Abstract
Graft versus host disease (GVHD) following allogeneic hematopoietic stem cell transplant (allo-HSCT) is caused by CD4+ and CD8+ donor T cells directed against mismatched recipient antigens, presented in the context of donor MHC-II (indirect pathway) and recipient MHC-I (direct pathway). Recently, the presence of 'cross-dressed' CD11c+ antigen presenting cells (APCs) expressing both donor and recipient type MHC-I molecules has been demonstrated in animal organ and HSCT transplant models supporting 'semi-direct' pathway of allo-activation (Wang et al, Blood. 2011).These APCs can efficiently present allo-antigens to both CD4+ and CD8+ T cells and activate immune responses that could lead to allograft rejection or GVHD. Exchange of membrane fragments and associated proteins between cells, termed trogocytosis, generates cross-dressed APCs.We sought to test whether cross-dressed APCs facilitate antigen presentation to donor T cells and initiate GVHD following allo-HSCT. Further, we tested an array of drugs as inhibitors of trogocytosis, to interrupt the semi-direct pathway of allo-antigen presentation.
In vivo experiments used a B6(H2Kb) ˆ B10.BR(H2Kk) murine transplant model. Spleens of transplanted mice were analyzed on days 10, 15, 20 post-transplant for presence of cross dressed CD11c+cells, and their expression of CD80, CD86 and MHC-II by flow cytometry. Cross dressed donor CD11c+ FACS sorted cells from recipient spleens were co-cultured with CFSE labeled donor type T-cells for 6 days, and T-cell proliferation was measured as dilution of CFSE by flow cytometry. In vitro experiments used primary MLR consisting of CFSE labeled B6 bone marrow cells co-cultured with PKH26 (membrane dye) labeled B10.BR splenocytes. B6 antigen presenting cells were analyzed by flow cytometry for the presence of CFSE+PKH26+ double positive cells generated by trogocytosis. Pharmacological inhibitors of cytoskeleton function were added to the primary MLR and their effect on trogocytosis as well as T cell proliferation was assessed.
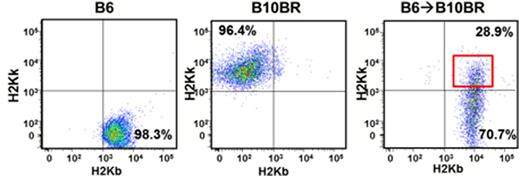
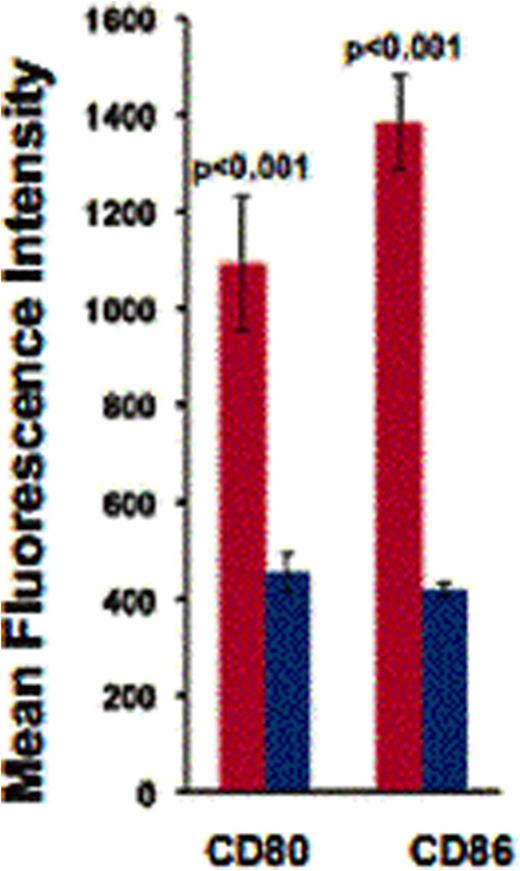
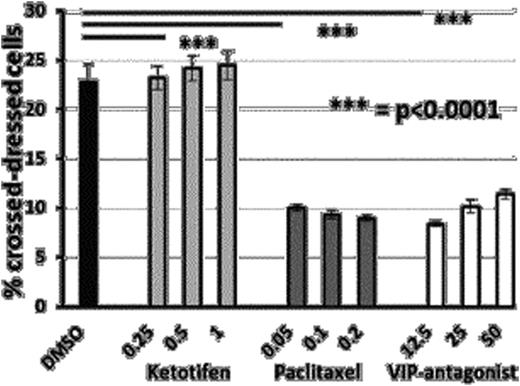
Cross-dressed donor CD11c+ APCs were generated in vivo following allo-HSCT (Figure 1). Recipient spleens showed that 50%, 28.6% (p=0.01) and 12% (p=0.02) of donor type CD11c+ cells were cross dressed on days 10, 15 and 20 respectively post transplant (n=5). These cross dressed APCs expressed higher levels of co-stimulatory molecules CD80 (p<0.001) and CD86 (p<0.001), and MHC-II compared to non-cross-dressed donor CD11c+ cells (Figure 2). Sorted cross dressed CD11c+ cells from recipient mice were able to induce in vitro proliferation of co-geneic CD8 T-cells, while their non-crossdressed counterparts did not. We demonstrated that cross-dressed CD11c+ cells were generated in vitro, by exchange of plasma membrane fragments and could be inhibited in vitro by low doses of paclitaxel and VIP antagonist (Figure 3), while preserving cell viability. Further more, bone marrow treated with 0.05uM of paclitaxel, caused significantly decreased T cell proliferation in primary MLR compared to non drug treated bone marrow.
Cross-dressed donor CD11c+ APCs were generated following allo-HSCT. Left and middle panels show expression of H2Kb and H2Kk in non-transplanted mice. Right panel shows cross-dressed APC on day 15 post allo-HSCT.
Cross-dressed donor CD11c+ APCs were generated following allo-HSCT. Left and middle panels show expression of H2Kb and H2Kk in non-transplanted mice. Right panel shows cross-dressed APC on day 15 post allo-HSCT.
Cross-dressed APCs (red) have increased expression of co-stimulatory molecules CD80 and CD86 compared to non-cross dressed donor CD11c+ APC (blue).
Cross-dressed APCs (red) have increased expression of co-stimulatory molecules CD80 and CD86 compared to non-cross dressed donor CD11c+ APC (blue).
Paclitaxel inhibited trogocytosis in MLR. The frequency of cross-dressed CD11c+ APC on day 6 of MLR was assessed by flow cytometry.
Paclitaxel inhibited trogocytosis in MLR. The frequency of cross-dressed CD11c+ APC on day 6 of MLR was assessed by flow cytometry.
The high frequencies of cross-dressed donor CD11c+ APCs following allo-HSCT suggests that semi-direct allo-antigen presentation may play a key role in the initiation of GVHD, while the decreasing trend could reflect replacement of host cells by donor hematopoetic cells. Reducing the generation of cross-dressed APCs by pharmacological inhibition of trogocytosis is a novel approach to reduce GVHD post allo-HSCT, targeting the semi-direct pathway of allo-antigen presentaion. Our data shows that very low doses of paclitaxel, a microtubule inhibitor and VIPHyb, an antagonist of Vasoactive Intestinal Peptide signaling, can reduce semi-direct presentaion of allo-antigen to Tcells and reduce alloreactivity without direct cytotoxic effect.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal