bleeding assays, and intravital imaging of laser-induced arterial thrombus formation. Microscopic analysis detected marked abnormalities in Nbeal2−/− bone marrow megakaryocytes, which when cultured showed delayed maturation, decreased survival, decreased ploidy, and developmental abnormalities, including abnormal extracellular distribution of VWF. Our results confirm that α-granule secretion plays a significant role in platelet function, and they also indicate that abnormal α-granule formation in Nbeal2−/− mice has deleterious effects on megakaryocyte survival, development, and platelet production.
Introduction
As bone marrow megakaryocytes (MKs) develop, they undergo increases in ploidy, nuclear lobulation, and cytoplasmic mass prior to the emergence of the demarcation membrane system. Their development culminates in the formation of proplatelets, microtubule-containing cell extensions1,2 that protrude through the vascular endothelium and are released into the bloodstream as nascent platelets.3 Mature platelets contain abundant stores of secretory vesicles that include dense (δ-) granules, lysosomes, and α-granules (50-80 per platelet), which carry endogenously synthesized or endocytosed cargo4,5 and arise from budding vesicles in the MK trans-Golgi network that mature into multivesicular bodies and nascent α-granules5,6 that are transported into proplatelets.7
Insights into platelet granule formation and function have come from investigating patients and mutant mice with secretory granule deficiencies. Studies of conditions associated with a lack of δ-granules—Hermansky-Pudlak syndrome and Chediak-Higashi syndrome (MIM214500)—have implicated several vesicular trafficking molecules in δ-granule formation.8-11 Studies of arthrogryposis, renal dysfunction, and cholestasis syndrome, in which platelets lack α-granules, have shown 2 proteins to be required for α-granule development: VPS33B and its binding partner VPS16B.12,13 Recently, we and others have identified loss of function mutation of NBEAL2 (neurobeachin-like 2) as the cause of gray platelet syndrome (GPS; MIM139090), an autosomal recessive bleeding disorder characterized by macrothrombocytopenia and gray-appearing platelets with decreased/absent α-granules14-18 and α-granule proteins.19,20 Patients with GPS have moderate bleeding symptoms, splenomegaly, and progressive bone marrow fibrosis characterized by abnormal reticulin deposition in the bone marrow.16,19
Although studies using mouse models of δ-granule deficiency have shown the importance of platelet δ-granule secretion during in vivo thrombus formation,21 the importance of isolated α-granule secretion has not been investigated because of lack of an appropriate model. Here we report the results of studies using the Nbeal2−/− mouse as a model of GPS. In comparison with wild-type (WT), Nbeal2−/− mice show splenomegaly, low platelet counts, and a profound deficiency of platelet α-granules and their soluble cargo proteins. Expression of the α-granule membrane protein P-selectin was decreased in Nbeal2−/− platelets, but it was observed to mobilize to the surface of activated cells. Nbeal2−/− platelets showed decreased response to agonists in vitro, whereas in vivo assays showed increased bleeding in Nbeal2−/− mice and impaired platelet thrombus formation in response to laser-induced arterial injury. These results confirm the importance of α-granule secretion for platelet function. Studies of bone marrow and cultured MKs also revealed significant abnormalities in Nbeal2−/− mice, providing the first indication that impaired α-granule formation due to the absence of NBEAL2 can have major consequences for MK development and platelet production.
Methods
Animals
Nbeal2−/− mice were generated from cryopreserved spermatozoa obtained from the Mutant Mouse Regional Resource Center at the University of California, Davis, from strain B6;129S5-Nbeal2tm1Lex/Mmucd, in which exons 4 to 11 from the 54-exon Nbeal2 gene were deleted by homologous recombination. Spermatozoa were used for in vitro fertilization of WT C57BL/6J mouse oocytes to generate embryos that were transferred into pseudopregnant C57BL/6J females by the Toronto Centre for Phenogenomics (TCP) to generate heterozygous litters, which were crossed to generate homozygous Nbeal2−/− mice. Sequencing of the targeted deletion region (exons 4-11 of Nbeal2) of ear punch samples was done via polymerase chain reaction using 1 5′ primer (5′-GTCCTGCTTGACCTACCGTC-3′) and 2 3′ primers (5′-CAGGGAGGATAACGAGATAGTCTT-3′ and 5′-CCTAGGAATGCTCGTCAAGA-3′). Two polymerase chain reaction reactions with the same 5′ primer and 1 of each 3′ primer results in predicted 223 bp + no product for WT and no product + 401 bp for the homozygous deletion, respectively. These confirmed Nbeal2+/+, Nbeal2+/−, or Nbeal2−/− status. Age- and sex-matched WT mice were C57BL/6J, obtained from the TCP. This study was approved by the TCP Animal Use Protocols (AUP #0215-H).
Platelet enumeration, bone marrow and spleen analysis, bright field and electron microscopy, immunoblotting, platelet preparations for immunofluorescence microscopy, megakaryocyte culture, immunostaining, high-resolution confocal laser immunofluorescence microscopy, in vitro assessments of platelet function, tail bleeding assay, intravital analysis of platelet accumulation, and activation after laser-induced injury to cremaster muscle arterioles
The experimental details are described in the supplemental Methods section and supplemental Figures, found on the Blood Website.
Results
Mice
Nbeal2−/− mice were fertile, born with expected Mendelian frequency, and did not reveal morphological, viability, or behavioral abnormalities. Comparison of spleen weights with age-matched (∼4 months old) WT (C57BL/6J) mice revealed significant splenomegaly (P = .0001, Mann-Whitney 2-tailed test) in Nbeal2−/− mice (WT: 0.09 g, standard deviation [SD] 0.02, n = 13; Nbeal2−/−: 0.16 g, SD 0.05, n = 13). Hematoxylin/eosin stained Nbeal2−/− spleens contained significantly more MKs (WT spleen slice: 67, SD 19, n = 3; Nbeal2−/− spleen slice: 330, SD 12, n = 3; P < .0001, 2-tailed t test). There was no increased reticulin staining in Nbeal2−/− spleens. Bone marrow morphology assessed by hematoxylin/eosin and reticulin staining was indistinguishable from WT (supplemental Figure 1), confirming the absence of myelofibrosis.
Platelet number and morphology
Automated blood analysis showed mean platelet counts for Nbeal2−/− mice lower than those for WT (WT: 848 × 109/L, SD 202, n = 3; Nbeal2−/−: 519 × 109/L, SD 55, n = 3), whereas mean platelet volume was greater (Nbeal2−/− 6.69 fL, SD 0.35, n = 3, vs WT: 5.10 fL, SD 0.85, n = 3). On blood films platelets from WT (Figure 1A) and Nbeal2+/−, heterozygous mice (Figure 1B) show typical punctate staining, whereas Nbeal2−/− platelets appear pale (Figure 1C). These observations are consistent with GPS.
Blood film and ultrastructure abnormalities of Nbeal2−/− mouse platelets. Blood films were prepared from WT (A), Nbeal2+/− (B), and Nbeal2−/− (C) mice and stained with Wright-Giemsa stain prior to light microscopy. WT (A) and Nbeal2+/− (B) blood films show typical platelet (black arrowheads) size and morphology with discernible granulation against light cytoplasmic staining, and the Nbeal2−/− film (C) shows gray-appearing platelets with indistinct granulation and visible vacuoles. Thin-section transmission electron micrographs of representative platelets are from WT mice (D) and Nbeal2−/− mice (E). Multiple α-granules (white arrowheads) were evident in WT platelets and absent in Nbeal2−/− platelets, which are larger on average. Magnification ×40 000; scale bars represent 500 nm.
Blood film and ultrastructure abnormalities of Nbeal2−/− mouse platelets. Blood films were prepared from WT (A), Nbeal2+/− (B), and Nbeal2−/− (C) mice and stained with Wright-Giemsa stain prior to light microscopy. WT (A) and Nbeal2+/− (B) blood films show typical platelet (black arrowheads) size and morphology with discernible granulation against light cytoplasmic staining, and the Nbeal2−/− film (C) shows gray-appearing platelets with indistinct granulation and visible vacuoles. Thin-section transmission electron micrographs of representative platelets are from WT mice (D) and Nbeal2−/− mice (E). Multiple α-granules (white arrowheads) were evident in WT platelets and absent in Nbeal2−/− platelets, which are larger on average. Magnification ×40 000; scale bars represent 500 nm.
Platelet ultrastructure was examined via thin-section transmission electron microscopy (TEM), in which Nbeal2−/− α-granule deficiency was clearly evident (Figure 1D-E; supplemental Figure 2A). Morphometric analysis12,22 revealed a virtual absence of α-granules in Nbeal2−/− platelets, with an average of 0.25 α-granules per platelet thin section in comparison with 4.8 in WT (Nbeal2−/− n = 100 platelets; WT n = 25; human platelets have approximately 5.5 α-granules per platelet thin section12 ). Whole-mount electron microscopy revealed a dense granule count of 2.6 per platelet in Nbeal2−/− mice in comparison with 4.9 per platelet in WT mice (n = 50 platelets).
Platelet proteins
Platelet lysates were examined for protein content via immunoblot analysis; equivalent sample loading was achieved by loading lysates from similar numbers of platelets, measuring total protein concentrations and probing for actin or glyceraldehyde-3-phosphate dehydrogenase. We observed (Figure 2) that the MK-synthesized α-granule cargo proteins VWF, platelet factor 4, and thrombospondin-1 were greatly reduced or absent in Nbeal2−/− platelet lysates (Figure 2A-C). Normal levels of endothelium-derived VWF were present in Nbeal2−/− mouse plasma (Figure 2E). Reduced amounts of plasma-derived fibrinogen were present in Nbeal2−/− platelets, whereas plasma levels were normal (Figure 2D,F). The α-granule membrane protein P-selectin is present at approximately 48% of WT levels (Figure 2G; 48.4, SD 5.3, n = 3 determinations), indicating that despite lacking mature granules and their soluble cargo, Nbeal2−/− platelets appear to contain the membrane constituents of α-granules. This contrasts with the effects of loss-of-function mutations in VPS33B and VPS16B, which result in human platelets lacking both α-granule cargo proteins and membrane constituents including P-selectin, suggesting that VPS33B/VPS16B are required for the formation of precursor α-granules.12,13 VPS33B and VPS16B are both present in approximately equivalent amounts in Nbeal2−/− and WT platelets (Figure 2H-I), suggesting that NBEAL2 affects a later stage of α-granule biogenesis than do these proteins.
Protein content in Nbeal2−/− mouse platelets and plasma. Immunoblots comparing platelet (PLT) whole-cell lysates or plasma from WT and Nbeal2−/− mice. MK-derived thrombospondin-1 (TSP1; A), platelet factor 4 (PF4; B), and von Willebrand factor (VWF; C) were undetectable or significantly reduced in Nbeal2−/− in comparison with WT platelet lysates, and plasma VWF (E) and fibrinogen (F) levels were normal. (D) Plasma-derived fibrinogen (Fgn) was present in decreased amounts in Nbeal2−/− platelets. (G) P-selectin in Nbeal2−/− platelets was present at approximately 48% of WT levels. VPS33B (H) and VPS16B (I) were present at similar levels in WT and Nbeal2−/− platelets. Lysate from equivalent numbers of platelets was loaded in each lane, and protein loading is indicated by probing for actin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH). See the “Methods” section for antibody details.
Protein content in Nbeal2−/− mouse platelets and plasma. Immunoblots comparing platelet (PLT) whole-cell lysates or plasma from WT and Nbeal2−/− mice. MK-derived thrombospondin-1 (TSP1; A), platelet factor 4 (PF4; B), and von Willebrand factor (VWF; C) were undetectable or significantly reduced in Nbeal2−/− in comparison with WT platelet lysates, and plasma VWF (E) and fibrinogen (F) levels were normal. (D) Plasma-derived fibrinogen (Fgn) was present in decreased amounts in Nbeal2−/− platelets. (G) P-selectin in Nbeal2−/− platelets was present at approximately 48% of WT levels. VPS33B (H) and VPS16B (I) were present at similar levels in WT and Nbeal2−/− platelets. Lysate from equivalent numbers of platelets was loaded in each lane, and protein loading is indicated by probing for actin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH). See the “Methods” section for antibody details.
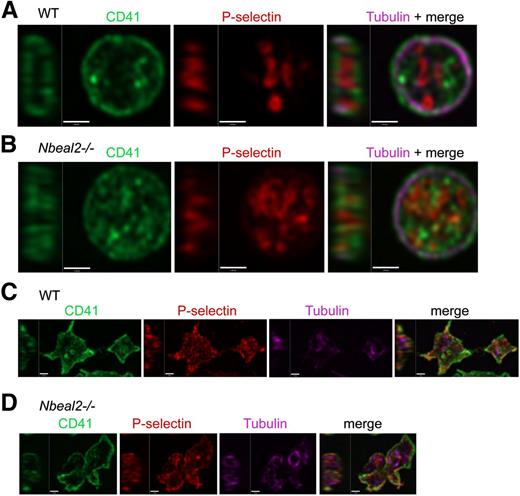
The intracellular distribution of platelet proteins was examined via high-resolution immunofluorescence confocal microscopy. Staining for α-granule cargo proteins was negative in Nbeal2−/− platelets (not shown), which showed normal distributions (Figure 3A-B) of surface membrane CD41/αIIb and circumferential ring cytoskeletal α-tubulin. Consistent with blood cell analysis, morphometric measurements of the long axis of microtubule rings showed Nbeal2−/− platelets to be significantly larger than WT (mean values for WT: 2.91 µm, SD 0.69, n = 50 platelets; for Nbeal2−/−: 3.21 µm, SD 0.57, n = 50 platelets; P < .01, Mann-Whitney 2-tailed test). The distribution of P-selectin within resting Nbeal2−/− platelets appeared less well defined than in WT cells (Figure 3A-B, middle panels), and both showed similar patterns of shape change and surface mobilization of P-selectin after thrombin activation (Figure 3C-D). The amount and distribution of the membrane protein LAMP1 did not differ between resting Nbeal2−/− and WT platelets (supplemental Figure 2B-C), indicating their lysosomal/late endosomal compartments are similar.
P-selectin in resting and thrombin-activated Nbeal2−/− platelets. High-resolution confocal laser immunofluorescence microscopy imaging of intracellular P-selectin membrane proteins in fixed resting platelets. Permeabilized cells were stained for α-tubulin (violet), P-selectin (red), and CD41/integrin αIIb (green) and imaged (final magnification = ×150, Z stepping = 250 nm). Single-channel and merged mid-cell ZY/XY slices of representative individual platelets are shown (A-B). Resting platelets from WT (A) and Nbeal2−/− (B) mice have similar flat, discoid morphology with a well-defined circumferential tubulin ring cytoskeleton. Both contain P-selectin, which defines compact looping structures of the α-granule secretory matrix typical of WT platelets that generally appear to be less orderly in Nbeal2−/− platelets. Thrombin activated WT (C) and Nbeal2−/− (D) platelets show characteristic activation-triggered changes in shape and surface mobilization of P-selectin.
P-selectin in resting and thrombin-activated Nbeal2−/− platelets. High-resolution confocal laser immunofluorescence microscopy imaging of intracellular P-selectin membrane proteins in fixed resting platelets. Permeabilized cells were stained for α-tubulin (violet), P-selectin (red), and CD41/integrin αIIb (green) and imaged (final magnification = ×150, Z stepping = 250 nm). Single-channel and merged mid-cell ZY/XY slices of representative individual platelets are shown (A-B). Resting platelets from WT (A) and Nbeal2−/− (B) mice have similar flat, discoid morphology with a well-defined circumferential tubulin ring cytoskeleton. Both contain P-selectin, which defines compact looping structures of the α-granule secretory matrix typical of WT platelets that generally appear to be less orderly in Nbeal2−/− platelets. Thrombin activated WT (C) and Nbeal2−/− (D) platelets show characteristic activation-triggered changes in shape and surface mobilization of P-selectin.
Platelet function in vitro
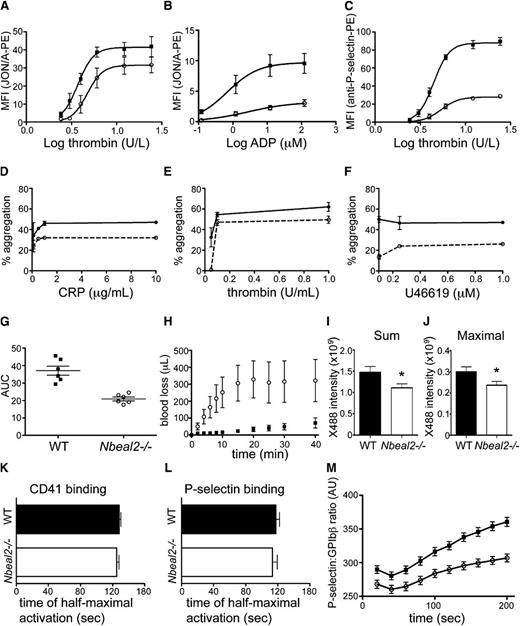
Flow cytometry was used to measure platelet activation via surface exposure of activated αIIbβ3 (detected via binding of JON/A-PE antibody; Figure 4A-B) and P-selectin (detected by binding of anti-P-selectin-PE antibody; Figure 4C). In both assays, platelets from Nbeal2−/− mice had impaired activation induced by thrombin in relation to WT (Figure 4A,C), and their αIIbβ3 activation response to adenosine 5′-diphosphate (ADP) was also weaker (Figure 4B). Nbeal2−/− maximal P-selectin exposure at high thrombin concentrations was one third that of WT (Figure 4C); thus although Nbeal2−/− platelets have half the P-selectin of WT (Figure 2G), its externalization appears to be impaired. The response of washed platelets to agonists was measured via light transmission aggregometry (Figure 4D-F), where activation by thrombin, collagen-related peptide (CRP), and the thromboxane-prostanoid receptor agonist U46619 produced weaker aggregation in Nbeal2−/− platelets at all concentrations. At 0.05 U/mL thrombin, Nbeal2−/− platelets showed no aggregation; addition of 200 µg/mL of human fibrinogen yielded 19% (SD 7.1) aggregation, in comparison with 35.7% (SD 8.5) for WT (not shown). Impedance aggregometry in the Multiplate analyzer (Roche, Basel, Switzerland) was used to measure platelet response to collagen in citrated whole blood, where platelets from Nbeal2−/− mice had significantly reduced (P = .04) area-under-the-curve aggregation in comparison with WT (Figure 4G). Lumiaggregometry was used to assess dense granule adenosine triphosphate (ATP) release. For thrombin (1 U), peak ATP release was 4.88 (SD 0.81) nmoles for WT and 4.07 (SD 0.30) for Nbeal2−/− platelets; for U46619 (2 µM) the values were 3.75 (SD 0.28) and 1.52 (SD 0.20) nmoles, respectively. Taken together, these results indicate that Nbeal2−/− platelets have impaired responses to activating stimuli.
Impaired Nbeal2−/− mouse platelet function in vitro and in vivo. Measurements of activation of platelets from WT (solid symbols) and Nbeal2−/− mice (open symbols). (A-C) Washed platelets were assessed by flow cytometry for activation in response to varying concentrations of agonists (X-axis) by measuring antibody binding (JON/A) to activated αIIbβ3 (A-B) or exposed P-selectin (C). Graphs show mean ± standard error of the mean (SEM) (n = 6 mice per group) for mean fluorescence intensity (MFI) after activation by thrombin (A,C) or ADP (B). (D-F) Optical aggregometry in physiological buffer of washed platelets exposed to varying concentrations of CRP (D), thrombin (E), or the thromboxane analog U46619 (F). The agonist concentration is plotted against the percentage of aggregation (see the “Methods” section). (G) Impedance aggregometry measurement of platelets in citrated blood exposed to collagen (7 μg/mL, n = 6 mice per group); horizontal lines represent mean ± SEM for area under the curve of Multiplate aggregation. (H) Nbeal2−/− mice showed greater cumulative blood loss in relation to WT in a tail transection bleeding assay; mean ± SEM is shown for WT (solid squares) and Nbeal2−/− mice (open circles; n = 11 for each). (I-M) Results of intravital videomicroscopy monitoring of platelet accumulation and activation in thrombi formed in response to laser injury of cremaster muscle arterioles. (I-J) Sum and maximal platelet accumulation in thrombi formed in response to injury were determined using a fluorescent anti-mouse GPIbβ antibody (×488); values shown are mean ± SEM for 170 thrombi in 12 WT mice and 155 thrombi in 12 Nbeal2−/− mice (*P < .05, unpaired t test). (K-L) The time to half-maximal activation ratio for CD41 was determined using an anti-mouse CD41 Fab fragment (K) and for P-selectin (L) using an anti-mouse P-selectin antibody. Values shown are mean ± SEM; for CD41, n = 119 thrombi in 6 WT mice and 93 thrombi in 6 Nbeal2−/− mice; for P-selectin, n = 69 thrombi in 6 WT mice and 60 thrombi in 6 Nbeal2−/− mice. (M) The time course of P-selectin activation after injury. Mean ± SEM for WT mice (solid squares) and Nbeal2−/− mice (open circles) are shown.
Impaired Nbeal2−/− mouse platelet function in vitro and in vivo. Measurements of activation of platelets from WT (solid symbols) and Nbeal2−/− mice (open symbols). (A-C) Washed platelets were assessed by flow cytometry for activation in response to varying concentrations of agonists (X-axis) by measuring antibody binding (JON/A) to activated αIIbβ3 (A-B) or exposed P-selectin (C). Graphs show mean ± standard error of the mean (SEM) (n = 6 mice per group) for mean fluorescence intensity (MFI) after activation by thrombin (A,C) or ADP (B). (D-F) Optical aggregometry in physiological buffer of washed platelets exposed to varying concentrations of CRP (D), thrombin (E), or the thromboxane analog U46619 (F). The agonist concentration is plotted against the percentage of aggregation (see the “Methods” section). (G) Impedance aggregometry measurement of platelets in citrated blood exposed to collagen (7 μg/mL, n = 6 mice per group); horizontal lines represent mean ± SEM for area under the curve of Multiplate aggregation. (H) Nbeal2−/− mice showed greater cumulative blood loss in relation to WT in a tail transection bleeding assay; mean ± SEM is shown for WT (solid squares) and Nbeal2−/− mice (open circles; n = 11 for each). (I-M) Results of intravital videomicroscopy monitoring of platelet accumulation and activation in thrombi formed in response to laser injury of cremaster muscle arterioles. (I-J) Sum and maximal platelet accumulation in thrombi formed in response to injury were determined using a fluorescent anti-mouse GPIbβ antibody (×488); values shown are mean ± SEM for 170 thrombi in 12 WT mice and 155 thrombi in 12 Nbeal2−/− mice (*P < .05, unpaired t test). (K-L) The time to half-maximal activation ratio for CD41 was determined using an anti-mouse CD41 Fab fragment (K) and for P-selectin (L) using an anti-mouse P-selectin antibody. Values shown are mean ± SEM; for CD41, n = 119 thrombi in 6 WT mice and 93 thrombi in 6 Nbeal2−/− mice; for P-selectin, n = 69 thrombi in 6 WT mice and 60 thrombi in 6 Nbeal2−/− mice. (M) The time course of P-selectin activation after injury. Mean ± SEM for WT mice (solid squares) and Nbeal2−/− mice (open circles) are shown.
Bleeding phenotype
In a tail transection assay (Figure 4H), Nbeal2−/− mice showed increased rate of blood loss in comparison with WT and a 5-fold higher cumulative blood loss after 40 minutes (Nbeal2−/− 322 ± 125 µL vs WT 71 ± 30 µL, n = 11 for both, P = .03, unpaired t test). These results point to a hemostatic deficiency in Nbeal2−/− mice.
Platelet function in vivo
Platelet accumulation in laser-injured cremaster arterioles was assessed using established methods. Platelet accumulation in thrombi (measured by Dylight-tagged anti-GPIbβ, X488), expressed as sum of accumulation 3 minutes after injury and maximal accumulation, was lower in Nbeal2−/− mice (Figure 4I, P = .03; Figure 4J, P = .04) in comparison with WT. These results confirm that Nbeal2−/− mice have impaired platelet accumulation in thrombi in vivo. As a measure of platelet activation at the site of injury, WT and Nbeal2−/− mice had similar time to half-maximal activation ratio of CD41 and of P-selectin (Figure 4K-L). However, further inspection of the P-selectin activation reveals the maximal anti-P-selectin to anti-GPIbβ ratio is lower in Nbeal2−/− mice than in WT mice (Figure 4M). Thus, although platelet accumulation in thrombi that form at sites of laser injury in Nbeal2−/− mice is impaired, the rate of activation of the platelets within the thrombi is not different from that in WT. The amount of P-selectin that Nbeal2−/− platelets can express in thrombi appears to be limited. These results show that the platelet activation time in thrombi is not altered in Nbeal2−/− mice, but their maximal P-selectin expression is impaired.
Abnormalities in megakaryocyte structure and development
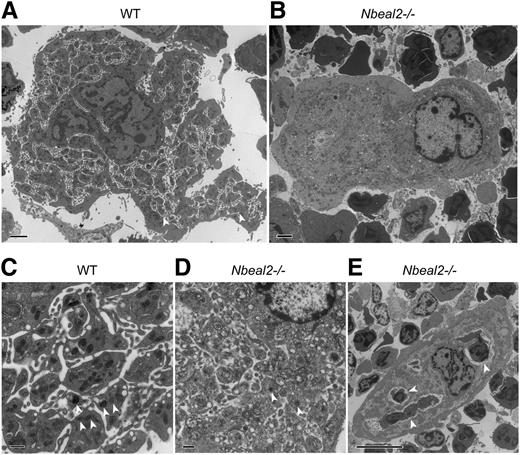
The α-granule deficiency observed in platelets was also noted in bone marrow MKs from Nbeal2−/− mice analyzed by TEM (Figure 5). Morphologically distinct α-granules were numerous in WT MKs (Figure 5A,C, n = 50 MK) and greatly reduced in Nbeal2−/− MKs (Figure 5B,D, n = 50). WT MKs also contain numerous “platelet territories”23 (Figure 5A,C) that were clearly deficient in Nbeal2−/− MKs (Figure 5B,D), where emperipolesis (the presence of an intact cell within the cytoplasm of another cell) was observed much more frequently (Figure 5E).
Abnormal ultrastructure of Nbeal2−/− bone marrow megakaryocytes. (A) Thin-section transmission electron micrographs of representative WT MKs show typical platelet territories (white arrowheads) and platelet-like structures containing α-granules. (B) In contrast, Nbeal2−/− MK are deficient in both α-granules and platelet territories (magnification ×4000, scale bars = 2 µm). (C) Higher-magnification (×15 000) images of WT MK reveal multiple α-granules (white arrowheads) and platelet territories, whereas (D) α-granules (white arrowheads) are rare in Nbeal2−/− MK, which show poorly defined platelet territories (scale bars = 500 nm). (E) Example of the emperipolesis that was frequently observed in Nbeal2−/− MK; here, 3 exogenous cells (white arrowheads) are present in the MK (magnification ×3000, scale bar = 10 µm).
Abnormal ultrastructure of Nbeal2−/− bone marrow megakaryocytes. (A) Thin-section transmission electron micrographs of representative WT MKs show typical platelet territories (white arrowheads) and platelet-like structures containing α-granules. (B) In contrast, Nbeal2−/− MK are deficient in both α-granules and platelet territories (magnification ×4000, scale bars = 2 µm). (C) Higher-magnification (×15 000) images of WT MK reveal multiple α-granules (white arrowheads) and platelet territories, whereas (D) α-granules (white arrowheads) are rare in Nbeal2−/− MK, which show poorly defined platelet territories (scale bars = 500 nm). (E) Example of the emperipolesis that was frequently observed in Nbeal2−/− MK; here, 3 exogenous cells (white arrowheads) are present in the MK (magnification ×3000, scale bar = 10 µm).
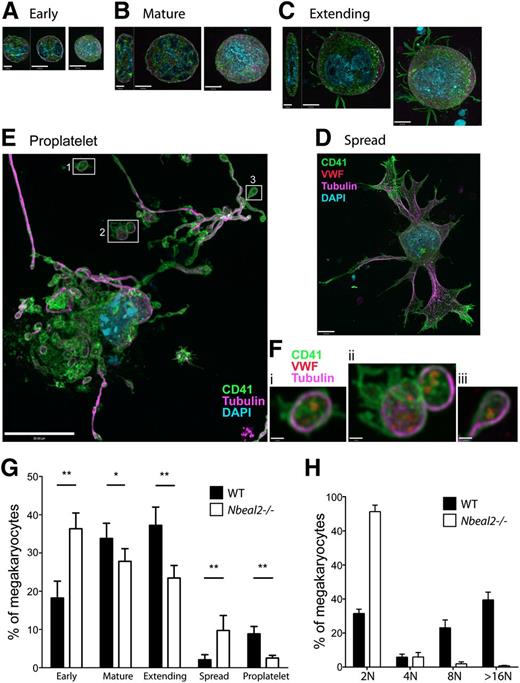
Our observations of ultrastructural abnormalities in marrow resident Nbeal2−/− MKs point to developmental abnormalities that may contribute to the lower platelet counts observed in these mice. We investigated this possibility by culturing MKs from primary bone marrow cells in the presence of thrombopoietin. Initial counts showed similar proportions of CD41+ cells among all cells recovered from marrow (WT: 0.228% ±0.094; Nbeal2−/−: 0.231% ± 0.051; n = 3 mice and 7500 cells for each). Examination of bone marrow MKs revealed reduced ploidy (16N, 32N) in Nbeal2−/− in comparison with WT (n = 3, not shown). MKs were observed to undergo differentiation leading to terminal proplatelet and platelet formation after 5 days (Figure 6), and though cells in this final stage of development appeared similar, comparisons of all stages (Figure 6A-F; supplemental Figure 3) detected by CD41 expression, size, and nuclear morphology within MK populations indicated developmental abnormalities. The relatively higher proportion of Nbeal2−/− MKs found in the earliest stages (Figure 6G) indicates that their development is delayed in relation to WT, whereas the high proportion of Nbeal2−/− cells that appeared to be stalled in the spread phase (Figure 6D)—which we presume precedes terminal proplatelet development (Figure 6E)—indicates that later stages may also be affected. Ploidy analysis of 5-day MK cultures revealed increased 2N but reduced 8N and >16N Nbeal2−/− MKs in comparison with WT (Figure 6H). In addition, we observed that although the proportion of MK-lineage cells present in 5-day WT cultures was 14.0% ±0.62, consistent with other reports,24 the proportion present in Nbeal2−/− cultures was significantly lower at 7.67% ±0.12 (P < .01, 2-tailed t test; n = 3 cultures and 900 cells for each). This indicates that Nbeal2−/− MKs may be overall less viable than are WT.
Abnormal Nbeal2−/− megakaryocyte development in populations of cultured bone marrow cells. Immunofluorescence images of megakaryocytes cultured to the terminal proplatelet stage. (A-E) Cells present in fixed 5-day cultures (n = 5 mice and 600 cells for both Nbeal2−/− and WT) were identified by immunofluorescence microscopy as MKs by size (>10 µm diameter), large/lobulated nuclei (light blue), and expression of lineage-specific CD41 (green) and VWF (red). Individual cells were classified by apparent developmental stage as (A) early, <20 µm in diameter (ZY sections show these cells to be spheroidal); (B) mature, >20 µm without projections (ZY sections show flattening with increased size); (C) extending, round cells with membrane projections; (D) spread, large cells with extensive membrane and tubulin (violet) projections but not showing clearly defined proplatelets; and (E) terminal proplatelets emanate from these very large megakaryocytes. (E) A distinctive pattern of nuclear retraction (light blue) and elaboration of nascent platelets defined by extensions of membrane (visualized by CD41, green) and cytoskeletal α-tubulin strands and loops (violet). (F) Higher-magnification views of nascent-free platelets (i,ii) and a proplatelet bud (iii) from the WT cell having the distinctive platelet tubulin cytoskeletal ring and the presence of VWF (red, not shown; E), which is absent in Nbeal2−/− platelets (Figure 2C). Panels A-C show left to right YZ (bars = 5 µm) and XY confocal midcell sections and extended focus images (bars = 10 µm); panels D,E show extended focus images (D scale bar = 10 µm; E scale bar = 20 µm; F scale bars = 1 µm). (G) Population distributions of mean proportions of cells in each stage showed significant differences between Nbeal2−/− and WT (*P < .05; **P < .01, 2-tailed t test), with Nbeal2−/− cultures showing a markedly higher proportion of cells in both the early and spread stages and fewer in the mature, extending, and proplatelet stages. (H) Distribution of Nbeal2−/− and WT CD41+ MK cells and ploidy (n = 3 per group) after 5-day cultured cells, revealing increased 2N and greatly reduced 8N and >16N Nbeal2−/− MKs.
Abnormal Nbeal2−/− megakaryocyte development in populations of cultured bone marrow cells. Immunofluorescence images of megakaryocytes cultured to the terminal proplatelet stage. (A-E) Cells present in fixed 5-day cultures (n = 5 mice and 600 cells for both Nbeal2−/− and WT) were identified by immunofluorescence microscopy as MKs by size (>10 µm diameter), large/lobulated nuclei (light blue), and expression of lineage-specific CD41 (green) and VWF (red). Individual cells were classified by apparent developmental stage as (A) early, <20 µm in diameter (ZY sections show these cells to be spheroidal); (B) mature, >20 µm without projections (ZY sections show flattening with increased size); (C) extending, round cells with membrane projections; (D) spread, large cells with extensive membrane and tubulin (violet) projections but not showing clearly defined proplatelets; and (E) terminal proplatelets emanate from these very large megakaryocytes. (E) A distinctive pattern of nuclear retraction (light blue) and elaboration of nascent platelets defined by extensions of membrane (visualized by CD41, green) and cytoskeletal α-tubulin strands and loops (violet). (F) Higher-magnification views of nascent-free platelets (i,ii) and a proplatelet bud (iii) from the WT cell having the distinctive platelet tubulin cytoskeletal ring and the presence of VWF (red, not shown; E), which is absent in Nbeal2−/− platelets (Figure 2C). Panels A-C show left to right YZ (bars = 5 µm) and XY confocal midcell sections and extended focus images (bars = 10 µm); panels D,E show extended focus images (D scale bar = 10 µm; E scale bar = 20 µm; F scale bars = 1 µm). (G) Population distributions of mean proportions of cells in each stage showed significant differences between Nbeal2−/− and WT (*P < .05; **P < .01, 2-tailed t test), with Nbeal2−/− cultures showing a markedly higher proportion of cells in both the early and spread stages and fewer in the mature, extending, and proplatelet stages. (H) Distribution of Nbeal2−/− and WT CD41+ MK cells and ploidy (n = 3 per group) after 5-day cultured cells, revealing increased 2N and greatly reduced 8N and >16N Nbeal2−/− MKs.
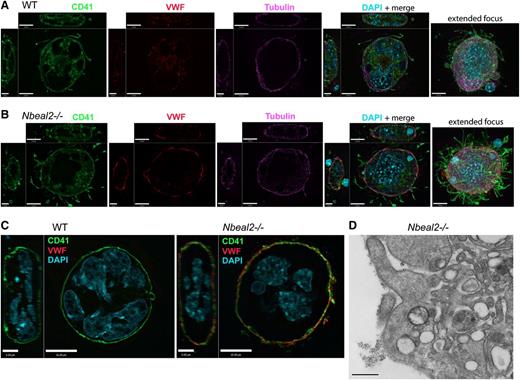
Developmental differences between WT and Nbeal2−/− MKs were explored in detail by examining expression of VWF, a large protein normally packaged in distinct regions of α-granules.25 We observed that although VWF is present in early Nbeal2−/− and WT MKs, by the mature stages there is a marked difference in intracellular VWF distribution (Figure 7; supplemental Figure 3A-B). In permeabilized WT MKs, VWF is distributed throughout the nascent platelet territories delineated by CD41 (Figure 7A), and in Nbeal2−/− MKs, VWF is concentrated at the cell periphery (Figure 7B). This pattern of VWF distribution was confirmed by examining unpermeabilized MKs (Figure 7C), where the absence of VWF staining in WT cells is contrasted by the strong colocalization of VWF and CD41 at the outer membrane of mature Nbeal2−/− MKs. In some cases we also saw evidence that VWF may accumulate in inclusions or be released from Nbeal2−/− cells. The abnormal presence of VWF outside of Nbeal2−/− MKs was also observed via TEM of pre-embed immunogold labeled sections (Figure 7D).
Abnormal VWF distribution in Nbeal2−/− megakaryocytes. (A) Immunofluorescence imaging of a representative permeabilized mature extending stage WT megakaryocyte shows elaboration of demarcation membranes stained with CD41 (green), dispersed VWF expression (red), and a peripheral tubulin cytoskeletal meshwork (violet). (B) A representative Nbeal2−/− MK at the same stage shows a strong peripheral distribution of VWF, which is absent in Nbeal2−/− platelets; this MK also contains the nucleus of an exogenous cell in its cytoplasm visible in the XZ view of the merged confocal panel. Comparisons of nonpermeabilized cells (C) confirm that VWF is abnormally concentrated near or on the surface of mature Nbeal2−/− megakaryocytes (scale bars in ZY panels = 5 µm; scale bars in XY, XZ, and extended focus panels = 10 µm). (D) Transmission electron micrograph of immunogold labeled VWF present on the surface of a Nbeal2−/− megakaryocyte. Magnification ×50 000; scale bar represents 500 nm.
Abnormal VWF distribution in Nbeal2−/− megakaryocytes. (A) Immunofluorescence imaging of a representative permeabilized mature extending stage WT megakaryocyte shows elaboration of demarcation membranes stained with CD41 (green), dispersed VWF expression (red), and a peripheral tubulin cytoskeletal meshwork (violet). (B) A representative Nbeal2−/− MK at the same stage shows a strong peripheral distribution of VWF, which is absent in Nbeal2−/− platelets; this MK also contains the nucleus of an exogenous cell in its cytoplasm visible in the XZ view of the merged confocal panel. Comparisons of nonpermeabilized cells (C) confirm that VWF is abnormally concentrated near or on the surface of mature Nbeal2−/− megakaryocytes (scale bars in ZY panels = 5 µm; scale bars in XY, XZ, and extended focus panels = 10 µm). (D) Transmission electron micrograph of immunogold labeled VWF present on the surface of a Nbeal2−/− megakaryocyte. Magnification ×50 000; scale bar represents 500 nm.
Discussion
As with human heterozygous carriers of GPS-associated NBEAL2 mutations, we observed no obvious differences between WT and Nbeal2+/− mice or their platelets, so we focused our efforts on comparisons of Nbeal2−/− and WT. As with GPS patients, Nbeal2−/− mice have a low platelet count (approximately 60% of WT), and their platelets are enlarged as assessed by both mean platelet volume and diameter of the cytoskeletal microtubule ring (see the “Results” section). Nbeal2−/− mice also have pale gray-appearing platelets in comparison with WT and Nbeal2+/− mice, and TEM examination confirmed the virtual absence of α-granules (Figure 1; supplemental Figure 2A). On average, 4.8 α-granules are seen in WT mouse platelet thin sections; this is slightly less than the 5.5 reported for human platelets,12 which may reflect the relatively smaller size of mouse platelets.26 These observations confirm that as with human NBEAL2,15,17,18 the absence of functional NBEAL2 protein in mice results in macrothrombocytopenia with platelets that are profoundly α-granule deficient. In contrast to their α-granule deficiency, Nbeal2−/− mouse platelets had normal lysosome constituents (LAMP1; supplemental Figure 2B-C). However, Nbeal2−/− mouse platelets had fewer dense granules per whole-mount electron microscopy than WT and lower ATP secretion in response to both thrombin (Nbeal2−/−/WT = 0.83) and U46619 (Nbeal2−/−/WT = 0.41). By comparison, human GPS platelets contain normal to increased dense granules as measured by whole-mount electron microscopy.27
Splenomegaly is observed in GPS patients16,19 and was also evident in 4-month-old Nbeal2−/− mice, in which the average spleen weight was 1.8× that of WT, containing increased numbers of MKs, suggesting augmented extramedullary megakaryopoiesis. Another feature of GPS is the prevalence of myelofibrosis in the bone marrow, indicated by increased reticulin staining.16,19 We did not detect differences in reticulin staining between Nbeal2−/− and WT mice in either the bone marrow (supplemental Figure 1) or spleen; however, myelofibrosis progresses with age in GPS,16 and it may be that 4-month-old mice are not old enough for this condition to become evident. We will continue to monitor our Nbeal2−/− mice as they age.
As in GPS, the absence of α-granules in Nbeal2−/− platelets is expected to be accompanied by a lack of cargo proteins. Immunoblot analysis of platelet lysates failed to detect the megakaryocyte-synthesized soluble cargo proteins platelet factor 4, thrombospondin-1, and VWF (Figure 2A-C), whereas plasma-derived fibrinogen was greatly reduced (Figure 2D). VWF and fibrinogen were present at normal levels in Nbeal2−/− plasma (Figure 2E-F). These cargo protein deficiencies have also been observed in GPS.16,19 The membrane-spanning α-granule protein P-selectin was present in Nbeal2−/− platelets, albeit at approximately half of the normal amount (Figure 2G), and granule membranes and membrane-associated proteins such as P-selectin have been observed in GPS platelets by us15 and others.16,19,20,25,28-31
The presence of P-selectin in platelets from Nbeal2−/− mice and GPS patients suggests that despite the lack of cargo, some of the membrane constituents and vesicular precursors of α-granules may be present. This contrasts with the situation in arthrogryposis, renal dysfunction, and cholestasis syndrome, where loss of function of VPS33B or VPS16B leads to platelets lacking P-selectin12,13 and presumably α-granule precursors. Loss of VPS16B expression results in decreased levels of VPS33B, suggesting that these proteins interact during α-granule biogenesis.13 We examined their possible interaction with NBEAL2 by assessing levels of VPS33B and VPS16B in Nbeal2−/− platelets (Figure 2H-I), which proved to be the same as WT. Because loss of NBEAL2 function appears to have no effect on VPS33B and VPS16B, we reason that these proteins and their human homologs likely act independently of NBEAL2 and are involved in an earlier stage of α-granule formation. Nbeal2 encodes a polypeptide of 2754 amino acids containing multiple domains15,17,18 (supplemental Figure 4), which as with other BEACH domain–containing proteins, has been implicated in cellular vesicular trafficking.15,32-35
High-resolution confocal immunofluorescence microscopy15 revealed that although P-selectin is present in the central region of both WT and Nbeal2−/− platelets, the latter show a less orderly and more diffuse P-selectin distribution (Figure 3B) in relation to the tightly packed loops visible in WT platelets (which presumably correlate with mature α-granules). These differences, however, do not prevent P-selectin from being externalized when Nbeal2−/− platelets are activated with thrombin (Figures 3D and 4C,L,M), although at lower amounts, as has also been observed in GPS platelets.28 This may relate to the decreased total levels of P-selectin in Nbeal2−/− platelets and abnormal intracellular distribution, possibly indicating altered precursor α-granule structures that cannot fuse as effectively with the plasma membrane as normal α-granules do in activated platelets.
Optical aggregometry revealed a decreased response of washed Nbeal2−/− platelets to CRP, thrombin, and the thromboxane analog U46619 (Figure 4D-F). Unlike WT, Nbeal2−/− platelets did not aggregate in the presence of 0.05 U/mL thrombin; weak aggregation was seen when exogenous fibrinogen was added. This indicates that lack of α-granule-borne fibrinogen may partially account for the relatively weak aggregation of Nbeal2−/− platelets. Because flow cytometry showed decreased thrombin and ADP-induced αIIbβ3 activation (Figure 4A-B), and impedance aggregometry showed a decreased collagen response (Figure 4G), impaired platelet activation independent of α-granule-borne fibrinogen is also evident. Taken together, these results show that Nbeal2−/− platelets have a lowered response in relation to WT to agonist-mediated activation. For comparison, in vitro platelet aggregation studies summarized by the Nurden group19 and Gunay-Aygun et al16 show that decreased responses to varying agonists—including collagen, thrombin, and ADP—have been observed in many GPS patients.
Several proteins secreted from platelet α-granules, including VWF and coagulation factor V, are known to be important for platelet hemostatic function at sites of blood vessel injury, where an absence of α-granules in GPS platelets is thought to contribute to a bleeding phenotype that is characteristically mild to moderate.16,36,37 The results of tail bleeding assays (Figure 4H) clearly show increased blood loss in Nbeal2−/− mice in comparison with WT, suggesting that their platelets do not function normally in vivo. Increased bleeding of Nbeal2−/− mice is not due to abnormalities in endothelial cell–derived VWF, because plasma levels are normal (Figure 2E). Nor are their decreased platelet counts likely to be the cause because reductions up to 97.5% have been shown to have little influence on bleeding in mice.38
The in vivo function of Nbeal2−/− mouse platelets was directly examined using high-speed confocal immunofluorescence microscopy to monitor response to laser-induced damage in cremaster muscle arterioles. We observed that Nbeal2−/− mice show relatively decreased platelet accumulation at injury sites (Figure 4I-J), whereas their rate of platelet activation within the thrombi is not significantly different from WT (Figure 4K-L). The future availability of an appropriate antibody will allow testing the possibility that absent NBEAL2 in the vasculature contributes to the decreased platelet accumulation at injury sites.
Splenectomy increases platelet counts in GPS patients,16 and a shortened mean platelet half-life has been described using 111In labeling,19 suggesting that splenic sequestration and increased platelet destruction are causes of GPS-associated thrombocytopenia. Because this thrombocytopenia is noted to be progressive with age and accompanied by myelofibrosis,16 the more severe thrombocytopenia seen in older GPS patients likely indicates that fibrotic bone marrow is a poor environment for MK maturation and platelet production. The overall bone marrow morphology of our 4-month-old Nbeal2−/− mice was normal (supplemental Figure 1), indicating that their thrombocytopenia may be related to abnormal MK development. The presence of ultrastructural abnormalities in Nbeal2−/− MKs was confirmed by transmission electron microscopy using direct fixation of bone marrow cells (Figure 5). Nbeal2−/− MKs revealed a marked reduction of α-granules and platelet territories, which is likely associated with abnormalities in the demarcation membrane system (Figure 5B,D). Vesicles within Nbeal2−/− MKs appeared empty with a vacuolated appearance (compare Figure 5C WT with Figure 5D Nbeal2−/−). Ultrastructural examination of GPS MKs has also revealed highly vacuolated cells with decreased α-granules.19 A striking feature noted in Nbeal2−/− MKs was the frequent observation of emperipolesis (the presence of an intact cell within the cytoplasm of another cell); for example, Figure 5E shows 3 cells within the same MK, and an Nbeal2−/− MK containing a nucleated cell is also shown in Figure 7B (4′6 diamidino-2-phenylindole + merge panel). MK emperipolesis has also been observed in GPS,31 MYH9-related disease,39 thrombocytosis, myelofibrosis, and other conditions.39 Although the mechanism is unclear, the involvement of aberrant P-selectin expression has been proposed to mediate emperipolesis.19
The presence of marked ultrastructural abnormalities in bone marrow Nbeal2−/− MKs suggests that their differentiation into platelets may be impaired, which is also indicated by their decreased ploidy. In a population analysis of bone marrow MKs using CD41 as a lineage-specific marker, we determined that the proportion of MK-lineage cells in relation to all nucleated marrow cells (ie, 4′6 diamidino-2-phenylindole staining) was the same (approximately 0.23%) in WT and Nbeal2−/− mice. However, when these cells were cultured in the presence of thrombopoietin under conditions that promoted MK maturation, after 5 days the proportion of nucleated cells scored as MKs was 14% in WT (comparable to other studies24 ) and 7.8% in Nbeal2−/− cultures. Both WT and Nbeal2−/− MK cultures contained large cells showing extension of microtubule-containing proplatelets that were capable of producing platelets (Figure 6E-F; supplemental Figure 3A-B). We evaluated their development by classifying populations of cells (n = 600) into visually distinguishable stages: early, mature, extending, spread, and proplatelet MKs (Figures 6A-E). The resulting population distributions (Figure 6G) show that in relation to WT, a significantly larger proportion of Nbeal2−/− MKs were in the early stage and fewer were in the mature, extending, and proplatelet stages, indicating a developmental delay in Nbeal2−/− MKs. In addition, the proportion of spread MKs was significantly higher in the Nbeal2−/− MK population, which may indicate that spread cells represent an abnormal, stalled, or abortive stage of MK development. The abnormal development of Nbeal2−/− MKs was also indicated by the presence of increased 2N and greatly reduced 8N and >16N MKs in 5-day cultures (Figure 6H).
Several mechanisms can be proposed to account for abnormal Nbeal2−/− MK development. One possibility is that in addition to being important for α-granule formation, NBEAL2 may also be involved in the development of other membrane compartments such as the demarcation membrane, because Nbeal2−/− MKs appear to be more vacuolated than are WT (Figure 5D). Another possibility is that NBEAL2 is involved in formation of the cytoskeletal organization of MKs, proplatelets, and platelets, which influences platelet size and production.40 Platelets from GPS patients are larger and rounder than normal15,20,40 and contain thicker peripheral microtubule coils.40,41 NBEAL2 contains multiple domains (supplemental Figure 4), which may interact with components of the MK and platelet cytoskeleton in unknown ways. Atypical Nbeal2−/− MK development could also be associated with abnormal intracellular protein distribution arising from the failure to incorporate cargo proteins into maturing α-granules, as we observed with VWF (Figure 7). In mature WT MKs, VWF is dispersed throughout the cytoplasm (Figure 7A), whereas in mature Nbeal2−/− MKs, VWF protein is concentrated at the periphery (Figure 7B), and in some cells was observed in concentrated structures that sometimes extended beyond the outer membrane delineated by CD41 labeling (Figure 7C) and is observed outside of native (not cultured) Nbeal2−/− MKs labeled with immunogold (Figure 7D). VWF forms multimeric complexes that are organized into highly structured tubules that are stored in either α-granules in MKs or Weibel-Palade bodies in endothelial cells in an acidic environment.42,43 The failure of VWF to enter α-granules in Nbeal2−/− MKs may lead to its deposition in other membrane compartments or the cytoplasm, where a neutral pH may cause VWF to degrade or form abnormal structures (eg, inclusions) that physically interfere with terminal MK differentiation.
In summary, we propose that Nbeal2−/− mice represent a plausible animal model of human GPS caused by loss of NBEAL2 expression, because they show many GPS-specific aspects of platelet and megakaryocyte structure, function, and development both in vitro and in vivo. Our observations of protein expression in Nbeal2−/− mouse platelets indicate that NBEAL2 affects a later stage of α-granule biogenesis than does the mouse homologs of 2 other proteins identified as essential for this process in humans: VPS33B and VPS16B. Finally, exploiting the potential of our mouse model, we have observed the development of Nbeal2−/− mouse MKs in culture and noted abnormalities that indicate that GPS-associated thrombocytopenia likely arises from delayed and aberrant platelet development that may be directly linked to the failure of MKs to form mature α-granules and properly package their cargo.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Kirin Brewery Company for their generous gift of recombinant pegylated human megakaryocyte growth and development factor (thrombopoietin).
This work was supported by grants from the Canadian Institutes of Health Research to W.H.A.K. (MOP-259952), and a Heart and Stroke Foundation of Ontario Grant-in-Aid to P.L.G.
Authorship
Contribution: W.H.A.K., C.L.-M., and P.L.G. designed experiments, performed research, and wrote and edited the manuscript; R.W.L. performed and analyzed mouse bone marrow cultures; L.L. performed sequencing, immunoblotting, and mouse work, and analyzed data; F.G.P. performed confocal immunofluorescence microscopy, analyzed data, and wrote and edited the manuscript; H.C. performed transmission electron microscopy; R.N. performed intravital microscopy experiments; N.V. performed tail bleeding assays; C.E.H. performed bone marrow pathology analysis; and A.S.W. and J.D.P. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Walter H. A. Kahr, Departments of Paediatrics & Biochemistry, Division of Haematology/Oncology, Program in Cell Biology, The Hospital for Sick Children, University of Toronto, 555 University Ave, Toronto, ON M5G 1X8, Canada; e-mail: walter.kahr@sickkids.ca.
References
Author notes
C.L.-M. and P.L.G. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal