Abstract
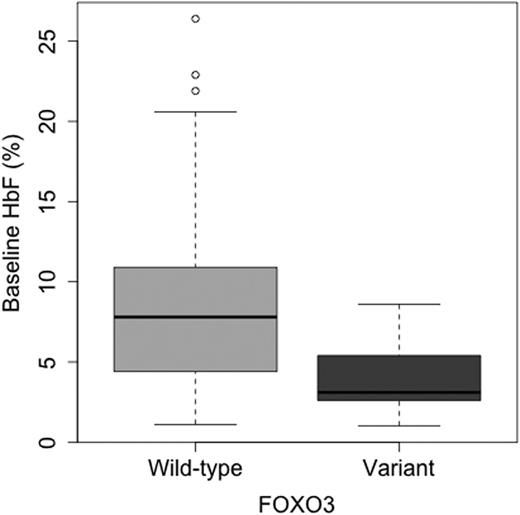
Although they ostensibly have a monogenetic disease, individuals with sickle cell anemia (SCA) exhibit wide variability in their degree of clinical severity. One of the most powerful and reproducible predictors of disease severity is the level of endogenous fetal hemoglobin (HbF). Several genetic modifiers of HbF levels have previously been identified, by association with beta globin haplotypes, or by genome wide association studies. These methods are only able to detect common variants, with a minor allele frequency (MAF) greater than 5%. We have applied whole exome sequencing (WES) to find new genetic variants associated with baseline HbF in SCA, using 171 pediatric SCA genomes from participants in two clinical trials, HUSTLE (NCT NCT00305175) and SWiTCH (NCT 00122980). WES allows identification of both common and rare exonic variants, and use of burden analysis testing. In order to capture the association between the phenotype variants with a MAF below 1%, burden tests maximize power by grouping low frequency variants together by gene. This allows us to transition from a single variant theory, in which a variant is associated with a phenotype, to a gene-based theory, in which a collection of rare variants within a single gene are associated with the phenotype. Burden analysis (T1), found seven unique non-synonymous variations in a Forkhead box O transcription factor, FOXO3, to be significantly associated with lower HbF (p=5.6x10-4, b-value ln HbF -0.66). All variants produced the same effect, a lowering of HbF. HbF values were normalized using natural log transformation to permit analysis, and adjusted for age, BCL11A, and XmnI variant status. The box-plot below shows the overall effect of any of the non-synonymous exonic variants in FOXO3 on baseline HbF compared to wild-type. Each individual was heterozygous for a variant. FOXO3 is involved in multiple cellular processes, including cell cycle arrest, removal of reactive oxygen species, and regulation of erythroid differentiation. Erythroid maturation is altered by changes in post-translational maturation (PTM) such as removal of a phosphorylation site, as the in Ser553Phe variant identified in our analysis (Bakker, et al, JCB 2004). Changes in erythroid maturation kinetics may affect the amount of HbF produced. Variations in FOXO3 may represent another contributor to the heritability of HbF in patients with SCA. Further functional genomics analysis of FOXO3 may add to our understanding of gamma globin induction.
Close modal
Table 1
Description of FOXO3 variants found to be associated with lower HbF in a cohort of pediatric sickle cell patients by WES and burden testing (T1).
| Chr 6 Location . | Amino Acid Change . | Altered PTM . | PolyPhen2 prediction . | %MAF . |
|---|---|---|---|---|
| 108882607 | Asp66Asn | none known | Possibly damaging | 0.3 |
| 108882830 | Ala140Val | none known | Benign | 0.6 |
| 108984883 | Asp283Asn | none known | Damaging | 0.3 |
| 108985057 | Ala121Thr | none known | Benign | 0.3 |
| 108985280 | Pro415Leu | none known | Damaging | 0.6 |
| 108985679 | Arg548His | methylation | Damaging | 0.3 |
| 108985694 | Ser553Phe | phosphorylation | Benign | 0.3 |
| Chr 6 Location . | Amino Acid Change . | Altered PTM . | PolyPhen2 prediction . | %MAF . |
|---|---|---|---|---|
| 108882607 | Asp66Asn | none known | Possibly damaging | 0.3 |
| 108882830 | Ala140Val | none known | Benign | 0.6 |
| 108984883 | Asp283Asn | none known | Damaging | 0.3 |
| 108985057 | Ala121Thr | none known | Benign | 0.3 |
| 108985280 | Pro415Leu | none known | Damaging | 0.6 |
| 108985679 | Arg548His | methylation | Damaging | 0.3 |
| 108985694 | Ser553Phe | phosphorylation | Benign | 0.3 |
Figure 1
Boxplot demonstrating effect of any FOXO3 variants on endogenous %HbF compared to wild-type individuals.
Figure 1
Boxplot demonstrating effect of any FOXO3 variants on endogenous %HbF compared to wild-type individuals.
Disclosures:
Off Label Use: Hydroxyurea is not FDA approved for use in pediatric sickle cell patients.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2013 by The American Society of Hematology
2013

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal