Abstract
Cytomegalovirus (CMV) reactivation frequently occurs during early immune reconstitution after hematopoietic stem cell transplantation (HSCT). Although CMV is associated with accelerated immune ageing in healthy individuals, the impact of early CMV reactivation on T-cell immunity long term post HSCT is unknown. In this study, we report the impact of early CMV reactivations on the reconstitution and composition of the T-lymphocyte compartment one to two year after HSCT in a large cohort of pediatric HSCT recipients.
We analyzed the lymphocyte compartment one and two year after transplantation in 131 consecutive (2002 - 2011) pediatric HSCT recipients that were eligible for follow up one year post HSCT. Viral infections were routinely monitored by weekly serum viral DNA PCR in the first 100 days. Peripheral blood mononuclear cells were routinely analyzed by multicolor flow cytometry. Six patients with early CMV reactivation and an HLA type for which CMV-tetramers were available were analyzed for the presence and phenotype of CMV-specific CD8+ T-lymphocytes.
One year post HSCT, patients with early CMV reactivation (n = 46, PCR ≥ 1x ≥ 200 copies / mL) had significantly higher lymphocyte counts compared to patients without CMV reactivation (n = 85, PCR always < 200 copies / mL). This could be attributed to a significant increase of CD3+ T-lymphocytes while NK- and B-cell numbers did not differ. Within the T-cell compartment, a three-fold expansion of CD8+ T-cells (median 1323 vs. 424 cells / μL, p < 0.0001, Mann-Whitney) and an increase in gamma-delta T-cells (median 104 vs. 47 cells / μL, p = 0.0005) was observed, while absolute numbers of CD4+ T-cells did not differ between the groups. This effect was not observed in relation to Epstein-Barr virus (EBV) or Adenovirus (HAdV) reactivation. In multivariate analysis, CD8+ T-cell numbers one year post HSCT were highly influenced by CMV reactivation (p < 0.0001, linear regression) but not affected by pre-transplant CMV serostatus of donor (p = 0.22) or recipient (p = 0.14).
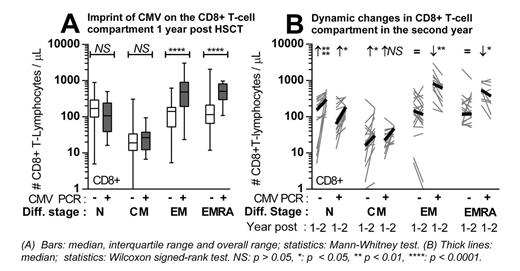
In a representative subcohort (n = 53, 2008 - 2010), we more closely analyzed the differentiation stages of T-cells based on expression of CD45RA and CCR7. In both the CD4+ and CD8+T-cell subset, the proportion of Effector Memory (EM) and EMRA T-cells was enlarged in patients with (n = 18) compared to patients without (n = 35) early CMV reactivation. In the CD8+ T-cell compartment, this was caused by a major expansion of CD8+ EM and EMRA T-cells (median 485 vs. 141, p < 0.0001 and 509 vs. 114 cells / μL, p < 0.0001, Figure A), while the Naive (median 105 vs. 169 cells / μL, p = 0.17) and Central Memory (CM) compartment did not differ. In the CD4+ compartment, a non-significant increase of EM and EMRA cells was accompanied by a non-significant decrease of N and CM cells. CMV-specific CD8+ T-cells (median 4.1, range 0.3 - 25.6% of CD8+ T-cells) were detected within the EM and EMRA compartment.
Two year after HSCT, data were available for 76 patients. Both in patients with (lymphocyte subsets: n = 34 / T-cell differentiation: n = 12) and without (n = 42 / n = 19) early CMV reactivation, a further reconstitution of CD4+ and CD8+ T-cells was observed, reflected by an expansion of Naive and CM cells (Figure B). However, the total number of CD8+ T-cells decreased in patients with early CMV reactivation, caused by a contraction of late differentiated CD8+ EM and EMRA cells (median 825 to 618, p = 0.0068 and 555 to 378 cells / μL, p = 0.0342, Wilcoxon).
Early CMV reactivation leaves a virus-specific and dynamic imprint on the reconstituting immune system 1 and 2 year after HSCT. The marked expansion of CD8 + EM and EMRA T-cells was not seen in patients with early EBV or HAdV reactivation and did not compromise the reconstitution of the Naive and CM compartment available to respond to neo- and recall antigens. Pediatric HSCT recipients differed from solid organ transplantation recipients in which CMV has been correlated to an accelerated and ongoing accumulation of late differentiated T-cells. The dynamic contraction of the CD8+ late differentiated memory T-cell compartment in the second year after HSCT implies that an ongoing process of immune-regulation and further reconstitution is modeling the cellular immune system after discontinuation of immunosuppressive medication.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal