Abstract
Bendamustine in combination with rituximab (BR) has shown to be similar, if not superior, in efficacy with more favorable side-effect profiles compared to R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisone) for front-line treatment of indolent B-cell lymphoma. In addition, bendamustine 120 mg/m2 in combination with rituximab 375 mg/m2 was associated with high response rates and acceptable toxicity even in older patients with relapsed or refractory aggressive B-cell lymphoma. Clinical evaluation of BR is warranted in the front-line setting for DLBCL patients not eligible for anthracyclines or very elderly, for whom the standard therapy has not been established.
In this single-arm phase II study, patients with ≥65 years of age who were deemed poor candidates for R-CHOP therapy at the discretion of the treating physician were enrolled through the UNC Cancer Network to determine the efficacy and safety of BR in previously untreated stage II-IV DLBCL (ClinicalTrials.gov #NCT01234467). Patients received bendamustine at a dose of 120 mg/m2 daily on days 1 and 2 of each 21-day cycle along with rituximab on day 1 for up to 8 cycles of therapy. Patients with ECOG score of 3 at baseline were allowed to receive bendamustine at a dose of 90 mg/m2 daily, and the dose would increase to 120 mg/m2 daily if their ECOG score improved to ≤ 2 after 3 cycles of BR. Pre-phase steroid therapy with prednisone 100 mg daily for five days was permitted prior to the initiation of BR in patients with poor functional status at the initial presentation.
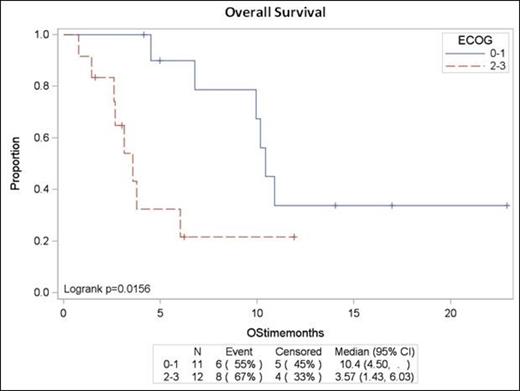
Twenty three patients were enrolled with the majority (83%) having stage III or IV disease at baseline. The median age was 80 years (range 65 – 89), and 78% of patients had IPI score of ≥3. More than half the patients (52%) presented with poor functional status with ECOG score of ≥2 prior to therapy, including six patients with ECOG score of 3. The overall response rate was 93% with a complete response rate of 60% for 15 evaluable patients. The median time to progression (TTP) was 7.4 months. The median survival was 9.9 months (95% CI of 3.6 – 10.9) for all patients, but for patients with ECOG score of ≥2, the median survival was 3.6 months (1.4 – 6.0). Grade 3/4 AEs observed in > 10% of patients were anemia (27%), neutropenia (18%), lymphopenia (68%), thrombocytopenia (18%), and fatigue (14%). Four grade 5 treatment-related AEs, including two cases of pneumonia and two cases of anorexia, were reported. Four deaths were directly related to disease progression during or after treatment. Six patients died of other causes, including cerebral vascular accidents, congestive heart failure, and hip fracture, which were felt to be related to the patients' underlying comorbidities. Overall survival at 12 months was 25% (8.2 – 46.9).
Combination therapy with BR demonstrates high response rates in older patients who were deemed poor candidates for the standard R-CHOP therapy for treatment of previously untreated DLBCL. However, the survival rates were not considered promising in this older frail patient population. BR, especially with a high dose of bendamustine, should be used with great caution in future clinical trials involving older DLBCL patients with poor functional status.
Park:Seattle Genetics, Inc.: Research Funding; TEVA: Research Funding. Off Label Use: bendamustine in previously untreated DLBCL.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal