Key Points
Highly electronegative LDL (L5), which is elevated in patients with STEMI, induces platelet activation and aggregation through LOX-1.
L5 may have a role in promoting thrombogenesis that leads to STEMI.
Abstract
Platelet activation and aggregation underlie acute thrombosis that leads to ST-elevation myocardial infarction (STEMI). L5—highly electronegative low-density lipoprotein (LDL)—is significantly elevated in patients with STEMI. Thus, we examined the role of L5 in thrombogenesis. Plasma LDL from patients with STEMI (n = 30) was chromatographically resolved into 5 subfractions (L1-L5) with increasing electronegativity. In vitro, L5 enhanced adenosine diphosphate–stimulated platelet aggregation twofold more than did L1 and induced platelet-endothelial cell (EC) adhesion. L5 also increased P-selectin expression and glycoprotein (GP)IIb/IIIa activation and decreased cyclic adenosine monophosphate levels (n = 6, P < .01) in platelets. In vivo, injection of L5 (5 mg/kg) into C57BL/6 mice twice weekly for 6 weeks shortened tail bleeding time by 43% (n = 3; P < .01 vs L1-injected mice) and increased P-selectin expression and GPIIb/IIIa activation in platelets. Pharmacologic blockade experiments revealed that L5 signals through platelet-activating factor receptor and lectin-like oxidized LDL receptor-1 to attenuate Akt activation and trigger granule release and GPIIb/IIIa activation via protein kinase C-α. L5 but not L1 induced tissue factor and P-selectin expression in human aortic ECs (P < .01), thereby triggering platelet activation and aggregation with activated ECs. These findings indicate that elevated plasma levels of L5 may promote thrombosis that leads to STEMI.
Introduction
Platelet activation triggers acute thrombosis, the main cause of acute coronary occlusion in patients with ST-elevation myocardial infarction (STEMI),1 and it predicts the degree of damage in acute MI.2,3 Additionally, collagen adenosine diphosphate (ADP) closure times are significantly shortened in patients with STEMI.3,4 ADP is an important soluble agonist that is released from adherent and activated platelets. Excess ADP has been shown to regulate the P2Y12/phosphatidylinositol-3 kinase (PI3K) pathway that is essential for stable platelet aggregation.5 Furthermore, the release of ADP enhances platelet aggregation by increasing lectin-like oxidized low-density lipoprotein (LDL) receptor-1 (LOX-1) expression and by mediating the inside-out integrin signaling–dependent activation of glycoprotein (GP)IIb/IIIa.6 Therefore, the use of pharmacologic therapies such as aspirin,7 clopidogrel,8 and GPIIb/IIIa inhibitors9 to inhibit platelet activation is paramount for preventing the onset and recurrence of acute coronary syndrome. However, complications are associated with the use of currently available antiplatelet drugs, and the efficacy of these drugs remains to be further improved. Thus, it is important to identify plasma factors that initiate platelet activation so that new targeted approaches can be developed.
We have shown that human LDL can be chromatographically resolved into 5 subfractions (L1-L5) with increasing electronegativity.10 As the electronegativity of LDL increases from L1 to L5, the content of apolipoprotein B decreases, and the content of other lipoproteins increases.11 L5 is not recognized by the normal LDL receptor but is internalized by LOX-1, which in turn leads to endothelial cell (EC) apoptosis.12 Circulating L5 has been shown to be proatherogenic13 and is the only subfraction of human LDL capable of inducing endothelial dysfunction and atherogenic responses in cultured vascular cells.10,14,15 L5 levels are moderately increased in patients with high cardiovascular risks such as hypercholesterolemia, type 2 diabetes mellitus, and smoking.10,14,16 In addition, we have recently shown that plasma levels of L5 are elevated in patients with STEMI compared with those in control subjects, in whom L5 levels are low or undetectable.17 Furthermore, L5 can induce methylation of the fibroblast growth factor-2 (FGF2) promoter to reduce the production of FGF2, a protein important for normal endothelial function.17 These findings indicate that L5 may play an important role in the pathogenesis of STEMI.
L5 has proatherogenic effects similar to those of laboratory-derived oxidized LDL (ox-LDL), which, interestingly, has been shown to render the endothelium highly thrombogenic by increasing tissue factor expression18 and EC apoptosis.19 In addition, ox-LDL–induced endothelial dysfunction is accompanied by an increase in P-selectin and E-selectin expression on cells, resulting in increased platelet-EC interactions.20
We hypothesized that, when elevated, L5 is a critical atherogenic LDL capable of enhancing the occurrence and progression of thrombotic events. We confirmed that L5 levels are elevated in patients with STEMI and identified a mechanism of platelet activation and endothelial dysfunction mediated by L5.
Patients and methods
Study subjects
This study was approved by the institutional review boards of Kaohsiung Medical University Hospital (KMUH), Kaohsiung Municipal Ta-Tung Hospital (KMTTH), and China Medical University Hospital (CMUH) in Taiwan, and all participants gave written informed consent in accordance with the Declaration of Helsinki. A total of 30 patients with STEMI were enrolled in this study between October 2010 and August 2012. The control group comprised 30 subjects without metabolic syndrome. All study patients were seen by cardiologists at KMUH, KMTTH, and CMUH. For patients with STEMI, blood samples were collected after the insertion of an intravenous catheter and before 0.09% saline was administered and primary percutaneous coronary intervention was performed for acute STEMI patients. Clinical and medication histories for the 3 months preceding case enrollment were recorded for each patient. Lipid parameters for all study subjects were measured in the Department of Laboratory Medicine at KMUH (accredited by the College of American Pathologists) according to standard operating procedures.
LDL isolation and separation of LDL subfractions
Whole blood samples (20 mL) were freshly collected and anticoagulated with 5 mM EDTA. LDL (density = 1.063-1.019) was isolated by sequential potassium bromide density centrifugation and treated with 5 mM EDTA and nitrogen to avoid ex vivo oxidation.21 LDL was further divided into subfractions L1, L2, L3, L4, and L5 against a graded salt gradient by using anion-exchange columns (Uno-Q12; Bio-Rad Laboratories, Inc., Hercules, CA) with the ÄKTA fast protein liquid chromatography system (GE Healthcare Life Sciences, Pittsburgh, PA) as previously described.12,14 The effluent was monitored at 280 nm and protected from ex vivo oxidation with 5 mM EDTA. The LDL subfractions from patients with STEMI were separately concentrated by using Centriprep filters (YM-30; EMD Millipore Corp., Billerica, MA) and sterilized by passing through 0.22-μm filters. Additional details are provided in the supplemental Methods on the Blood Web site.
Preparation of human platelet-rich plasma
Whole blood (40 mL) was drawn from control subjects and added into 1:10 sodium citrate anticoagulant buffer (170 mM sodium citrate and 83 mM citrate acid). Platelet-rich plasma (PRP) was prepared by centrifugation at 150g for 25 minutes at room temperature.
Aggregation study for human platelets
Platelet aggregation was studied as previously described.22 PRP was adjusted to a concentration of 3.5 × 108 platelets/mL and pretreated with 5, 25, or 50 μg/mL L1 or L5 from STEMI patients at 37°C for 15 minutes. Platelet aggregation was initiated by the addition of 4 μM ADP, and the extent of platelet aggregation was measured as the turbidity change by using an aggregometer (Lumi-Aggregometer; Payton Scarborough, Canada) at 37°C under stirring at 900 rpm. To abolish platelet aggregation, PRP was pretreated with different pharmacologic blockades, including 10 μg/mL TS-92 (from the laboratory of T.S., National Cerebral and Cardiovascular Center, Japan), 10 μM platelet-activating factor receptor (PAFR) antagonist ABT-491 (Calbiochem, La Jolla, CA), 0.3 µM PI3K inhibitor Wortmannin, and 1 μM protein kinase C (PKC) inhibitor RO31820 (Sigma-Aldrich, St. Louis, MO).
Parallel plate flow chamber assay for human platelets
To mimic the flow of blood in vitro, we used a parallel plate flow chamber assay (Glycotech, Rockville, MD) similar to that described by Cruz and colleagues.23 PRP samples were stimulated by adding 50 μg/mL L1 or L5 from STEMI patients or phosphate-buffered saline (PBS; control) at 37°C for 10 minutes; then, the samples were perfused for 2 minutes, and the fibrinogen-coated bottom of the parallel flow chamber was washed with PBS. Attached platelets were observed by using a phase-contrast microscope (Eclipse TE300; Nikon, Garden City, NY) equipped with an image recording system (Model Quantix Photometrics, Tucson, AZ). To study the interaction of platelets with activated bovine aortic ECs (BAECs), we treated BAECs with or without 50 µg/mL L1 or L5 from STEMI patients at 37°C for 24 hours. Treated BAECs replaced fibrinogen to form the bottom of the parallel flow chamber. We perfused calcein AM–labeled PRP through the chamber for 2 minutes at a wall shear rate of 750 S−1. To examine adherence, platelets were visualized by using a fluorescence microscope (IX70; Olympus, Tokyo, Japan) equipped with a medical-grade micro-imaging system (MacroFire; Optronics, Goleta, CA). The nuclei of BAECs were stained with 1 μM Hoechst 33342 (Invitrogen, Molecular Probes, Carlsbad, CA) for 5 minutes. Additional details are provided in the supplemental Methods.
Tail bleeding time assay
L5 or L1 (5 mg/kg each) from STEMI patients or PBS (negative control) was injected into the tail vein of adult male C57BL/6 mice or LOX-1 knockout mice twice a week for 6 weeks (n = 3 for each treatment group). Mice were anesthetized with an intraperitoneal injection of 25 mg/kg pentobarbital. Tails were cut 2.0 mm from the end and were immersed in prewarmed PBS. Tail bleeding time was determined as previously described24 and was recorded as the time required from the start of bleeding to the cease of bleeding. The body weight of each mouse was measured at baseline and at the end of the 6-week study.
Western blot analysis
PRP was treated with L1 (50 μg/mL) or L5 (25 or 50 μg/mL) from STEMI patients at 37°C for 15 minutes and solubilized in NETN buffer. Platelet lysates were separated by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were electrotransferred to polyvinylidene fluoride membrane and probed with an antibody against the protein of interest. Additional details are provided in the supplemental Methods.
Detection of cellular cyclic adenosine monophosphate in human platelets
The concentration of PRP was adjusted to 3.5 × 108 platelets/mL, and PRP was treated with various concentrations of L1 or L5 from STEMI patients at 37°C for 15 minutes. To determine the concentration of cyclic adenosine monophosphate (cAMP), potassium hydroxide and acetic anhydride were added to acetylate each sample and standard. An enzyme immunoassay (Cayman Chemical, Ann Arbor, MI) was used to quantify cAMP.
Flow cytometry–based platelet activation assay
Staining of human samples
PRP (3.6 × 108/mL) was exposed to ADP (4 M), 50 µg/mL L1 or L5 from STEMI patients, or vehicle control at 37°C for 15 minutes. Phycoerythrin-labeled P-selectin antibody (BD, San Jose, CA) and free-form Oregon green 488–labeled fibrinogen antibody (Molecular Probes) were used to detect P-selectin and activated GPIIb/IIIa on platelets. The fluorescein-labeled platelets were detected by using a flow cytometer (FACScan System; BD). Data were collected from 50 000 platelets per experimental group, and the platelets were identified on the basis of their characteristic forward and orthogonal light-scattering profile. The mean fluorescence intensities of the experimental groups were plotted against that of the PBS control group.
Staining of mouse samples
Mouse whole blood was drawn from the carotid artery of 3 adult male C57BL/6 mice and collected into tubes containing sodium citrate buffer. The samples were then incubated with phycoerythrin-labeled P-selectin antibody (BD) and free-form Oregon green 488–labeled fibrinogen antibody (Molecular Probes). According to the methodology described above, the fluorescein-labeled platelets were detected by using flow cytometry.
Human platelet–cultured EC interactions
To examine platelet-EC interactions, human aortic ECs (HAECs) were exposed to L5 (25 μg/mL) or L1 (500 μg/mL) from STEMI patients at 37°C for 24 hours. Culture media that contained L5 or L1 was removed, and HAECs were washed with PBS 3 times. Subsequently, whole blood samples drawn from control subjects were cocultured with treated HAECs at 37°C for 15 minutes. Unbound platelets were washed away with PBS. Images were acquired by using an inverted microscope (BX51; Olympus).
Immunofluorescence staining
HAECs were treated with 50 μg/mL L5 or L1 from STEMI patients or PBS at 37°C for 24 hours. In situ P-selectin and tissue factor expression was detected by using specific antibodies and fluorescence-conjugated probes. Nuclei were counterstained with 1 μM Hoechst 33342 (Invitrogen, Molecular Probes) for 5 minutes. Fluorescence was visualized by using a fluorescence microscope (IX70; Olympus).
Statistical analysis
All data are presented as relative frequencies for discrete responses and as the mean ± standard deviation for continuous responses. A Student t test and χ2 test were used to compare the difference between 2 groups. P < .05 was considered statistically significant. The Statistical Package for Social Science (version 19.0; SPSS, Chicago, IL) was used to perform all statistical analyses.
Results
Plasma L5 concentration is significantly elevated in patients with STEMI
Patient characteristics and biochemical profiles are shown in supplemental Table 1. Cardiac enzyme levels that were used for the diagnosis of STEMI are also shown in supplemental Table 1. LDL-cholesterol (C) levels were similar between patients with STEMI and control subjects (116.7 ± 32.4 vs 108.1 ± 28.4 mg/dL, respectively; supplemental Table 1). However, the mean L5% was significantly elevated in STEMI patients compared with that in control subjects (15.4 ± 14.5% vs 1.5 ± 1.1%, respectively; P < .001), in whom L5 was undetectable or present only in trace amounts (Figure 1; supplemental Table 1). Furthermore, the mean plasma L5 concentration ([L5]), defined as the L5% multiplied by the LDL-C (in mg/dL), was significantly higher in STEMI patients than in control subjects (18.9 ± 21.0 vs 1.7 ± 1.5 mg/dL, respectively; Figure 1; supplemental Table 1; P < .001).
Elevation of plasma L5 concentration in patients with STEMI. Fast-protein liquid chromatography analysis of LDL showing the content of each subfraction (L1-L5) in (A) normal control subjects with no cardiovascular risk factors and (B) patients with STEMI. The (C) L5% and (D) L5 plasma concentration ([L5]) for each subject is plotted. The line represents the mean of the group. The mean [L5] was significantly higher in STEMI patients (n = 30) than in control subjects (n = 30). ***P < .001, determined by using the Wilcoxon rank-sum test.
Elevation of plasma L5 concentration in patients with STEMI. Fast-protein liquid chromatography analysis of LDL showing the content of each subfraction (L1-L5) in (A) normal control subjects with no cardiovascular risk factors and (B) patients with STEMI. The (C) L5% and (D) L5 plasma concentration ([L5]) for each subject is plotted. The line represents the mean of the group. The mean [L5] was significantly higher in STEMI patients (n = 30) than in control subjects (n = 30). ***P < .001, determined by using the Wilcoxon rank-sum test.
To determine whether L5 from STEMI patients is similar to previously described L5 from diabetes and familial hypercholesterolemia patients,14,25,26 we examined the apolipoprotein content of LDL subfractions L1 and L5 from STEMI patients by using SDS-PAGE analysis. We found that the apolipoprotein content increased from L1 to L5 (data not shown). Furthermore, when we tested the toxicity of L1 and L5 from STEMI patients, we found that 25 to 50 μg/mL L5 but not L1 from STEMI patients induced EC apoptosis (data not shown). However, PAF components in L1 from STEMI patients were significantly different than those in L1 from healthy subjects (data not shown).
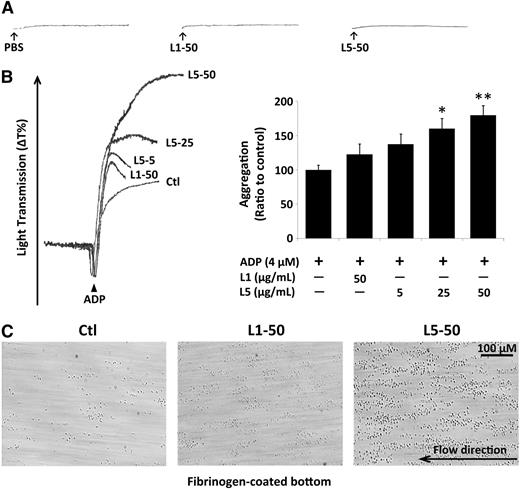
L5 augments platelet adhesion and aggregation
We examined platelet aggregation by using an aggregometer and found that neither L1 nor L5 (50 µg/mL) from STEMI patients induced platelet aggregation (Figure 2A). However, L5 in the presence of ADP dramatically increased platelet aggregation in a dose-dependent manner at 5, 25, and 50 µg/mL (Figure 2B), whereas L1 in the presence of ADP had only a slight effect on platelet aggregation, suggesting that the addition of L5 can prime and enhance ADP-induced platelet aggregation. L5 also significantly enhanced collagen-induced platelet aggregation when compared with the PBS-treated control (data not shown).
L5-induced platelet aggregation and adhesion. The results of platelet aggregation detected by using an aggregometer are shown. (A) Compared with the PBS control, 50 µg/mL L1 or L5 alone induced no platelet aggregation. (B) ADP-induced platelet aggregation was enhanced by the addition of 5, 25, or 50 µg/mL L5 in a dose-dependent manner. The quantification of aggregation is shown as the ratio of the treated group to the ADP-treated control (Ctl). Data are presented as the mean ± standard deviation (n = 6). *P < .05; **P < .01 vs ADP-treated control, determined by using 1-way analysis of variance with the Bonferroni post hoc test. (C) Parallel plate flow chamber analysis showed that platelets treated with 50 µg/mL L5 adhered to the fibrinogen-coated bottom of the chamber at a flow rate of 1500 S−1 (n = 3), whereas those treated with 50 µg/mL L1 or PBS (Ctl) did not.
L5-induced platelet aggregation and adhesion. The results of platelet aggregation detected by using an aggregometer are shown. (A) Compared with the PBS control, 50 µg/mL L1 or L5 alone induced no platelet aggregation. (B) ADP-induced platelet aggregation was enhanced by the addition of 5, 25, or 50 µg/mL L5 in a dose-dependent manner. The quantification of aggregation is shown as the ratio of the treated group to the ADP-treated control (Ctl). Data are presented as the mean ± standard deviation (n = 6). *P < .05; **P < .01 vs ADP-treated control, determined by using 1-way analysis of variance with the Bonferroni post hoc test. (C) Parallel plate flow chamber analysis showed that platelets treated with 50 µg/mL L5 adhered to the fibrinogen-coated bottom of the chamber at a flow rate of 1500 S−1 (n = 3), whereas those treated with 50 µg/mL L1 or PBS (Ctl) did not.
Additionally, we examined the effects of L5 on platelet adhesion by using a parallel plate flow chamber and found that L5-treated platelets adhered to the fibrinogen-coated bottom of the chamber at a flow rate of 1500 S−1 (Figure 2C), whereas L1- or PBS-treated platelets did not.
Platelets are activated in L5-injected mice
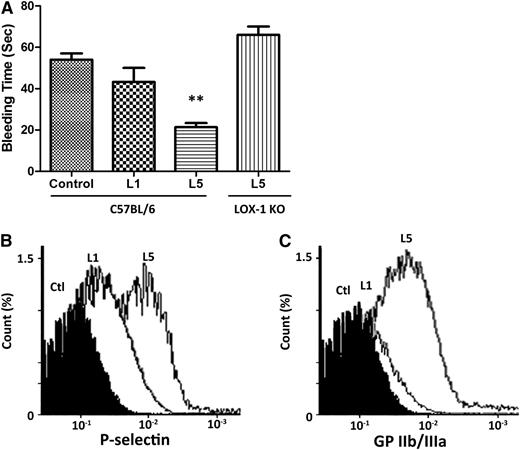
In adult male C57BL/6 mice, 5 mg/kg L5 or L1 from STEMI patients or PBS (negative control) was intravenously injected through the tail vein twice a week for 6 weeks. The mean body weights of PBS-, L1-, and L5-injected mice were not significantly different at baseline and at 6 weeks (data not shown). However, the tail bleeding time was significantly shorter in L5-injected mice (23.0 ± 2.8 s) than in PBS- or L1-injected mice (54 ± 5.3 and 43.3 ± 11.5 s, respectively; P = .01: L5 vs PBS; Figure 3B). Furthermore, the effect of L5 on tail bleeding time was attenuated when the same experiment was performed in LOX-1 knockout mice (Figure 3A). In addition, flow cytometry analysis of platelets showed that P-selectin expression and GPIIb/IIIa activation were higher in L5-injected mice than in PBS-injected mice but not L1-injected mice (Figure 3B-C), indicating that L5 induces platelet activation.
Induction of platelet activation in L5-injected mice. L5, L1 (5 mg/kg each), or PBS (Ctl) was injected into the tail vein of adult male C57BL/6 mice twice a week for 6 weeks (n = 3 for each treatment group). (A) Tail bleeding time was significantly shortened in L5-injected mice. **P < .01, determined by using 1-way analysis of variance with the Bonferroni post hoc test. When the same experiment was performed in LOX-1 knockout mice, the effect of L5 on tail bleeding time was attenuated. Whole blood drawn from mice was anticoagulated with heparin. Platelets were collected, stained with P-selectin and GPIIb/IIIa monoclonal antibodies, and subjected to flow cytometry. (B) P-selectin and (C) GPIIb/IIIa activation was significantly higher in L5-injected mice than in PBS-injected mice.
Induction of platelet activation in L5-injected mice. L5, L1 (5 mg/kg each), or PBS (Ctl) was injected into the tail vein of adult male C57BL/6 mice twice a week for 6 weeks (n = 3 for each treatment group). (A) Tail bleeding time was significantly shortened in L5-injected mice. **P < .01, determined by using 1-way analysis of variance with the Bonferroni post hoc test. When the same experiment was performed in LOX-1 knockout mice, the effect of L5 on tail bleeding time was attenuated. Whole blood drawn from mice was anticoagulated with heparin. Platelets were collected, stained with P-selectin and GPIIb/IIIa monoclonal antibodies, and subjected to flow cytometry. (B) P-selectin and (C) GPIIb/IIIa activation was significantly higher in L5-injected mice than in PBS-injected mice.
L5-mediated activation of human platelets requires the PKCα signaling pathway
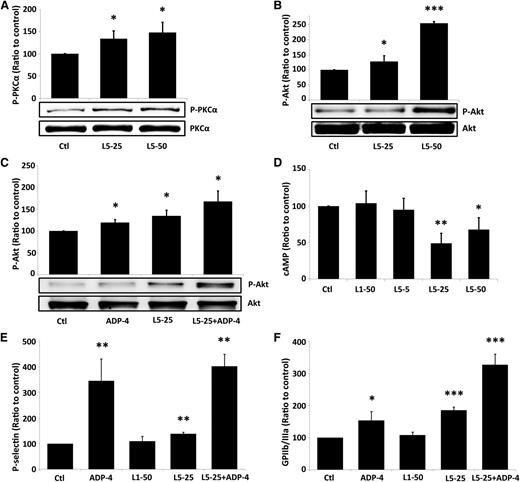
PKCα-mediated Akt phosphorylation regulates the secretion of platelet granules and the conformational change of GPIIb/IIIa.27 To determine whether L5 activates platelets through the PKCα signaling pathway, we examined the activity and activation of PKCα in human platelets exposed to L5 from STEMI patients. In the lysate of L5-treated platelets, total PKC activity was increased (data not shown). In addition, L5 increased PKCα phosphorylation in a dose-dependent manner (Figure 4A). L5 also induced Akt phosphorylation (Figure 4B) and augmented ADP-induced Akt phosphorylation (Figure 4C). Akt converts cAMP into inactivated 5′AMP; therefore, we examined the level of cAMP in platelets treated with L1 or L5 from STEMI patients. Compared with L1 (50 μg/mL), L5 (25 or 50 μg/mL) reduced cAMP levels (Figure 4D). We also found using flow cytometry that L5 alone (25 μg/mL) induced the expression of P-selectin and the activation of GPIIb/IIIa on human platelets and augmented ADP-induced expression of P-selectin and activation of GPIIb/IIIa (Figure 4E-F).
The requirement of the PKC signaling pathway for L5-mediated platelet activation. (A) L5 (25 or 50 μg/mL) increased PKCα phosphorylation in platelets in a dose-dependent manner. (B) L5 (25 or 50 μg/mL) alone induced Akt activation in a dose-dependent manner. (C) L5 (25 μg/mL) augmented ADP-induced (4 μM ADP) Akt phosphorylation. (D) L5 (25 or 50 μg/mL) decreased the expression of cAMP in platelets. Flow cytometry analysis of platelets, treated as indicated, showing the number of cells in which (E) P-selectin is expressed and (F) GPIIb/IIIa is activated, expressed as a ratio to that of the PBS-treated control (Ctl) group. All data shown represent the mean ± standard deviation; *P < .05; **P < .01; ***P < .001 vs PBS-treated control (n = 5), determined by using 1-way analysis of variance with the Bonferroni post hoc test.
The requirement of the PKC signaling pathway for L5-mediated platelet activation. (A) L5 (25 or 50 μg/mL) increased PKCα phosphorylation in platelets in a dose-dependent manner. (B) L5 (25 or 50 μg/mL) alone induced Akt activation in a dose-dependent manner. (C) L5 (25 μg/mL) augmented ADP-induced (4 μM ADP) Akt phosphorylation. (D) L5 (25 or 50 μg/mL) decreased the expression of cAMP in platelets. Flow cytometry analysis of platelets, treated as indicated, showing the number of cells in which (E) P-selectin is expressed and (F) GPIIb/IIIa is activated, expressed as a ratio to that of the PBS-treated control (Ctl) group. All data shown represent the mean ± standard deviation; *P < .05; **P < .01; ***P < .001 vs PBS-treated control (n = 5), determined by using 1-way analysis of variance with the Bonferroni post hoc test.
L5-induced PKCα pathway is mediated by PAFR and LOX-1 in platelets
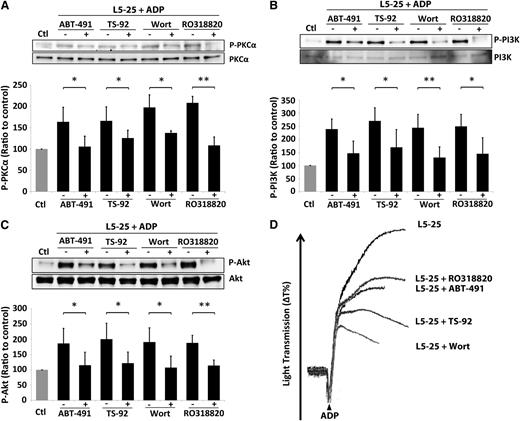
PAFR and LOX-1 mediate the L5-induced activation of signaling pathways.10,12 We examined the role of PAFR and LOX-1 in the L5-induced PKCα signaling pathway in human platelets by using the pharmacologic blockades ABT-491 (blocks PAFR), TS-92 (neutralizes LOX-1), Wortmannin (inhibits PI3K), and RO318820 (inhibits PKC activation). In platelets treated with ADP and 25 μg/mL L5 from STEMI patients, the addition of each pharmacologic blockade prevented the phosphorylation of PKCα, PI3K, and Akt (Figure 5A-C). Furthermore, platelet aggregation induced by the combination of ADP and 25 μg/mL L5 was attenuated in the presence of ABT-491, TS-92, Wortmannin, or RO318820 (Figure 5D).
Mediation of the L5-induced signaling pathway by PAFR and LOX-1. Phosphorylation of (A) PKCα, (B) PI3K, and (C) Akt induced by the combination of 4 μM ADP and 25 μg/mL L5 was prevented by ABT-491 (blocks PAFR), TS-92 (neutralizes LOX-1), Wortmannin (Wort; inhibits PI3K), and RO318820 (inhibits PKC activation). The expression of protein is shown as a ratio to that of the PBS-treated control (Ctl) group. (D) Platelet aggregation induced by ADP and 25 μg/mL L5 was attenuated in the presence of ABT-481, TS-92, Wortmannin, and RO318820. Black bars represent the mean ± standard deviation. *P < .05; **P < .01 vs ADP + L5-treated group (n = 5), determined by using 2-way analysis of variance with the Bonferroni post hoc test.
Mediation of the L5-induced signaling pathway by PAFR and LOX-1. Phosphorylation of (A) PKCα, (B) PI3K, and (C) Akt induced by the combination of 4 μM ADP and 25 μg/mL L5 was prevented by ABT-491 (blocks PAFR), TS-92 (neutralizes LOX-1), Wortmannin (Wort; inhibits PI3K), and RO318820 (inhibits PKC activation). The expression of protein is shown as a ratio to that of the PBS-treated control (Ctl) group. (D) Platelet aggregation induced by ADP and 25 μg/mL L5 was attenuated in the presence of ABT-481, TS-92, Wortmannin, and RO318820. Black bars represent the mean ± standard deviation. *P < .05; **P < .01 vs ADP + L5-treated group (n = 5), determined by using 2-way analysis of variance with the Bonferroni post hoc test.
L5 causes EC dysfunction by increasing the procoagulant activity of ECs and EC-platelet interactions
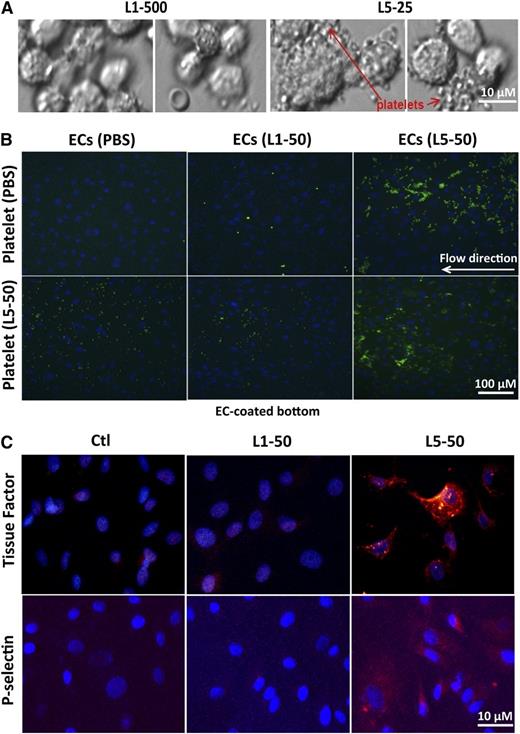
To examine the effect of L5 on the procoagulation activity of ECs, HAECs were exposed to L1 (500 μg/mL) or L5 (25 μg/mL) from STEMI patients for 24 hours and then incubated with freshly collected whole blood from control subjects for 15 minutes. Platelets were aggregated and attached to L5-treated HAECs but not to L1-treated HAECs (Figure 6A). To further examine EC-platelet interactions, we conducted parallel plate flow chamber analysis using BAECs. BAECs were treated with 50 µg/mL L1 or L5 from STEMI patients or PBS and were perfused with unstimulated platelets or L5-stimulated platelets. Unstimulated platelets and L5-stimulated platelets attached to L5-treated BAECs (Figure 6B), whereas L5-stimulated platelets attached only slightly to L1-treated or PBS-treated BAECs (Figure 6B). Furthermore, 50 μg/mL L5 but not L1 induced a marked increase in the expression of tissue factor and P-selectin, as shown by immunofluorescence staining (Figure 6C).
L5-mediated EC dysfunction. (A) HAECs were cultured in the presence of vehicle (endothelial growth media), L1 (500 μg/mL), or L5 (25 μg/mL) for 24 hours. Cells were then incubated with freshly collected whole blood from control subjects for 15 minutes, followed by 5 washes with PBS. Platelets were attached and aggregated on L5-treated HAECs but not L1-treated HAECs. Olympus BX51; bar = 10 µm. (B) Results of parallel plate flow chamber analysis with BAECs are shown. Cells were stained with 1 µM Hoechst (blue) to visualize cell nuclei. Human PRP samples containing 1 part acid sodium citrate (an anticoagulant), 10 µM calcein AM (makes platelets green), and unstimulated (treated with PBS) or L5-stimulated platelets were perfused at a flow rate of 750 S−1, and platelet-EC interactions were analyzed by fluorescence microscopy. (B, upper) Unstimulated (PBS-treated) platelets were attached to L5-treated BAECs. (B, lower) L5-stimulated platelets were slightly attached to PBS- or L1-treated BAECs but attached to L5-treated BAECs. Olympus IX70; bar = 100 µm. (C) Immunofluorescence staining with Alexa Fluor 555 (red) shows the expression of (upper) tissue factor and (lower) P-selectin in BAECs treated with PBS (Ctl), 50 μg/mL L1, or 50 μg/mL L5 for 24 hours. Cell nuclei were stained with DAPI (n = 3). Olympus IX70; bar = 10 µm.
L5-mediated EC dysfunction. (A) HAECs were cultured in the presence of vehicle (endothelial growth media), L1 (500 μg/mL), or L5 (25 μg/mL) for 24 hours. Cells were then incubated with freshly collected whole blood from control subjects for 15 minutes, followed by 5 washes with PBS. Platelets were attached and aggregated on L5-treated HAECs but not L1-treated HAECs. Olympus BX51; bar = 10 µm. (B) Results of parallel plate flow chamber analysis with BAECs are shown. Cells were stained with 1 µM Hoechst (blue) to visualize cell nuclei. Human PRP samples containing 1 part acid sodium citrate (an anticoagulant), 10 µM calcein AM (makes platelets green), and unstimulated (treated with PBS) or L5-stimulated platelets were perfused at a flow rate of 750 S−1, and platelet-EC interactions were analyzed by fluorescence microscopy. (B, upper) Unstimulated (PBS-treated) platelets were attached to L5-treated BAECs. (B, lower) L5-stimulated platelets were slightly attached to PBS- or L1-treated BAECs but attached to L5-treated BAECs. Olympus IX70; bar = 100 µm. (C) Immunofluorescence staining with Alexa Fluor 555 (red) shows the expression of (upper) tissue factor and (lower) P-selectin in BAECs treated with PBS (Ctl), 50 μg/mL L1, or 50 μg/mL L5 for 24 hours. Cell nuclei were stained with DAPI (n = 3). Olympus IX70; bar = 10 µm.
Discussion
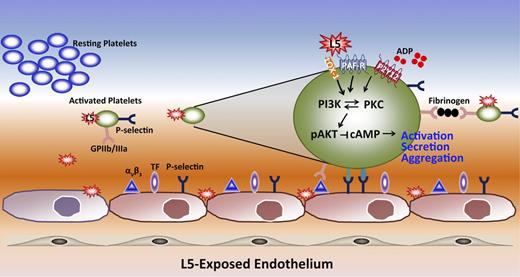
In this study, we confirmed that plasma L5 levels are significantly elevated in patients with STEMI. Furthermore, we found that when L5 isolated from the plasma of STEMI patients was injected into the tail vein of mice, it induced platelet activation and shortened tail bleeding time. These results indicate that L5 is prothrombotic, which was also demonstrated by the ability of L5 to increase tissue factor and P-selectin expression on ECs, enhance platelet aggregation, and promote platelet-EC interactions. In addition, L5 enhanced ADP-induced platelet activation through the PAFR- and LOX-1–mediated PKCα signaling pathway, summarized in Figure 7. Our findings suggest that L5 may be a key factor in promoting acute thrombosis that leads to STEMI.
A schematic diagram showing the mechanism by which L5 triggers platelet activation and aggregation. L5 promotes thrombogenesis by increasing endothelial expression of tissue factor and P-selectin, which are prone to enhance platelet adherence and activation. In addition, L5 activates platelets through the receptors LOX-1 and PAFR and enhances ADP-induced signaling. The receptor signaling through LOX-1 and PAFR mediates PI3K/PKC activation, which leads to the phosphorylation of AKT and reduces cAMP levels while promoting the release of granules. P-selectin is expressed on both the surface of platelets and ECs to increase platelet-EC interactions.
A schematic diagram showing the mechanism by which L5 triggers platelet activation and aggregation. L5 promotes thrombogenesis by increasing endothelial expression of tissue factor and P-selectin, which are prone to enhance platelet adherence and activation. In addition, L5 activates platelets through the receptors LOX-1 and PAFR and enhances ADP-induced signaling. The receptor signaling through LOX-1 and PAFR mediates PI3K/PKC activation, which leads to the phosphorylation of AKT and reduces cAMP levels while promoting the release of granules. P-selectin is expressed on both the surface of platelets and ECs to increase platelet-EC interactions.
We previously showed that L5% is moderately elevated to up to 5% to 6% in asymptomatic individuals with high cardiovascular risk10,12 and is elevated up to 12% to 13% in patients with STEMI.17 In the present study, we confirmed in a larger group of patients that L5 is elevated in patients with STEMI, showing that L5% was increased to 15.4 ± 14.5% in these patients. Although the mean LDL-C in these patients with STEMI was only 116.7 ± 32.4 mg/dL, the mean [L5] was 18.9 ± 21.0 mg/dL, or 189 ± 210 μg/mL, which far exceeds the plasma L5 levels in asymptomatic patients suggested in our previous studies. According to our data, an [L5] of 2.5-5.0 mg/dL was sufficient to induce apoptosis in HAECs and to trigger platelet activation. Therefore, a high concentration of L5 is more likely a causative factor in the prothrombotic phenotype than a consequence of it. However, the reason for the wide variation in [L5] between individuals in the STEMI group is still unclear. We are currently conducting more comprehensive studies, including time-course analysis, to further investigate these findings.
We compared the composition and activity of L1 and L5 from STEMI patients to those of previously described subjects.14,25,26 Using SDS-PAGE analysis and EC apoptosis assays, we found that L1 and L5 from STEMI patients were similar to those of diabetes and familial hypercholesterolemia patients. However, PAF components in L1 from STEMI patients were significantly different than those in L1 from previously described healthy subjects. Therefore, L1 from STEMI patients may not be truly representative of normal LDL. This may also explain why we observed a slight but nonsignificant effect of L1 on platelet activation in our in vitro and in vivo platelet activation studies. Intact ECs are essential for preventing platelet clot formation, which can be disrupted by several inflammatory mediators involved in atherosclerosis that increase levels of prothrombotic proteins and the risk of thrombogenesis.28 Of these inflammatory mediators, tissue factor is critical for triggering the extrinsic coagulation pathway and is expressed on cytokine-stimulated ECs.29 We previously found that L5 activates ECs to release inflammatory cytokines that, in turn, induce vascular smooth muscle cell damage.30 In the present study, we showed that the treatment of ECs with 50 µg/mL L5 stimulated the expression of tissue factor on the surface of ECs. Furthermore, we found that quiescent platelets aggregated on the tissue factor–exposed surface of ECs. Together, these results indicate that L5 increases the thrombotic potential by increasing the expression of tissue factor on dysfunctional ECs.
We found that the expression of P-selectin was augmented in platelets collected from L5-injected mice and that L5 induced P-selectin expression on human platelets and ECs in vitro. P-selectin, which increases platelet-EC interactions, is stored in α-granules in platelets and in Weibel-Palade bodies in ECs. On activation of the inflammatory response, P-selectin is released from cells, expressed on platelets and ECs,31 and bound to its receptors.32 The adhesion of platelets to the endothelium has been shown to accelerate the formation of atherosclerotic lesions in hypercholesterolemic mice.33 In addition, data derived from mice doubly deficient in P-selectin and E-selectin have provided strong evidence that platelet-EC interactions accelerate atherogenesis.34 Studies have suggested that P-selectin expressed on activated ECs attracts platelets to adhere to the surface of ECs by engaging P-selectin GP ligand-1 (PSGL-1) and GPIbα receptors on platelets.35,36 On the other hand, P-selectin expressed on activated platelets has been shown to interact with PSGL-1 on monocytes, resulting in the initiation of platelet-monocyte aggregation and outside-in signaling of the inflammatory response.37 In another study, P-selectin expressed on activated platelets was rapidly cleaved off and detected in the circulation in soluble form.38 Soluble P-selectin may be a circulating procoagulant protein, thus playing dual roles in promoting both the activation of platelets and coagulation.39 Our findings suggest that L5 may promote atherogenesis by increasing platelet-EC interactions and procoagulant activity through the upregulation of P-selectin.
Importantly, we showed that GPIIb/IIIa activation was significantly increased in L5-injected mice and that L5 increased the activation of GPIIb/IIIa in platelets in vitro. Furthermore, we observed the adhesion of L5-treated platelets to unstimulated, inactivated ECs. Activated GPIIb/IIIa (αIIbβ3) is an important receptor on platelets that mediates platelet aggregation by bridging with fibrinogen.40 However, GPIIb/IIIa also interacts with vascular ECs through α5β3. In the presence of soluble fibrinogen, platelets firmly adhere to the EC surface by forming a heterotypic interaction with ECs expressing integrin α5β3.41 Thus, activated platelets increase the activation of GPIIb/IIIa, which promotes platelets to adhere to ECs and to form platelet plaques during the progression of atherothrombosis. Thus, our findings indicate that L5 may promote the progression of atherothrombosis by upregulating GPIIb/IIIa.
We examined the L5 signaling pathways that mediate L5 function in platelet activation and aggregation. Previously, we found that L5 induces cell signaling pathway activation through LOX-1 and PAFR.10,12 In addition, Chen and colleagues42 reported that LOX-1 is expressed on human platelets in an activation-dependent manner. Subsequently, Marwali et al6 showed that LOX-1 expression and PKCα activity is increased in ADP-stimulated platelets; thus, platelet activation and aggregation can be induced through an autocrine or paracrine mechanism on stimulation with platelet agonists. ADP mediates the activation of the PKCα and PI3K signaling pathways through the G protein–coupled receptors P2Y1 and P2Y12 to phosphorylate downstream Akt.43 PKCα in turn mediates cAMP hydrolysis, which decreases the level of intracellular cAMP.44 Here, we showed that L5 amplified ADP-mediated platelet activation and aggregation by increasing PKCα and PI3K activity to phosphorylate downstream Akt and decrease the level of cAMP. In addition, we showed that L5-induced platelet activation was prevented by LOX-1 neutralizing antibody (TS-92) and PAFR blocker (ABT-491), indicating that L5 induces platelet activation through LOX-1 and PAFR. Thrombin generation is considered an important factor in the development of atherothrombosis because it triggers platelet activation45,46 ; however, our data showed that L5 has paradoxical effects on thrombin-induced platelet activation, indicating that more detailed studies are needed. Together, these findings support a mechanism for L5-induced platelet activation.
Finally, the purpose of performing the tail bleeding time assay was to provide in vivo evidence of platelet activation specifically caused by L5. Our results showed that the mean tail bleeding time was significantly shorter in L5-injected mice than in L1-injected mice and that the effect of L5 was attenuated in LOX-1 knockout mice. In a parallel study, we also found that L5 but not L1 shortened the tail bleeding time in C57BL/6 mice just 30 minutes after a single injection (unpublished data). These results support a relationship between high [L5] and platelet activation.
In conclusion, our results show that, through the activation of both platelets and EC, elevated plasma L5 levels may promote thrombosis formation that leads to STEMI. Our findings indicate that L5 may play an important role in the pathogenesis of STEMI and may serve as a therapeutic target for the prevention of STEMI.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nicole Stancel, PhD, ELS, of the Texas Heart Institute in Houston, Texas, for editorial assistance. We are also grateful for help from the Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital and Kaohsiung Medical University.
This work was supported by grants from the American Diabetes Association (1-04-RA-13), the National Institutes of Health, Heart, Lung, and Blood Institute (HL-63364), Merck/Schering-Plough Pharmaceuticals (research grant), the Mao-Kuei Lin Research Fund of Chicony Electronics, National Science Council (NSC 100-2314-B-039-040-MY3), China Medical University Hospital (DMR-100-184, DMR-102-091), Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH102-TD-B-111-004), and Biosignature Project Grant of Academia Sinica, Taiwan (BM101100888 and BM102021169).
Authorship
Contribution: H.-C.C. conceived and performed cell studies and animal experiments, analyzed data, and participated in writing the manuscript; L.-Y.K. performed clinical experiments, purified L5, analyzed data, and participated in writing the manuscript; C.-S.C. performed case enrollment and PCI for STEMI patients, interpreted data, and participated in writing the manuscript; K.-H.C., W.-T.L., and S.-H.S. performed case enrollment and PCI for STEMI patients; A.-S.L. performed animal experiments; M.-Y.S., J.L., and H.-C.B.C. performed experiments; J.-F.H. performed statistical analysis; T.S. contributed vital reagents; M.A.C. instructed the design of platelet studies; J.-H.Y. supervised students at the College of Medicine at KMU, revised the manuscript, and participated in the study design; and C.-H.C. supervised students at the Texas Heart Institute and at CMU, revised the manuscript, and participated in the study design.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chu-Huang Chen, Vascular and Medicinal Research, Texas Heart Institute, 6770 Bertner Ave., Houston, TX 77030; e-mail: cchen@texasheart.org; or Jeng-Hsien Yen, Department of Internal Medicine, Kaohsiung Medical University Hospital, No. 100, Tzyou 1st Rd, Kaohsiung, 807 Taiwan; e-mail:jehsye@cc.kmu.edu.tw.
References
Author notes
H.-C.C. and L.-Y.K. contributed equally to this work.

![Figure 1. Elevation of plasma L5 concentration in patients with STEMI. Fast-protein liquid chromatography analysis of LDL showing the content of each subfraction (L1-L5) in (A) normal control subjects with no cardiovascular risk factors and (B) patients with STEMI. The (C) L5% and (D) L5 plasma concentration ([L5]) for each subject is plotted. The line represents the mean of the group. The mean [L5] was significantly higher in STEMI patients (n = 30) than in control subjects (n = 30). ***P < .001, determined by using the Wilcoxon rank-sum test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/22/10.1182_blood-2013-05-504639/4/m_3632f1.jpeg?Expires=1769097836&Signature=jRTPyan5xGmccQNr8cX3QFwFdGXyrIjlOoGxps8emxCU5wne2gi8aYOCTLBg734UKLswfnz2JPZuC6f2tQgEVJREVIT8kWe-ulrzm37o1q6OPTIwJzp9w9W4aOQ71Lpp-B1RvJWBzsr4ZudJ5~KHHMuruXe3LiGdoBnca9v-YDbj3MWAhZFMubaj1ScHQ4LWcELfMM997qsz~jTu2T4KP8eesg7gohgP0yqRBShrFSev5gAXiHRyC5Y8Hok7UKre43HTPgl2aKJaNH~LLyS4nrKj2pfnqlBJKWh~tv7ldgVumGjogcCHkbawCwMWguHWnPkWVDqgmU7DooFfSIWCww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal