Abstract
Detection of sub-microscopic levels of disease (minimal residual disease; MRD) in childhood acute lymphoblastic leukaemia (ALL) during treatment is an important prognostic factor. Currently, stratification of therapy for the new frontline trial in childhood ALL (UKALL 2011) is provided by MRD analysis using real time quantitative PCR (RQ-PCR) to identify and quantitate the patient specific rearrangements of the immunoglobulin (Ig) and T-cell receptor (TCR) genes. The current methodology is expensive, time-consuming and complex to perform. Although MRD has proven to be a powerful and essential tool in stratification of ALL patients, 8% of individuals in the current UKALL 2011 trial do not have an informative MRD result. Recently, Next Generation Sequencing (NGS) has led to the opportunity to improve the sensitivity and specificity of Ig/TCR based MRD analysis.
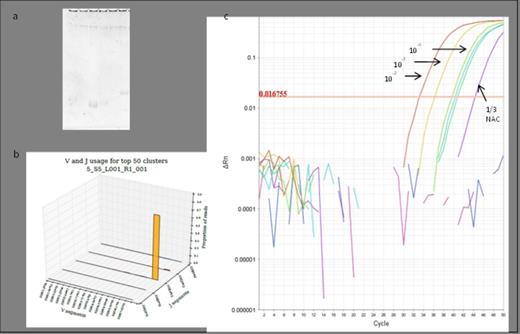
(a) Polyacrylamide electrophoresis could not recognise a target to use in current MRD methodologies (well 1 containing the products from a PCR reaction that would amplify VH1 and VH7, and wells 2-6 amplifying VH2-6, respectively), while the NGS pipeline could identify a VH7 rearrangement (b). (c) ASOs were designed to amplify the NGS-identified VH7-81*01 DH3-9*01 JH4*02 rearrangement and optimised to correctly identify 10-2, 10-3, 10-4 dilutions with a single NAC (non-amplification control; monocytes from 20 normal individuals) replicate amplified, therefore meeting current guidelines for a MRD target.
(a) Polyacrylamide electrophoresis could not recognise a target to use in current MRD methodologies (well 1 containing the products from a PCR reaction that would amplify VH1 and VH7, and wells 2-6 amplifying VH2-6, respectively), while the NGS pipeline could identify a VH7 rearrangement (b). (c) ASOs were designed to amplify the NGS-identified VH7-81*01 DH3-9*01 JH4*02 rearrangement and optimised to correctly identify 10-2, 10-3, 10-4 dilutions with a single NAC (non-amplification control; monocytes from 20 normal individuals) replicate amplified, therefore meeting current guidelines for a MRD target.
Having established NGS for identifying clonal targets in ALL, we are currently assessing the ability of the method and pipeline to quantify disease levels in end of induction and relapse samples, previously analysed by RQ-PCR, to determine the concordance between the methodologies. Indeed, logarithmic dilution series of patient DNA in a normal background revealed that stratification based on a clinical threshold of 1 in 1,000,000 is possible using this methodology. Further investigation into the clinical utility of NGS for MRD analysis will focus on analysing earlier time points in treatment and studying the potential use of blood rather than bone marrow. Altogether, this will further improve the predictive value and specificity of MRD testing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal