Adolescents and young adults (AYA) with cancer have been designated as a vulnerable population by the National Cancer Institute. This group, defined by the ages of 16-39 years, has not enjoyed the same survival improvements over the past several decades as older and younger cohorts. Most of the AYA patients with lymphoma are curable, even in advanced stage. After first-line therapy, 15% to 20% do not respond to treatment and relapse. Busulfan based preparative regimens have been effective in ASCT for a Hodgkin lymphoma (HL) and non- Hodgkin lymphoma (NHL). This study aims to determine the long term outcomes among AYA patients with lymphoma who received Busulfan based regimen for ASCT.
This was a retrospective study for AYA patients (age 16-39) undergoing ASCT for lymphoma consolidation between Jan/2000 and Dec/2010 at Taussig’s Cancer Center. Patients were identified from our Bone Marrow Transplantation database. Most of the patients (98.6%) had received Bu-Cy-VP16. Briefly, Busufan (Bu) total dose was 14 mg/kg, Etoposide 60mg/kg and cyclophosphamide dose 120mg/kg. Busulfan was not targeted and it was administered orally or IV. Pre-transplant patient and transplant characteristics are presented in table-1 and table-2 respectively.
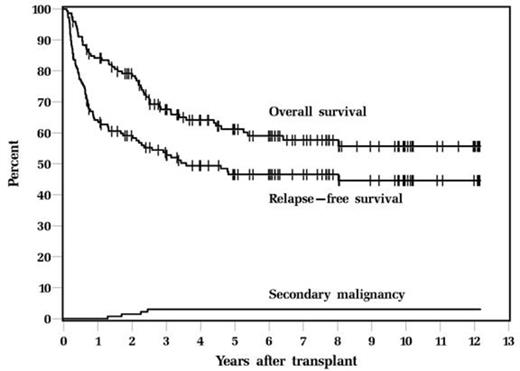
There were total 146 patients who received Bu-Cy-VP16 (80 HL, 66 NHL). The median age was 32 years (range, 16-39 years). Only 37 patients (25.3%) were in CR, 89 patients (61.0%) were PR and 20 patients (13.7%) had refractory disease. Most patients had at least 2 regimens of chemotherapy prior to ASCT. The regimen was well tolerated, with day 100 mortality 4.1% and non-relapse mortality 2.7%. With a median follow up of 6.2years, 5-year relapse-free survival (RFS) and overall survival (OS) were 46.6 % and 61.2%; 10-year RFS and OS were 44.6% and 55.7% (figures 1).
Four patients developed secondary malignancy (2.7%); the secondary malignancy were: ALL at 1.7 yrs after transplant, AML at 2.3 yrs, MDS at 2.5 yrs. One patient developed lung carcinoma. Cumulative incidence of secondary malignancy at 10 years is 3% which is considerably less than 9% that we reported in our whole patient population who gets this regimen which could be attributed to age and also due to previous chemotherapy regimens.
Worse ECOG performance status (hazard ratio [HR] 1.60, 95% confidence interval [CI] 1.13-2.28, P=0.009) and HL diagnosis (HR 1.78, CI 1.01-3.14, P=0.048) were the only significant risk factors for mortality in multivariable Cox analysis. Worse ECOG performance status (HR 1.53, CI 1.11-2.10, P=0.010 was the only prognostic factor for relapse/mortality (RFS).
The study confirms that Bu-Cy-VP16 is very well tolerated in AYA population with low 100-day NRM and very low incidence of secondary malignancy.
Overall Survival and Relapse-Free Survival, and secondary malignancy after ASCT
Patient characteristics and Pre-Transplant variables
| Variable . | Number . | % . |
|---|---|---|
| ECOG performance (N=139) | ||
| 0 | 82 | 59 |
| 1 | 51 | 36.7 |
| 2 | 4 | 2.9 |
| 3 | 2 | 1.4 |
| Diagnosis | ||
| HL/NHL | 80/66 | 54.8 / 45.2 |
| Number of prior Chemotherapy regimens | ||
| 1 | 18 | 12.3 |
| 2 | 104 | 71.2 |
| ≥3 | 24 | 16.4 |
| Disease status at Transplant | ||
| CR1 | 7 | 4.8 |
| ≥CR2 | 30 | 20.5 |
| PR1 | 19 | 13.0 |
| ≥PR2 | 70 | 47.9 |
| Refractory | 20 | 13.7 |
| Variable . | Number . | % . |
|---|---|---|
| ECOG performance (N=139) | ||
| 0 | 82 | 59 |
| 1 | 51 | 36.7 |
| 2 | 4 | 2.9 |
| 3 | 2 | 1.4 |
| Diagnosis | ||
| HL/NHL | 80/66 | 54.8 / 45.2 |
| Number of prior Chemotherapy regimens | ||
| 1 | 18 | 12.3 |
| 2 | 104 | 71.2 |
| ≥3 | 24 | 16.4 |
| Disease status at Transplant | ||
| CR1 | 7 | 4.8 |
| ≥CR2 | 30 | 20.5 |
| PR1 | 19 | 13.0 |
| ≥PR2 | 70 | 47.9 |
| Refractory | 20 | 13.7 |
Transplant Variables and outcomes
| Variable . | N (%) . |
|---|---|
| CD34 dose x10^6/kg | |
| Median (range) | 9.30 (2.08-45.39) |
| Days to PMN >500 | |
| Median (range) | 10 (9-12) |
| Days to PLT >20,000 | |
| Median (range) | 13 (7-129) |
| Length of stay | |
| Median (range) | 21 (19-34) |
| Status at last follow up | |
| Alive | 90 (61.6) |
| Dead | 56 (38.4) |
| Relapse | |
| Yes | 63 (43.2) |
| No | 83 (56.8) |
| Causes of death | |
| Relapse | 44 (78.6) |
| Infection | 3 (5.4) |
| Secondary malignancy | 2 (3.6) |
| ARDS | 1 (1.8) |
| Cardiac tamponade | 1 (1.8) |
| GI hemorrhage | 1 (1.8) |
| Unknown | 4 (7.1) |
| Variable . | N (%) . |
|---|---|
| CD34 dose x10^6/kg | |
| Median (range) | 9.30 (2.08-45.39) |
| Days to PMN >500 | |
| Median (range) | 10 (9-12) |
| Days to PLT >20,000 | |
| Median (range) | 13 (7-129) |
| Length of stay | |
| Median (range) | 21 (19-34) |
| Status at last follow up | |
| Alive | 90 (61.6) |
| Dead | 56 (38.4) |
| Relapse | |
| Yes | 63 (43.2) |
| No | 83 (56.8) |
| Causes of death | |
| Relapse | 44 (78.6) |
| Infection | 3 (5.4) |
| Secondary malignancy | 2 (3.6) |
| ARDS | 1 (1.8) |
| Cardiac tamponade | 1 (1.8) |
| GI hemorrhage | 1 (1.8) |
| Unknown | 4 (7.1) |
Univariable Prognostic Factors for Overall Survival and Relapse-Free Survival
| Variable . | Overall survival . | Relapse-Free survival . | ||||
|---|---|---|---|---|---|---|
| HR . | 95%CI . | P . | HR . | 95%CI . | P . | |
| ECOG status (Per 1 point increase) | 1.56 | 1.10-2.22 | 0.013 | 1.53 | 1.11-2.10 | 0.01 |
| Diagnosis: HL/NHL | 1.62 | 0.94-2.79 | 0.08 | 1.34 | 0.85-2.13 | 0.21 |
| Disease status at transplant | ||||||
| PR/CR | 1.85 | 0.86-3.98 | 0.12 | 2.01 | 1.07-3.78 | 0.029 |
| Refractory/CR | 3.07 | 1.25-7.51 | 0.014 | 2.40 | 1.08-5.35 | 0.032 |
| Variable . | Overall survival . | Relapse-Free survival . | ||||
|---|---|---|---|---|---|---|
| HR . | 95%CI . | P . | HR . | 95%CI . | P . | |
| ECOG status (Per 1 point increase) | 1.56 | 1.10-2.22 | 0.013 | 1.53 | 1.11-2.10 | 0.01 |
| Diagnosis: HL/NHL | 1.62 | 0.94-2.79 | 0.08 | 1.34 | 0.85-2.13 | 0.21 |
| Disease status at transplant | ||||||
| PR/CR | 1.85 | 0.86-3.98 | 0.12 | 2.01 | 1.07-3.78 | 0.029 |
| Refractory/CR | 3.07 | 1.25-7.51 | 0.014 | 2.40 | 1.08-5.35 | 0.032 |
Duong:Celgene: Honoraria, Research Funding. Hill:Celgene: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal