Abstract
The current WHO Classification defines acute myeloid leukemia (AML) in most cases as a myeloid neoplasm with ≥20% bone marrow (BM) or peripheral blood (PB) blasts. However, it is controversial whether AML cases with 20-29% BM blasts, referred to as oligoblastic AML or refractory anemia with excess blasts in transformation (RAEBT), should be considered as AML or myelodysplastic syndrome (MDS) in terms of treatment approach and prognosis.
We retrospectively studied 573 patients aged ≥50 years diagnosed after the year 2004 with de novo AML, including 142 with 20-29% BM blasts (RAEBT) and 431 with ≥30% BM blasts (AML30). Patients with t(15;17), t(8;21), inv(16)/t(16;16), or history of any prior myeloid neoplasm were excluded. Clinicopathologic and genetic features, treatments, and outcome of RAEBT were compared to AML30. The clinicopathologic and genetic features of RAEBT were also compared to 152 de novo MDS patients with 10-19% BM blasts (RAEB2) diagnosed over the same time period. Fisher's exact and Wilcoxon tests were used to compare categorical and continuous variables between groups, respectively. Overall survival (OS) was estimated using the method of Kaplan and Meier and the log-rank test was used to compare OS between groups. Univariate and multivariable Cox proportional hazards regression was used to assess the impact of the two groups along with other risk factors in OS.
Compared to AML30 patients, RAEBT patients were older and had lower white blood count and BM cellularity, but had higher hemoglobin, platelet count, and UKMRC karyotype risk score and greater morphologic dysplasia (Table 1).RAEBT and RAEB2 patients were similar with respect to age, peripheral counts, BM cellularity, and karyotype risk scores. In the subsets of patients in whom they were assessed, FLT3 and NPM1 mutations and monocytic features occurred more commonly in AML30 than in RAEBT (Table 1). RAEBT patients were treated more often with hypomethylating agents and less often with intensive therapy (anthracycline/cytarabine) compared to AML30 patients, whereas allogeneic stem cell transplant frequency was similar. With 55.5 months median follow-up, OS of RAEBT patients was superior to that of AML30 patients (p=0.003)(Figure 1). Multivariable analysis showed that RAEBT (p<0.0001), hemoglobin ≥10.3 g/dL (p=0.045), intermediate UKMRC karyotype score (p<0.0001), normal bone marrow karyotype (p=0.0005), intensive therapy (p<0.0001), and stem cell transplant (p<0.0001) were associated with longer OS.
Comparison of RAEBT and AML30 groups
| . | RAEBT (n=142) . | AML30 (n=431) . | p value . |
|---|---|---|---|
| Median age in years | 67 | 63 | <0.0001 |
| Median white blood count (x 109/L) | 2.38 | 9.20 | <0.0001 |
| Median hemoglobin (g/dL) | 9.80 | 9.20 | 0.0007 |
| Median platelets (x 109/L) | 76 | 60 | 0.01 |
| Median BM cellularity (%) | 60 | 90 | <0.0001 |
| Morphologic dysplasia (% with >10% erythroid / myeloid / megakaryocytic dysplasia) | 53 / 53 / 46 | 31 / 25 / 27 | <0.0001 / <0.0001 / <0.0001 |
| Monocytic or myelomonocytic differentiation | 23/115 (20%) | 137/342 (40%) | <0.0001 |
| UKMRC karyotype scores (% intermediate / adverse / missing) | 61 / 35 / 5 | 69 / 26 / 5 | 0.049 |
| FLT3-ITD mutation | 3/94 (3%) | 59/247 (23%) | <0.0001 |
| NPM1 mutation | 1/55 (2%) | 46/151 (30%) | <0.0001 |
| Supportive care only | 11/142 (8%) | 38/431 (9%) | 0.86 |
| Hypomethylating agent as initial therapy | 36/142 (25%) | 36/431 (8%) | <0.0001 |
| Intensive therapy as initial therapy | 73/142 (51%) | 310/431 (72%) | <0.0001 |
| Stem cell transplant | 41/142 (29%) | 118/431 (27%) | 0.74 |
| 12-month OS [95% CI] | 60% [46-74%] | 51% [47-55%] | 0.003 |
| 48-month OS [95% CI] | 29% [22-36%] | 20% [17-24%] |
| . | RAEBT (n=142) . | AML30 (n=431) . | p value . |
|---|---|---|---|
| Median age in years | 67 | 63 | <0.0001 |
| Median white blood count (x 109/L) | 2.38 | 9.20 | <0.0001 |
| Median hemoglobin (g/dL) | 9.80 | 9.20 | 0.0007 |
| Median platelets (x 109/L) | 76 | 60 | 0.01 |
| Median BM cellularity (%) | 60 | 90 | <0.0001 |
| Morphologic dysplasia (% with >10% erythroid / myeloid / megakaryocytic dysplasia) | 53 / 53 / 46 | 31 / 25 / 27 | <0.0001 / <0.0001 / <0.0001 |
| Monocytic or myelomonocytic differentiation | 23/115 (20%) | 137/342 (40%) | <0.0001 |
| UKMRC karyotype scores (% intermediate / adverse / missing) | 61 / 35 / 5 | 69 / 26 / 5 | 0.049 |
| FLT3-ITD mutation | 3/94 (3%) | 59/247 (23%) | <0.0001 |
| NPM1 mutation | 1/55 (2%) | 46/151 (30%) | <0.0001 |
| Supportive care only | 11/142 (8%) | 38/431 (9%) | 0.86 |
| Hypomethylating agent as initial therapy | 36/142 (25%) | 36/431 (8%) | <0.0001 |
| Intensive therapy as initial therapy | 73/142 (51%) | 310/431 (72%) | <0.0001 |
| Stem cell transplant | 41/142 (29%) | 118/431 (27%) | 0.74 |
| 12-month OS [95% CI] | 60% [46-74%] | 51% [47-55%] | 0.003 |
| 48-month OS [95% CI] | 29% [22-36%] | 20% [17-24%] |
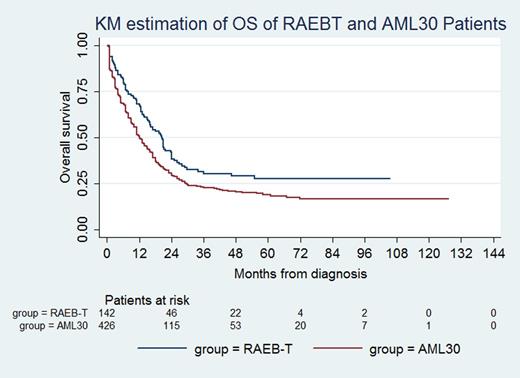
RAEBT is more similar to RAEB2 than to AML30 in terms of its clinicopathologic and cytogenetic features. In spite of less frequent treatment with intensive chemotherapy and higher karyotype risk, RAEBT patients had superior OS compared with AML30 patients. In a prior study (Estey E et al. Blood 1997;90:2969), RAEBT and AML30 patients treated with intensive therapy had similar outcomes; the advent of hypomethylating agent therapy may in part account for the differences found in our study. Our findings favor considering RAEBT in a disease category separate from AML with higher blast counts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal