Abstract
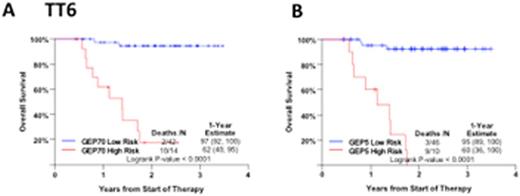
Gene expression profiling (GEP) reliably predicts overall and progression free survival in multiple myeloma. Driven by the concept that therapy will reveal biology, we applied the GEP70 risk model to 56 patients enrolled in Total Therapy 6 (TT6), a phase 2 trial for previously treated patients. One year survival estimates were 62% vs.97%, p<0.0001, Figure 1A). To investigate whether fewer than the 70 genes could predict this difference in outcomes, the probe sets of the GEP70 risk model were ranked by p-values, based on univariate Cox regression analysis for OS. The five probe sets with the smallest P values (corresponding to genes ENO1, FABP5, TRIP13, TAGLN2, and RFC4) were combined to create a continuous score (Figure1B). Association of several of these genes with different cancers has previously been reported by others.
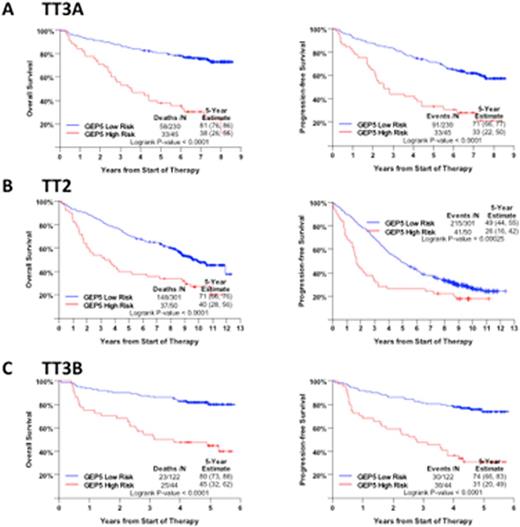
We re-trained this 5 gene model (GEP5) on a dataset of 275 uniformly treated patients on Total Therapy 3A (TT3A) and identified a new optimal cutoff of 10.68. We validated this new cutoff with patients enrolled in Total Therapy 2 (TT2) (n=351) and Total Therapy 3B (TT3B) (n=166). For TT2 patients, the dataset from which the GEP70 model was developed, clinical outcomes of the GEP5defined low risk patients were very similar to the GEP70 defined low risk patients. Survival estimates were higher for GEP5-defined high risk than for GEP70 high risk patients (5-year estimated OS: 40%, GEP5; 28%, GEP70; 5-year estimated PFS: 26%, GEP5; 15%, GEP70) (Figure 2A and 2B). This was also seen in both TT2 treatment arms. For the second validation cohort (TT3B), GEP5 and GEP70 risk distinction were similar to the TT3A discovery cohort (Figures 2C and 2D). On multivariate analysis, the GEP5-defined high-risk designation was the most adverse variable for PFS, with an estimated hazard ratio of 3.44 (95% CI: 2.02-5.86), whereas the GEP70 model was selected first for OS. (Table 1 ).
Multivariate stepwise Cox regression analysis performed on the TT3B validation set
| . | . | . | Overall survival . | Progression-free survival . | ||
|---|---|---|---|---|---|---|
| . | Variable . | n/N (%) . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Multivariate | ||||||
| GEP70 high-risk | 36/159 (23%) | 4.45 (2.47, 8.02) | <.001 | |||
| B2M > 5.5 mg/L | 49/159 (31%) | 1.73 (1.01, 2.95) | 0.045 | |||
| GEP5 high-risk | 42/159 (26%) | 3.44 (2.02, 5.86) | <.001 | |||
| . | . | . | Overall survival . | Progression-free survival . | ||
|---|---|---|---|---|---|---|
| . | Variable . | n/N (%) . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Multivariate | ||||||
| GEP70 high-risk | 36/159 (23%) | 4.45 (2.47, 8.02) | <.001 | |||
| B2M > 5.5 mg/L | 49/159 (31%) | 1.73 (1.01, 2.95) | 0.045 | |||
| GEP5 high-risk | 42/159 (26%) | 3.44 (2.02, 5.86) | <.001 | |||
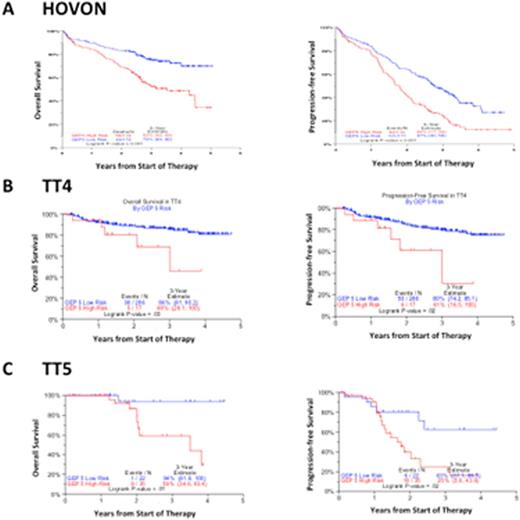
Applied to the publicly available dataset from the HOVON group, GEP5 identified a high risk group with a 3-year estimate OS survival of 52% compared to 75% for the low risk group (p<0.001).
TT4 and TT5 are phase 2 trials for previously untreated GEP70 defined low-risk and high-risk patients, respectively. GEP5 identified in TT4 a subset (17/303) of high risk patients with significantly worse 3-year estimated OS (69% vs. 86%, p=0.03), and in TT5 GEP5 identifies a low-risk subset (22/57) of patients with significantly better 3-year estimate OS (94% vs. 59%, p=0.01).
Recently a large-scale proteomics experiment involving 85 patients with MM identified ENO1, FABP5, and TAGLN2 among a set of 24 proteins that are associated with short OS. It was further shown that gene expression levels correlated closely with protein abundance.
In summary, we have identified 5 genes that have the greatest influence on GEP defined risk. The GEP5 score maintains prognostic power even in patients who have been risk stratified using other risk models. The correlation of expression at both mRNA and protein levels indicate that the genes identified in GEP5 are not simply an artifact of the microarray methodology, but rather supports their biologic relevance. This simplified risk model with a reduced number of genes has the potential to open molecular risk testing to a larger audience.
Overall Survival in TT6 according to A) GEP70 risk score and B) GEP5 risk score
Overall survival (left panels) and progression-free survival (right panels) according to GEP5 risk score in A) TT3A training set set, B) TT2 test set and C) a second test set TT3B
Overall survival (left panels) and progression-free survival (right panels) according to GEP5 in A) the publicly available HOVON dataset B) TT4, for previously untreated GEP70 defined low-risk myeloma and C) TT5, for previously untreated GEP70 defined high-risk myeloma
van Rhee:Jansen & Jansen: Research Funding. Usmani:Celgene: Consultancy, Research Funding, Speakers Bureau; Onyx: Research Funding, Speakers Bureau. Epstein:University of Arkansas for Medical Sciences: Co-inventor of the DNA probes for FISH of IGHC/IGHV (14q32), MMSET/FGFR3 (4p16), CCND3 (6p21), CCND1 (11q13), MAF (16q23), and MAFB (20q12) loci, sub. to the US Patent & Trademark Office as Prov. App# 61/726,327: Methods of Detecting 14q32 Translocations, Co-inventor of the DNA probes for FISH of IGHC/IGHV (14q32), MMSET/FGFR3 (4p16), CCND3 (6p21), CCND1 (11q13), MAF (16q23), and MAFB (20q12) loci, sub. to the US Patent & Trademark Office as Prov. App# 61/726,327: Methods of Detecting 14q32 Translocations Patents & Royalties. Zhang:University of Arkansas for Medical Sciences: Co-inventor of the DNA probes for FISH of IGHC/IGHV (14q32), MMSET/FGFR3 (4p16), CCND3 (6p21), CCND1 (11q13), MAF (16q23), and MAFB (20q12) loci, sub. to the US Patent & Trademark Office as Prov. App# 61/726,327: Methods of Detecting 14q32 Translocations, Co-inventor of the DNA probes for FISH of IGHC/IGHV (14q32), MMSET/FGFR3 (4p16), CCND3 (6p21), CCND1 (11q13), MAF (16q23), and MAFB (20q12) loci, sub. to the US Patent & Trademark Office as Prov. App# 61/726,327: Methods of Detecting 14q32 Translocations Patents & Royalties. Barlogie:Celgene: Consultancy, Honoraria, Research Funding; Myeloma Health, LLC: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal