In this issue of Blood, Betapudi and colleagues report that anti–β2-glycoprotein I (β2GPI) antibodies stimulate the release of endothelial cell microparticles (EMPs) via a nonmuscle myosin II motor protein-dependent pathway that involves phosphorylation of its regulatory light chains. The results of this study suggest that inhibiting nonmuscle myosin II phosphorylation in endothelial cells (ECs) may block potentially deleterious microparticles from being released into the circulation.1
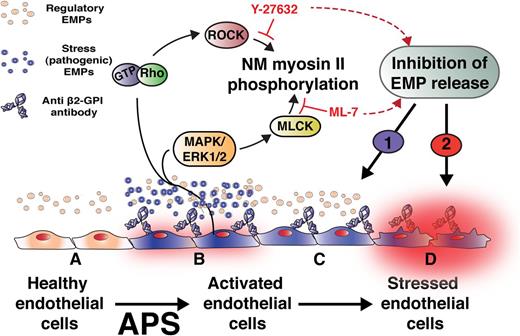
Constitutive release of microparticles from endothelium (regulatory EMPs, beige) may be an adaptive response to cell senescence and membrane turnover (A). In contrast, binding of anti-β2GPI antibodies that mediate APS induces a procoagulant, proinflammatory phenotype (B). The study by Betaputi et al shows that anti-β2GPI antibodies activate an intracellular signal transduction pathway that leads to phosphorylation of nonmuscle (NM) myosin II and the release of potentially pathogenic EMPs (blue) that reflect these alterations in endothelial function. Release of these pathogenic or “stress” EMPs may promote thrombosis and lead to gestational and other complications of APS. It is proposed that inhibition of NM myosin II phosphorylation will attenuate stress EMP-dependent pathogenic mechanisms (C) (1), but the effect of preventing release of regulatory EMPs on cellular adaptation to stress (D) (2) requires further investigation. The authors thank Dr Rudy Fuentes (University of North Carolina-Chapel Hill) for assistance in the development of this figure.
Constitutive release of microparticles from endothelium (regulatory EMPs, beige) may be an adaptive response to cell senescence and membrane turnover (A). In contrast, binding of anti-β2GPI antibodies that mediate APS induces a procoagulant, proinflammatory phenotype (B). The study by Betaputi et al shows that anti-β2GPI antibodies activate an intracellular signal transduction pathway that leads to phosphorylation of nonmuscle (NM) myosin II and the release of potentially pathogenic EMPs (blue) that reflect these alterations in endothelial function. Release of these pathogenic or “stress” EMPs may promote thrombosis and lead to gestational and other complications of APS. It is proposed that inhibition of NM myosin II phosphorylation will attenuate stress EMP-dependent pathogenic mechanisms (C) (1), but the effect of preventing release of regulatory EMPs on cellular adaptation to stress (D) (2) requires further investigation. The authors thank Dr Rudy Fuentes (University of North Carolina-Chapel Hill) for assistance in the development of this figure.
There is increasing evidence to implicate antibodies to β2GPI in the development of thrombosis and recurrent fetal loss in patients with the antiphospholipid antibody syndrome (APS), but the effector mechanisms are far from clear. Proposed pathways are plentiful and include activation of platelets, monocytes, and ECs; acceleration of coagulation reactions; interference with anticoagulants and thrombolysis; and activation of complement, among others. In addition to generating EMPs, binding of anti-β2GPI antibodies to cultured endothelial cells induces expression of adhesion molecules and tissue factor, creating a proinflammatory and prothrombotic phenotype that is imprinted onto the microparticles (MPs) they generate. Hence, identifying the role of EMPs in the complications of APS is an important albeit complex undertaking.
MPs are operationally defined as 100- to 1000-nm vesicles released from all studied cells in response to activation, apoptosis, necrosis, complement-mediated membrane injury, and other danger signals. Any generalization with respect to MPs, and EMPs specifically, may be confounded by their biophysical and functional heterogeneity. Nanoparticle tracking analysis and atomic force microscopy, which can resolve particle sizes 1 to 3 orders of magnitude lower than the 200-nm threshold for flow cytometry, indicate that >90% of MPs are below this detection limit. Whether these smaller vesicles are more or less biologically impactful or differ in function from those detected by conventional flow cytometry is unknown.
Indeed, MPs comprise one subset amid a complex array of subcellular vectors of intercellular communication that includes exosomes and immune complexes, which mediate transfer of membranes, receptors, DNA, messenger RNA, microRNA, transcription factors, and other biologically relevant mediators. Exosomes can transfer antigen and peptide–major histocompatibility complex (MHC) II complexes that stimulate antigen-presenting cells or CD4+ regulatory cells, or peptide–MHC I complexes that stimulate CD8+ T cells to the advantage of, or in the case of systemic lupus and perhaps APS, to the disadvantage of the host.2
EMPs are also heterogeneous in composition and function, purveying prothrombotic, anticoagulant and fibrinolytic, or cytoprotective activities. The endothelium may also have some capacity to recycle MPs from other cell types and invest them with an endothelial phenotype.3 The composition of EMPs may depend on whether their origination is constitutive, from healthy endothelium (regulatory MPs), or from regions of cell death or injury (stress MPs), where they are more likely to express tissue factor, anionic phospholipids, nuclear proteins, and inducible adhesion molecules that might spread damage to downstream vasculature.
MPs are cleared from the circulation within minutes,4 whereas their endocytosis and most reported in vitro effects on cell behavior require hours to days; this is in contrast to information transfer by trogocytosis, nibbling, and nanotubes,2 which can occur within minutes. This would suggest EMPs are more likely to act in confined spaces (eg, inflammatory joints5 ) or on the surface of injured vessels, where they are incorporated in nascent clots and foster thrombosis.6 Whether there is a tonic release and uptake pathway that permits EMPs to modify cell behavior over longer time frames requires additional study.
However, MPs may be more than a biomarker and conveyer of stress involving their cells of origin. Release of MPs may comprise part of an adaptive response that promotes cell survival by removing dangerous or redundant intracellular compounds, such as tumor necrosis factor α. For example, release of EMPs helps protect the endothelium from complement-mediated lysis.7 In one study, inhibition of MP release from stressed cultured ECs by Y-27632 or calpeptin compromised their survival accompanied by accumulation of caspase-3 in detached cells.8 EMPs may also participate in physiologic cell maintenance and vascular repair programs, such as angiogenesis.9 Therefore, the net benefit of interrupting the generation of EMPs may depend upon the balance between the consequences of their release and protection of the donor cells (see figure).
The mechanisms by which EMPs are released may also vary, depending in part on the inciting mechanism. Activation of Toll-like receptor signaling pathways, Rho/Rho kinase (ROCK), p38 mitogen-activated protein kinase, and nuclear factor κB pathways, and perhaps others, have all been implicated in various aspects of EMP production.10 Importantly, blocking ROCK inhibited the stimulated release of potentially pathogenic EMPs but not their constitutive release from ECs.11 On the other hand, neither the number of ECs releasing EMPs,11 nor the expression of E-selectin1 was affected, so the net effect of successful inhibition in vivo can only be conjectured.
Hence, release of EMPs may relieve stress on donor endothelium by removing dangerous membrane and intracellular material, but potentially at the expense of amplifying comparably or even more dangerous processes such as intravascular coagulation, autoimmunity, and inflammation and adversely affecting the phenotype and survival of recipient cells. The data reported by Betaputi et al are an important contribution toward understanding this rapidly emerging area of investigation. Dissection of the signal transduction pathways leading to EMP release is the first step toward selective interference in experimental models that will help to resolve how these particles contribute to thrombosis and inflammation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal