Abstract
Three target-specific oral anticoagulants (TSOA's) have been recently compared to standard therapy with a vitamin K antagonist (VKA) for the treatment of acute venous thromboembolism (VTE). Dabigatran (D) is a direct thrombin inhibitor, while rivaroxaban (R) and apixaban (A) block activated coagulation factor X. Those agents have not been compared head-to-head and minimal data exist to guide the choice of drug for any given patient.
We performed an indirect comparison between TSOA's using all phase III trials comparing D, R or A to a VKA for the acute treatment of VTE. Studies including patients with deep vein thrombosis (DVT), pulmonary embolism (PE) or both were considered. The method used to compute the measure of effect has been described elsewhere (Altman DG et al); briefly, the relative risk (RR) of an event for patients receiving treatment X versus Y (RRXY) is estimated by dividing the RR for treatment X versus Z by the RR for treatment Y versus Z. The standard error (SE) on the logarithmic scale for the indirect comparison XY can be easily computed (SEXY=√[SEXZ2+SEYZ2]). This approach has been previously applied to compare the results of atrial fibrillation trials for D, R and A (Mantha S et al). We employed the SAS 3.0.1 statistical platform, along with the “meta” package. The hazard ratio (HR) of an event during the study period was used for computations when provided in the original journal article since it reflected adjustment for pertinent factors and because for infrequent events the HR and RR have similar values.
Four phase III trials were identified, consisting of RE-COVER (Schulman S et al), EINSTEIN-DVT (EINSTEIN Investigators), EINSTEIN-PE (EINSTEIN-PE Investigators) and AMPLIFY (Agnelli G et al). The distribution of patient characteristics was similar between studies (see Table). VTE outcomes were reported for the intention-to-treat population and results for major bleeding were given for the safety (ie “on-treatment”) groups. The measures of effect for EINSTEIN-DVT and EINSTEIN-PE where combined using inverse variance weights and the fixed effect meta-analysis model.
Characteristics of Patients
| Trial . | RE-COVER* . | EINSTEIN-DVT† . | EINSTEIN-PE† . | AMPLIFY† . | p‡ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drugs Compared . | D . | VKA . | R . | VKA . | R . | VKA . | A . | VKA . | ||
| Number of Patients | 1273 | 1266 | 1731 | 1718 | 2419 | 2413 | 2691 | 2704 | - | |
| Age – mean ± SD (years) | 55.0±15.8 | 54.4±16.2 | 55.8±16.4 | 56.4±16.3 | 57.9±7.3 | 57.5±7.2 | 57.2±16.0 | 56.7±16.0 | >0.99 | |
| Female Gender – % | 42.0 | 41.1 | 42.6 | 43.7 | 45.9 | 48.3 | 41.7 | 40.9 | <0.001 | |
| Weight – mean ± SD (kg) | 85.5±19.2 | 84.2±18.3 | NA | NA | NA | NA | NA | NA | - | |
| Creatinine Clearance – mean ± SD (ml/min) | 105.8±40.7 | 104.4±39.9 | NA | NA | NA | NA | NA | NA | - | |
| Index Event – % | DVT | 69.1 | 68.6 | 98.7 | 98.8 | 0 | 0 | 65.0 | 65.9 | -¶ |
| PE | 21.2 | 21.4 | 0 | 0 | 74.9 | 75.5 | 25.2 | 25.2 | ||
| DVT and PE | 9.5 | 9.8 | 0.7 | 0.6 | 25.1 | 24.5 | 9.4 | 8.3 | ||
| TTR – %± SD | - | 59.9±22.9 | - | 57.7±NA | - | 62.7±NA | - | 61±NA | - | |
| Trial . | RE-COVER* . | EINSTEIN-DVT† . | EINSTEIN-PE† . | AMPLIFY† . | p‡ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drugs Compared . | D . | VKA . | R . | VKA . | R . | VKA . | A . | VKA . | ||
| Number of Patients | 1273 | 1266 | 1731 | 1718 | 2419 | 2413 | 2691 | 2704 | - | |
| Age – mean ± SD (years) | 55.0±15.8 | 54.4±16.2 | 55.8±16.4 | 56.4±16.3 | 57.9±7.3 | 57.5±7.2 | 57.2±16.0 | 56.7±16.0 | >0.99 | |
| Female Gender – % | 42.0 | 41.1 | 42.6 | 43.7 | 45.9 | 48.3 | 41.7 | 40.9 | <0.001 | |
| Weight – mean ± SD (kg) | 85.5±19.2 | 84.2±18.3 | NA | NA | NA | NA | NA | NA | - | |
| Creatinine Clearance – mean ± SD (ml/min) | 105.8±40.7 | 104.4±39.9 | NA | NA | NA | NA | NA | NA | - | |
| Index Event – % | DVT | 69.1 | 68.6 | 98.7 | 98.8 | 0 | 0 | 65.0 | 65.9 | -¶ |
| PE | 21.2 | 21.4 | 0 | 0 | 74.9 | 75.5 | 25.2 | 25.2 | ||
| DVT and PE | 9.5 | 9.8 | 0.7 | 0.6 | 25.1 | 24.5 | 9.4 | 8.3 | ||
| TTR – %± SD | - | 59.9±22.9 | - | 57.7±NA | - | 62.7±NA | - | 61±NA | - | |
on-treatment population; patients in the dabigatran group received a parenteral anticoagulant for a median of 6.0 days after randomization
intention-to-treat population
chi-square test for counts, ANOVA F-test for means; comparisons made across trials, not treatment groups
not computed because proportions varied by design
D: dabigatran
R: rivaroxaban
A: apixaban
VKA: vitamin K antagonist
SD: standard deviation
NA: information not available
DVT: deep vein thrombosis
PE: pulmonary embolism
TTR: INR time in the therapeutic range
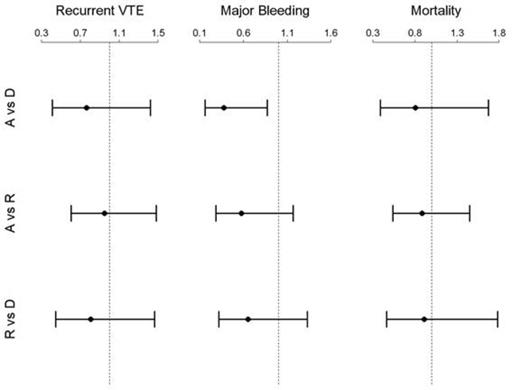
The estimated RR of recurrent VTE was 0.76 (95% CI=0.41-1.42, p=0.39) for A vs D, 0.95 (95% CI=0.61-1.48, p=0.81) for A vs R and 0.81 for R vs D (95% CI=0.44-1.47, p=0.48). For major bleeding, the RR of an event was 0.38 for A vs D (95% CI=0.16-0.87, p=0.02), compared with 0.58 for A vs R (95% CI=0.29-1.17, p=0.13) and 0.65 for R vs D (95% CI=0.32-1.33, p=0.24). Finally, the RR of all-cause mortality was 0.81 for A vs D (95% CI=0.39-1.67, p=0.56), 0.88 for A vs R (95% CI=0.54-1.45, p=0.63) and 0.91 for R vs D (95% CI=0.47-1.78, p=0.79). See Figure for corresponding plots.
Indirect Comparison of Dabigatran, Rivaroxaban and Apixaban for Venous Thromboembolic Disease
Indirect Comparison of Dabigatran, Rivaroxaban and Apixaban for Venous Thromboembolic Disease
Except for major bleeding, there was no significant difference in evaluated outcomes between dabigatran, rivaroxaban and apixaban. A dedicated randomized trial directly comparing the new agents would be required to confirm those results.
Ansell:Bristol-Myers Squibb: Consultancy; Pfizer: Consultancy; Janssen: Consultancy; Boehringer Ingelheim: Consultancy; Daiichi: Consultancy; Alere: Consultancy; Perosphere: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees; Instrumentation Laboratories: Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal