Abstract
The use of high-dose methotrexate (HD MTX) as first-line treatment for patients with primary central nervous system lymphoma (PCNSL) has resulted in dramatic improvements in survival and functional status for immunocompetent individuals. However, there is minimal data describing either its efficacy or safety in patients with AIDS-related (AR) PCNSL, a uniformly fatal disease in the era preceding combined anti-retroviral therapy (cART). Furthermore, it is unclear whether HD MTX and cART may be safely co-administered. Here we report our experience in treating patients with AR PCNSL in the pre-cART, cART, and HD MTX eras at San Francisco General Hospital (SFGH).
The medical records of 93 patients with biopsy-proven, AR PCNSL diagnosed between April 1988 and August 2012 were culled via electronic tumor registry, electronic medical record, and physical chart review. All patients required documentation of biopsy-proven disease for inclusion in this analysis, and all but one patient record originated from SFGH, the exception being an HIV+ individual who received treatment at our university hospital. Patients receiving a diagnosis prior to 1997 were grouped into the pre-cART era as this was the year in which combined anti-retroviral therapy was widely available and incorporated into treatment regimens at SFGH. The HD MTX era began in 2002 at SFGH with its routine use as first-line treatment for PCNSL.
Median age at diagnosis for the entire cohort (N = 93) was 36 years, and 95% of the patients were male. The majority of diagnoses were made in the pre-cART era (N = 75, or 81%). With regard to histology, all patients were diagnosed with either diffuse large B-cell lymphoma (DLBCL) or large cell lymphoma NOS, and there were no T-cell nor indolent B-cell histologic subtypes recorded. Median CD4 count at diagnosis was 11 (range 0 to 386) for the 37 patients with this data recorded. The median Karnofsky Performance Status (KPS) score at diagnosis was 30 for the entire cohort with no differences between pre-cART or cART eras. A total of 56 patients (60%) suffered from opportunistic infections (OIs) prior to PCNSL diagnoses, and the proportion was higher among the cART era patients (15 of 18, or 83%). Only 14 patients (15%) were on anti-retroviral (ARV) therapy of any kind prior to diagnosis, and while the majority (10/14) of these patients were diagnosed in the cART era, none were compliant or otherwise receiving treatment on a consistent basis.
Regarding treatment, of the 57 patients with documented whole-brain XRT (WBXRT) doses, 55 were treated in the pre-HD MTX era (N = 81) and received a median dose of 3100 cGy with a median of 26 days from diagnosis to completion of therapy. Among the 12 patients diagnosed in the era of HD MTX + cART, 10 patients had clinical data available to calculate a median IELSG score of 4. Ten patients in the HD MTX era received a median 7 cycles of HD MTX with a median dose of 8 gm/m2 per cycle; two patients expired prior to receiving any therapy. No patient in this era received WBXRT as part of first line therapy. One patient experienced grade 4 thrombocytopenia, otherwise there were no noted grade 3/4 toxicities. There were 6 complete remissions (CRs) and 2 partial remissions (PRs) for an overall response rate (ORR) of 80% among patients treated with HD MTX. The median post-treatment KPS among patients treated with HD MTX was 80.
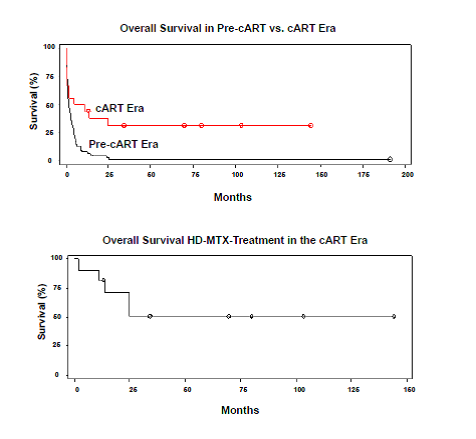
Finally, the median OS for the entire cohort (N = 93) was 2.2 months. Among patients diagnosed in the pre-cART era (N = 75), median OS was 2 months. Patients diagnosed in the cART era (N = 18) and patients treated with HD MTX in the cART era (N = 10) had median OS of 8.1 and 29.7 months, respectively. Notably, among survivors (N = 6) of patients treated with HD MTX in the cART era, the median OS was 75 months.
AR PCNSL was a uniformly and rapidly fatal disease at SFGH between the years 1988 and 1997 with the use of WBXRT as first-line treatment. In contrast, for patients diagnosed with AR PCNSL in the cART era, HD MTX has been safely and effectively administered with concomitant anti-retroviral therapy at similar doses used for immunocompetent patients. Furthermore, such treatment can lead to durable remissions even in patients with a history of non-compliance or sustained periods off cART therapy. Supported by Leukemia and Lymphoma Society and NIH R01CA139-83-01A1.
Rubenstein:Genentech: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal