Abstract
Adoptive immunotherapy is an increasingly effective modality of cancer therapy. The ability to redirect the antigenic specificity of patient-derived T cells toward autologous tumor cells through introduction of T-cell receptors (TCRs) or chimeric antigen receptors (CARs) enables reproducible manufacturing of tumor-reactive T cell products even in patients who carry few, if any, tumor-reactive T cells in their peripheral blood repertoire. We present the results of our pre-clinical studies of adoptive therapy with T cells transduced with a retroviral vector that encodes an enhanced-affinity (a95:LY) variant of the HLA-A*02:01-restricted, NY-ESO-1157-165-specific 1G4 TCR to redirect CD8+ T cells from HLA-A*02:01+ multiple myeloma patients to HLA-A*02:01+, NY-ESO-1-expressing myeloma cells.
CD3-stimulated peripheral blood mononuclear cells from HLA-A*02:01+ multiple myeloma patients were retrovirally transduced with the NY-ESO-1157-165-specific 1G4 a95:LY TCR. CD8+ TCR-transduced cells were isolated by flow cytometric sorting with a NY-ESO-1157-165/HLA-A*02:01 tetramer. The cytolytic activity of CD8+tetramer+ cells was evaluated by 51Cr release assay using as target cells the multiple myeloma cell lines U266 (HLA-A*02:01+ NY-ESO-1+) and UM-9 (HLA-A*02:01- NY-ESO-1+), and T2 cells with or without exogenous NY-ESO-1157-165 peptide. The U266 cell line was stably transduced with luciferase-containing retrovirus and used to develop a xenograft model of diffuse myeloma in NOD/scid/IL-2Rg-null (NSG) mice in order to evaluate the anti-myeloma activity of adoptive therapy with CD8+ TCR-transduced T cells. Mice that received TCR-transduced CD8+cells and developed disease were sacrificed, and human CD138+ cells were harvested from marrow and other sites for evaluation by flow cytometry, HLA-A typing, NY-ESO-1 expression, and loss of heterozygosity (LOH) analysis of the Major Histocompatibility Complex (MHC) on chromosome 6 with short tandem repeat (STR) probes to determine the mechanism of immune escape.
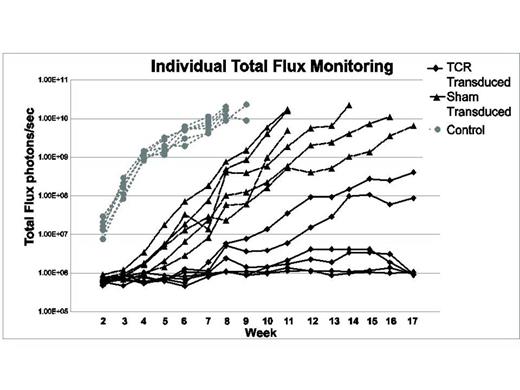
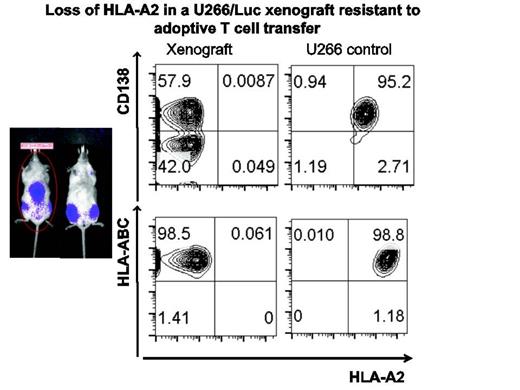
CD8+ TCR-transduced cells were specifically cytolytic against HLA-A*02:01+, NY-ESO-1+ tumor cells. Intravenous injection of luciferase-transduced U266/Luc in sub-lethally irradiated NSG mice led to the development of a multiple myeloma-like disease. Mice that received U266/Luc without T cells (control) developed progressive disease within 2 weeks, and met criteria for euthanasia by week 9. Mice that received U266/Luc with sham-transduced cells developed myeloma more slowly, yet all met criteria for euthanasia by week 18 after U266/Luc injection. Of the 6 mice that received U266/Luc and NY-ESO-1-specific TCR-transduced CD8+ T cells, 4 did not have any evidence of myeloma by bioluminescence at the end of study (week 18), and 2 had low burden disease at that point. Kaplan-Meier survival analysis demonstrated significant improvement of overall survival in the mice that received TCR-transduced T cells (Log-rank test p< 0.0001). Flow cytometric analysis of human CD138+ cells isolated from the 2 mice that developed myeloma despite adoptive therapy with NY-ESO-1-specific T cells demonstrated selective loss of surface HLA-A*02 expression, with preserved expression of other MHC class I molecules. Real-time PCR analysis also confirmed preserved expression of HLA-A, B2M, and NY-ESO-1. Low resolution HLA-A typing of genomic DNA from myeloma cells from these 2 mice revealed loss of HLA-A*02, but retention of HLA-A*03. LOH analysis using 7 STR markers mapping to the MHC on chromosome 6p21.3 and 2 markers on chromosome 15 (control) demonstrated LOH in the MHC involving the HLA-A locus in myeloma cells from both of the mice that developed disease despite TCR-transduced T cells. The extent of LOH in the myeloma cells from the 2 mice was distinct.
LOH in the MHC as a mechanism of immune scape has been described in allogeneic transplantation for AML, but has not been described in multiple myeloma. We identified LOH affecting the HLA-A allele targeted by adoptively transferred TCR-transduced T cells. Given that NY-ESO-1-specific TCR-transduced cells have recently entered clinical testing, this mechanism of immune escape should be evaluated in patients that fail therapy despite persistence of adoptively transferred T cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal