Abstract
The outcomes of patients (pts) with relapsed/refractory acute lymphoblastic leukemia (ALL) remain poor. Dysregulation of the PI3K/AKT/mTOR signal transduction pathway is central to leukemic cell growth and survival. Everolimus is an orally active inhibitor of mTOR approved for the treatment of pts with several tumor types.
The purpose of this phase I/II study was to establish the safety and efficacy of everolimus in combination with hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone) in pts with relapsed/refractory ALL and lymphoblastic lymphoma (LL).
Pts ≥ 10 years of age with relapsed/refractory ALL or LL, ECOG performance status 2 2 and adequate organ function were eligible. Pts were treated with oral everolimus at 5 or 10 mg (phase 1 dosing) continuously concurrently with 8 cycles of the standard hyper-CVAD regimen, starting day 0 of course 1. Everolimus 5 mg daily was established as maximum tolerated dose (MTD) after 2 cycles. Comprehensive proteomic profiling of 10 proteins along with their phosphorylated forms was performed using reverse-phase protein arrays (RPPA). Total RNA was isolated from patient samples using TRIzol reagent and gene expression analysed using a HumanHT-12 v4 gene expression chip from Illumina (N = 5). Pharmacokinetic (PK) studies were performed on day 0 and day 15 of course 1 (N = 8).
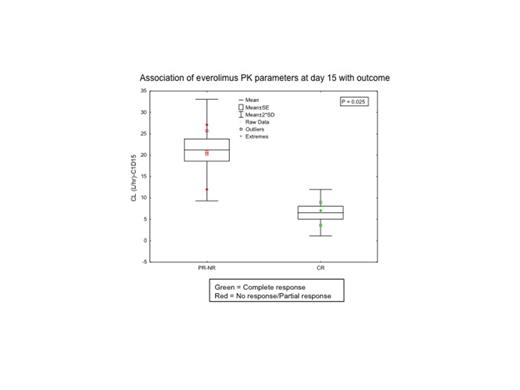
Twenty pts were enrolled between April 2010 and June 2013, with accrual ongoing. Clinical characteristics included 9 pts in first salvage, 2 in second salvage and 9 beyond second salvage (Table 1). Median (Med) number of cycles given was 2 (1-5). Med follow-up was 19 months (1-35). Seven of 20 pts responded for an overall response rate of 35% with complete remission (CR) in 6 pts (30%), CR with incomplete count recovery (CRi) in 1 (5%), and partial remission (PR) in 2 (10%). Six of 9 pts (66%) in first salvage achieved CR, and one of 2 pts in second salvage achieved CRi. Four pts proceeded to stem cell transplant in CR. Med event-free survival (EFS) and med overall survival (OS) were 6 and 7 months, respectively, for pts in 1st salvage, and 2 and 4 months, respectively for pts in second salvage and beyond (P=0.01 and P=0.002). The 1-year OS is 47% for 1st salvage pts and 9% for pts in second salvage and beyond. Grade 3 mucositis was dose-limiting toxicity. Other grade 3/4 toxicities including infections, transaminitis, diarrhea, headache, and increased bilirubin occurred in 18 (90%), 6 (30%), 2 (10%), 2 (10%), and 2 (10%) pts. Among the 10 pts profiled by RPPA, inhibition of mTOR signaling (p-pS6K) was observed in 7/10 (70%). Everolimus significantly inhibited phosphorylation of S6K on both, pS235-S236 and pS240-S244 sites (P=0.007 and P=0.01, respectively). Inhibition of pS6 was observed at both 5 mg and 10 mg doses. No statistically significant difference in phosphorylation of 4EBP1, AKT or other proteins was noted. Gene set enrichment analysis performed in paired PB samples from 5 pts showed enrichment of the ABC transporter gene set in pts who failed to respond. Analysis of PB samples 24 hrs post everolimus exposure was associated with reduction of the signature for miR-21, miR-30 and miR-507 gene sets, irrespective of a biological response to mTOR inhibition as assessed by S6K phosphorylation. PK analysis showed that area under the curve (AUC) for everolimus was dose-dependent with higher AUC at 10 mg versus 5 mg. Those achieving CR had significantly higher AUC and lower clearance at steady state compared to pts with PR/no response (Fig. 1, P=0.025), but it was not associated with toxicity.
Pt characteristics (N = 20)
| Characteristic | N (%) / [range] |
| Med age, years | 24 [11-64] |
| Male | 13(65%) |
| Med prior chemotherapies | 2 [1-7] |
| Prior radiotherapy | 3(15%) |
| Prior stem cell transplant | 4(20%) |
| ECOG PS | |
| 0-1 | 17(85%) |
| Diagnosis | |
| B-ALL | 12(60%) |
| T-ALL | 8(40%) |
| Med WBC X 109 | 5.7 [0.3-100.7] |
| Med hemoglobin g/dL | 9.6 [6.9-12.7] |
| Med platelets x 109 | 57 [5-240] |
| Med bone marrow blasts % | 67 [0-98] |
| Med peripheral blood blasts % | 19.5 [0-100] |
| CNS involvement at enrollment | 3 (15%) |
| Cytogenetics | |
| Diploid | 5(25%) |
| Hyperdiploid | 4(20%) |
| Miscellaneous | 4(20%) |
| Insufficient | 7(35%) |
| Characteristic | N (%) / [range] |
| Med age, years | 24 [11-64] |
| Male | 13(65%) |
| Med prior chemotherapies | 2 [1-7] |
| Prior radiotherapy | 3(15%) |
| Prior stem cell transplant | 4(20%) |
| ECOG PS | |
| 0-1 | 17(85%) |
| Diagnosis | |
| B-ALL | 12(60%) |
| T-ALL | 8(40%) |
| Med WBC X 109 | 5.7 [0.3-100.7] |
| Med hemoglobin g/dL | 9.6 [6.9-12.7] |
| Med platelets x 109 | 57 [5-240] |
| Med bone marrow blasts % | 67 [0-98] |
| Med peripheral blood blasts % | 19.5 [0-100] |
| CNS involvement at enrollment | 3 (15%) |
| Cytogenetics | |
| Diploid | 5(25%) |
| Hyperdiploid | 4(20%) |
| Miscellaneous | 4(20%) |
| Insufficient | 7(35%) |
The combination ofhyper-CVAD plus everolimus was well tolerated, and findings indicate that further testing of mTOR inhibitors with standard chemotherapy in the frontline therapy of ALL and LL is warranted.
Kantarjian:Sanofi-Aventis: Research Funding. Ravandi:Sunesis: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Teva: Consultancy, Honoraria; Pfizer: Honoraria; Merck: Research Funding; Bayer/Onyx: Consultancy, Honoraria; EMD Serono: Research Funding; Medimmune: Research Funding; Amgen: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal