To the editor:
BCR-ABL translocation and JAK2-V617F mutation can sometimes be found concomitantly in the same patient (reviewed in Hummel et al1 ). To date, 4 studies have examined the chronology and architecture of BCR-ABL and JAK2-V617F clones at the molecular level. In 3 studies, the 2 mutations were sequentially acquired by the same stem cell, with JAK2-V617F preceding the acquisition of BCR-ABL.2-4 Another report concluded that BCR-ABL and JAK2-V617F represented 2 distinct clones.5
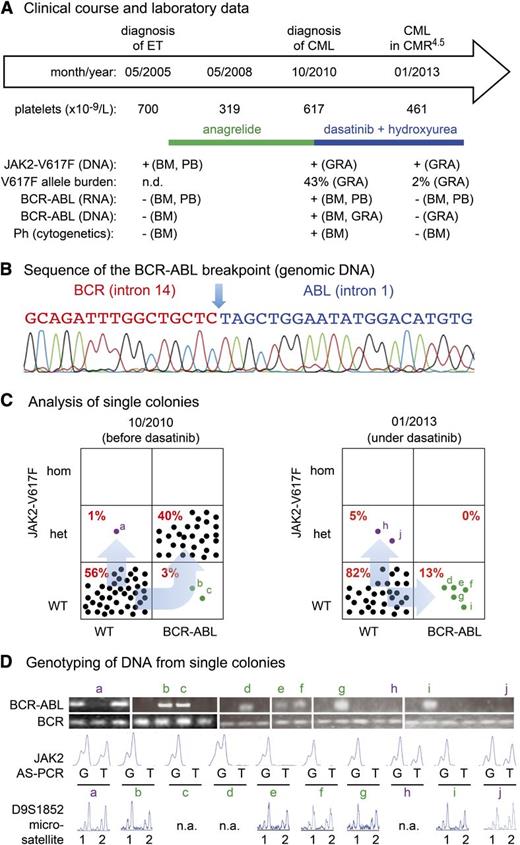
Here, we studied a 56-year-old female patient diagnosed in May 2005 with JAK2-V617F–positive essential thrombocythemia (ET), normal cytogenetics, and absence of BCR-ABL (Figure 1A). With anagrelide (2 mg/day), platelet levels normalized. However, in October 2010, thrombocytosis, leukocytosis (white blood cell count = 30 × 109/L) and the presence of myelocytes and metamyelocytes in the peripheral blood was noted. Bone marrow showed typical features of chronic myeloid leukemia (CML) and cytogenetic analysis revealed the Philadelphia chromosome translocation t(9;22)(q34;q11). Molecular analysis confirmed expression of a BCR-ABL fusion (b3a2). Treatment with dasatinib induced remission of CML, but thrombocytosis persisted. Therefore, low-dose hydroxyurea (500 mg every second day) was added. A complete molecular remission with a 10−4 to 10−5 reduction of BCR-ABL transcripts (CMR4.5) was reached within 12 months.
Patient characteristics, BCR-ABL breakpoint sequence, and genotyping of single colonies. (A) Clinical diagnoses, treatment history, and diagnostic workup of a 56-year-old female patient with coexisting JAK2-V617F mutation and BCR-ABL rearrangement. BM, bone marrow; CMR4.5, complete molecular remission with a log4 to log5 reduction of BCR-ABL transcripts; GRA, granulocytes; n.d., not determined; Ph, Philadelphia chromosome. (B) Sequencing chromatogram of a 157-bp amplicon derived from granulocyte DNA revealed the breakpoint sequence in the intron 14 of BCR and intron 1 of ABL. The same sequences were obtained in colonies positive for BCR-ABL. (C) Analysis of single colonies for mutation in JAK2-V617F and BCR-ABL rearrangement in the genomic DNA before and after tyrosine kinase (dasatinib) therapy. Mononuclear cells from peripheral blood were grown in methylcellulose in the presence of erythropoietin. Single burst-forming units erythroid (BFU-E) and non–BFU-Es (ie, CFU-G and CFU-GM) were picked and analyzed individually for the presence of JAK2-V617F mutation by allele-specific PCR. Each colony is represented by a dot that is placed into 1 of 6 quadrangles representing the 6 possible genotypes: wild-type (WT), heterozygous (het) and homozygous (hom) for JAK2-V617F on the vertical axis, and for BCR-ABL on the horizontal axis. Percentages are shown in red for each possible genotype. (D) Genotyping of DNA from single colonies. Ethidium-bromide–stained PCR fragments for the BCR-ABL breakpoint and for BCR (loading control) were separated by agarose gel electrophoresis (top panel). The chromatograms of the allele-specific PCR assay (AS-PCR) show the presence or absence of the wild-type “G” or mutant sequence “T” in codon 617 of JAK2. The results of individual colonies marked with small letters in panel C are shown. Results of microsatellite analysis for a marker on chromosome 9p are shown below. Note that all colonies tested retained both alleles (1 and 2), excluding uniparental disomy as the reason for the loss of BCR-ABL/JAK2-V617F double-mutant colonies. n.a., not available.
Patient characteristics, BCR-ABL breakpoint sequence, and genotyping of single colonies. (A) Clinical diagnoses, treatment history, and diagnostic workup of a 56-year-old female patient with coexisting JAK2-V617F mutation and BCR-ABL rearrangement. BM, bone marrow; CMR4.5, complete molecular remission with a log4 to log5 reduction of BCR-ABL transcripts; GRA, granulocytes; n.d., not determined; Ph, Philadelphia chromosome. (B) Sequencing chromatogram of a 157-bp amplicon derived from granulocyte DNA revealed the breakpoint sequence in the intron 14 of BCR and intron 1 of ABL. The same sequences were obtained in colonies positive for BCR-ABL. (C) Analysis of single colonies for mutation in JAK2-V617F and BCR-ABL rearrangement in the genomic DNA before and after tyrosine kinase (dasatinib) therapy. Mononuclear cells from peripheral blood were grown in methylcellulose in the presence of erythropoietin. Single burst-forming units erythroid (BFU-E) and non–BFU-Es (ie, CFU-G and CFU-GM) were picked and analyzed individually for the presence of JAK2-V617F mutation by allele-specific PCR. Each colony is represented by a dot that is placed into 1 of 6 quadrangles representing the 6 possible genotypes: wild-type (WT), heterozygous (het) and homozygous (hom) for JAK2-V617F on the vertical axis, and for BCR-ABL on the horizontal axis. Percentages are shown in red for each possible genotype. (D) Genotyping of DNA from single colonies. Ethidium-bromide–stained PCR fragments for the BCR-ABL breakpoint and for BCR (loading control) were separated by agarose gel electrophoresis (top panel). The chromatograms of the allele-specific PCR assay (AS-PCR) show the presence or absence of the wild-type “G” or mutant sequence “T” in codon 617 of JAK2. The results of individual colonies marked with small letters in panel C are shown. Results of microsatellite analysis for a marker on chromosome 9p are shown below. Note that all colonies tested retained both alleles (1 and 2), excluding uniparental disomy as the reason for the loss of BCR-ABL/JAK2-V617F double-mutant colonies. n.a., not available.
To allow detecting the translocation in DNA samples, we sequenced the BCR-ABL breakpoint and designed polymerase chain reaction (PCR) primers across the junction. The identity of the amplified fragment was confirmed by sequencing (Figure 1B). DNA derived from single colonies grown from the patient’s peripheral blood in methylcellulose was analyzed for the presence of JAK2-V617F by allele-specific PCR,6,7 and for BCR-ABL by PCR primers bridging the breakpoint. In October 2010, before dasatinib/hydroxyurea treatment, the majority of the colonies were either wild-type or carried both JAK2-V617F and BCR-ABL. However, 2 colonies harbored only BCR-ABL and 1 colony showed only JAK2-V617F (Figure 1C, left panel). Because the BCR-ABL breakpoint in this patient is unique, this pattern suggested that BCR-ABL was acquired before JAK2-V617F and implied that JAK2-V617F occurred twice. Genotyping of individual colonies with a microsatellite marker for chromosome 9p allowed excluding uniparental disomy as an alternative explanation for the single-mutant BCR-ABL colonies (Figure 1D). This biclonal pattern was confirmed in colonies derived from a second blood sample taken under dasatinib/hydroxyurea treatment 26 months later. Interestingly, the double-positive colonies completely disappeared, while several colonies positive for either JAK2-V617F or BCL-ABL persisted, suggesting that the double mutant may be more sensitive to treatment (Figure 1C, right panel). Despite the presence of BCR-ABL single-mutant colonies, BCR-ABL was undetectable in messenger RNA from bone marrow and peripheral blood and absent in DNA from granulocytes. We can only speculate that the BCR-ABL single-mutant progenitors can divide in vitro and form colonies but are unable to efficiently expand in vivo in the presence of dasatinib.
This patient displayed a novel pattern with 2 separate clones: 1 that first acquired BCR-ABL followed by a JAK2-V617F mutation within the same clone, and a second clone positive for JAK2-V617F only. Our data show that subclones can respond differentially to therapy. Knowledge of the clonal architecture in patients with MPN/CML could be useful to monitor remission and select therapy.
Authorship
Acknowledgments: The authors thank Jürg Schwaller for helpful comments on the manuscript as well as Asensio Gonzalez and Manuela Nickler for technical support. They further thank Anne Schneider, Anne-Claire Voegeli, and Bassam Isaac (Plateforme Moléculaire des Cancers d’Alsace, Hôpital de Hautepierre, Strasbourg, France) for molecular analyses. The collection of blood and bone marrow samples was performed at the study center in Mulhouse, France. Written consent was obtained from the patient in accordance with the Declaration of Helsinki. The study was approved by the Ethik Kommission Beider Basel. This work was supported by grants 310000-120724/1 and 32003BB_135712/1 from the Swiss National Science Foundation and the Swiss Cancer League (KLS-02398-02-2009) to R.C.S.
Contribution: J.G. designed and performed research, analyzed data and wrote the paper, M.O.U. designed research, provided clinical data, and analyzed data, R.L., H.H.S. performed research, P.L., A.D., E.J., and A.K. analyzed data, R.C.S. designed research, analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Radek C. Skoda, Department of Biomedicine, Experimental Hematology, University Hospital Basel, Hebelstr 20, 4031 Basel, Switzerland; e-mail: radek.skoda@unibas.ch; and Mario Ojeda-Uribe, Service d’Hématologie Clinique et Unité de Thérapie Cellulaire, Hôpital Emile Müller, 20 Ave du Docteur René Laennec, 68100 Mulhouse, France; e-mail: ojedam@evhr.net.