Abstract
Eltrombopag is an oral, non-peptide thrombopoietic receptor-agonist (TPO-RA).
In chronic immune thrombocytopenic purpura (ITP) randomized–controlled trials proved to be effective, safe and well tolerated with reported response rates of 59%-88%.
When eltrombopag is discontinued, platelet counts usually return to baseline within 2 weeks. However, certain patients may be able to discontinue TPO-RA and still maintain platelet counts above baseline without additional treatment.
We report here 12 patients who presented sustained responses after discontinuing eltrombopag without substituting additional anti-ITP therapy.
Primary ITP was defined as a platelet count < 100 x 109/L in the absence of other causes or disorders that may be associated with thrombocytopenia.
Patients received daily oral eltrombopag, at a starting dose of 50 mg/day adjusting the dose as needed up to a maximum of 75 mg/day based on the patient’s platelet count.
Successful discontinuation of eltrombopag treatment was defined as a platelet count of 30,000/μl and 20,000/μl above initial baseline for at least 6 months off eltrombopag without substituting additional anti-ITP therapy.
Our patients were 4 males and 8 females, with a mean disease onset age of 55 years (range, 28–79 years). The median time from diagnosis to eltrombopag start was 24 months (range, 1-480). 5 cases had ITP since less than 1 year.
The median prior number of therapies was 5 (range, 1-7). All patients were refractory to corticosteroids. Six patients had received rituximab: Patient (P) 1, P2, P3, P6, P7 and P8. Seven patients were splenectomized.
Three patients (P2, P6 and P8) who failed to respond to romiplostim were switched to eltrombopag. One romiplostim responder (P12) switched to eltrombopag because patient request. The median platelet count before starting treatment was 7 x 109/L (range, 1-97 x 109/l).
At start, concomitant treatment was administered in 4 patients: P2, P3 and P9 corticosteroids. P5, intravenous immunoglobulin.
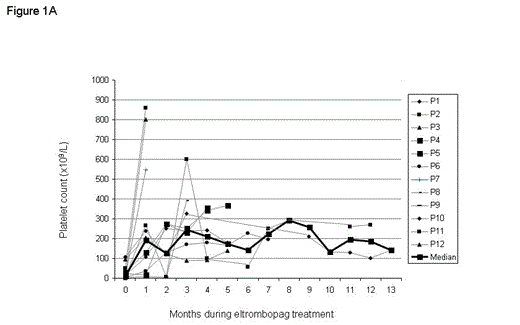
The median maximum platelet count during treatment was 482 x109/l (range, 251-858 x109/l). One patient had a transient increase in leukocyte count reaching 12x109/L.
The median duration of treatment was 5 months (range, 1-13) (Fig 1A): only one month in three patients. Nine patients stopped treatment due to platelets higher than 250 x 109/l.
Initial stop of eltrombopag in P2 was failed because of low platelet counts and eltrombopag was reinitiated. After 8 months of re-treatment, eltrombopag could be stopped with no other treatments needed for over 6 months.
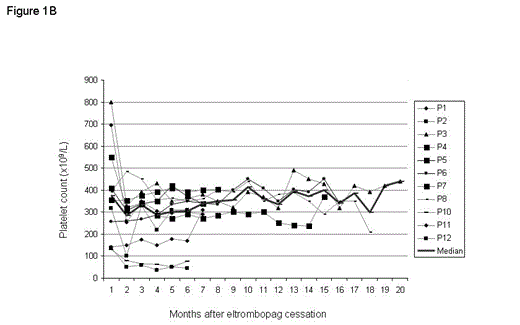
In P1, P10 and P11 eltrombopag was stopped at 140, 178 and 138 x109/L platelets, respectively. After a median follow-up of 7 months (range, 6 – 20 months), ten patients maintain a platelet count greater than 100 x 109/L (Fig 1B) without any anti-ITP treatment.
The possibility of eltrombopag cessation in a specific subset of patients has emerged.
Recently, a prospective ongoing study has demonstrated that approximately 1/3 of patients (5 of 15) appear able to successful elective discontinuation of eltrombopag after 2 or more years of treatment.
Nevertheless we have showed that the remission of ITP is feasible after short term treatment with eltrombopag (3 patients treated for only 1 month).
Repeated short-term use of eltrombopag in chronic ITP has been reported. Patient P2 could succesfully reintroduce eltrombopag after initial treatment stop.
In our data no factors predict which patients may discontinue eltrombopag.
Summary of 12 ITP patients with successful discontinuation of eltrombopag
| . | P1 . | P2 . | P3 . | P4 . | P5 . | P6 . | P7 . | P8 . | P9 . | P10 . | P11 . | P12 . | Median (range) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | 64 | 28 | 59 | 71 | 46 | 51 | 61 | 79 | 40 | 50 | 66 | 45 | 55 (28-79) |
| Gender (M/F) | F | F | F | F | F | M | M | F | M | F | M | F | - |
| Prior lines therapies for ITP | 4 | 6 | 7 | 7 | 5 | 5 | 5 | 6 | 2 | 1 | 1 | 2 | 5 (1-7) |
| Splenectomy (yes/no) | Y | Y | Y | Y | N | Y | Y | Y | N | N | N | N | - |
| ITP duration before TPO-RA (months) | 13 | 39 | 9 | 108 | 47 | 35 | 216 | 480 | 1 | 1 | 2 | 3 | 24 (1-480) |
| Platelet count before TPO-RA (x109/L) | 10 | 5 | 1 | 22 | 29 | 3 | 5 | 2 | 2 | 12 | 50 | 97 | 7 (1 - 97) |
| Maximum doses of TPO-RA (mg/day) | 50 | 25 | 50 | 75 | 50 | 50 | 50 | 50 | 50 | 75 | 50 | 50 | 50 (25-75) |
| Maximum platelet count during TPO-RA treatment (x109/L) | 413 | 600 | 800 | 354 | 416 | 255 | 548 | 392 | 550 | 251 | 858 | 580 | 482 (251-858) |
| Duration of TPO-RA (months) | 13 | 12 | 1 | 4 | 5 | 9 | 1 | 3 | 1 | 5 | 2 | 5 | 5 (1-13) |
| Side effect of TPO-RA | None | Y | None | None | None | None | None | None | None | None | None | None | - |
| Months after cessation of TPO-RA | 7 | 6 | 20 | 8 | 7 | 16 | 15 | 18 | 7 | 6 | 7 | 6 | 7 (6-20) |
| Platelet count, last visit (x109/L) | 310 | 310 | 438 | 402 | 338 | 340 | 368 | 208 | 208 | 76 | 290 | 44 | 310 (44-438) |
| . | P1 . | P2 . | P3 . | P4 . | P5 . | P6 . | P7 . | P8 . | P9 . | P10 . | P11 . | P12 . | Median (range) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | 64 | 28 | 59 | 71 | 46 | 51 | 61 | 79 | 40 | 50 | 66 | 45 | 55 (28-79) |
| Gender (M/F) | F | F | F | F | F | M | M | F | M | F | M | F | - |
| Prior lines therapies for ITP | 4 | 6 | 7 | 7 | 5 | 5 | 5 | 6 | 2 | 1 | 1 | 2 | 5 (1-7) |
| Splenectomy (yes/no) | Y | Y | Y | Y | N | Y | Y | Y | N | N | N | N | - |
| ITP duration before TPO-RA (months) | 13 | 39 | 9 | 108 | 47 | 35 | 216 | 480 | 1 | 1 | 2 | 3 | 24 (1-480) |
| Platelet count before TPO-RA (x109/L) | 10 | 5 | 1 | 22 | 29 | 3 | 5 | 2 | 2 | 12 | 50 | 97 | 7 (1 - 97) |
| Maximum doses of TPO-RA (mg/day) | 50 | 25 | 50 | 75 | 50 | 50 | 50 | 50 | 50 | 75 | 50 | 50 | 50 (25-75) |
| Maximum platelet count during TPO-RA treatment (x109/L) | 413 | 600 | 800 | 354 | 416 | 255 | 548 | 392 | 550 | 251 | 858 | 580 | 482 (251-858) |
| Duration of TPO-RA (months) | 13 | 12 | 1 | 4 | 5 | 9 | 1 | 3 | 1 | 5 | 2 | 5 | 5 (1-13) |
| Side effect of TPO-RA | None | Y | None | None | None | None | None | None | None | None | None | None | - |
| Months after cessation of TPO-RA | 7 | 6 | 20 | 8 | 7 | 16 | 15 | 18 | 7 | 6 | 7 | 6 | 7 (6-20) |
| Platelet count, last visit (x109/L) | 310 | 310 | 438 | 402 | 338 | 340 | 368 | 208 | 208 | 76 | 290 | 44 | 310 (44-438) |
San Miguel:Jansen, Celgene Corporation, Onyx, Novartis, Millenium: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal