Abstract
Severe combined immunodeficiency (SCID) arises from different genetic defects associated with lymphocyte development and function and presents with severe infections. Allogeneic hematopoietic stem cell transplantation is an extremely effective way of restoring immunity in these individuals. Numerous multicenter studies have identified the factors determining successful outcome, and survival for SCID has shown great improvement. Advances in understanding the genetic basis of disease also mean that we increasingly tailor transplant protocols to the specific SCID form. Wherever possible, we attempt to transplant SCID patients without the use of cytoreductive conditioning, but it is clear that this is only successful for specific SCID forms and, although survival is good, in specific patients there are ongoing humoral defects. We aim to use matched related and unrelated donors (including cord blood) whenever possible and have limited the use of mismatched haploidentical donors. The development of autologous hematopoietic stem cell gene therapy provides another treatment of the X-linked and adenosine deaminase–deficient forms of SCID, and we discuss how we have integrated gene therapy into our treatment strategy. These developments together with the advent of universal newborn screening for SCID should allow for a highly favorable outcome for this otherwise lethal condition.
Introduction
Severe combined immunodeficiencies (SCIDs) are a genetically heterogeneous group of inherited defects characterized by severe abnormalities of immune system development and function. Affected infants present in the first few months of life with severe, recurrent, and opportunistic infections, and without definitive treatment, the condition is invariably fatal.1 The genetic defects in approximately 90% of the different forms of SCID have now been identified and, despite genetic heterogeneity, all patients are characterized by abnormalities of thymopoiesis and T-cell maturation and function. The severity of the clinical and immunologic phenotype requires prompt intervention, and for most patients, the only curative treatment is allogeneic hematopoietic stem cell transplantation (HSCT). Gene therapy offers a cure for two specific forms of SCID and, although other SCID forms may become amenable to this form of treatment in the future, it is likely that HSCT will continue to be the mainstay of treatment of the majority of SCID patients.
The first ever successful transplant for SCID was performed in 1968. Over the past several decades, the outcome for SCID as a whole has improved dramatically, and a number of large retrospective registry studies have documented the success in improving overall survival (OS) and immunologic recovery. The improvements are due to a number of different factors, including earlier diagnosis, better pre- and post-HSCT supportive care, improved HLA typing, the availability of compatible donors from unrelated volunteer and cord blood banks, and less toxic chemotherapy regimens to prepare patients for HSCT. Many of the major studies have tended to look at SCID as a whole, and outcomes have been presented for all types of SCID. However, the rapid advances in gene identification technology now allow us to make a genetic diagnosis in the vast majority of SCID patients, and we increasingly base our HSCT strategy on the underlying genetic defect. For this reason, while we will outline some generic issues regarding HSCT in SCID, we will also look more closely at how transplant strategies can be tailored to the different forms of SCID.
Genetic basis and immunologic phenotype of the different forms of SCID
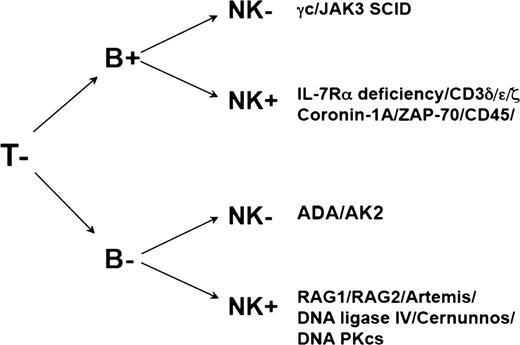
The genetic basis of at least 18 different forms of SCID has now been identified2-18 (summarized in Table 1), and in all cases, the defect leads to a failure of T-cell development or function. The different genetic defects can be categorized according to pathways affected by the molecular defect (Table 1) or by the specific immunologic phenotype arising from the genetic defect (Figure 1). In the majority of cases, a precise molecular genetic diagnosis can be made on the basis of the phenotype. We also use radiation sensitivity testing on cultured skin fibroblasts to aid in the phenotypic characterization by identifying the radiation-sensitive varieties of SCID.19 In the small proportion of cases in which molecular genetic diagnosis proves difficult, the availability of stored fibroblasts also provides a source of DNA for further studies. The distribution of the different genetic defects among the SCID population suggests that X-linked SCID (SCID-X1) arising from mutations in the IL2RG gene or other T−B+ forms accounts for approximately 40% to 50% of all forms of SCID, T−B− forms arising from VDJ recombination defects account for ∼30%, and adenosine deaminase (ADA)-deficiency for ∼10% to 15%. However, these data are taken from a single-center study in the United States20 and from the European immunodeficiency transplantation database (SCETIDE; Stem Cell Transplant for Immunodeficiencies in Europe), and the distribution of genetic defects may be biased by the nature of the population. In countries where parental consanguinity is higher, a higher percentage of autosomal recessive conditions may be expected. Indeed, in our cohort of 117 patients transplanted since 2000, the percentage of molecularly confirmed γ chain–deficient patients was equivalent to that of ADA-deficient and recombinase-activating gene (RAG)-deficient patients (γ chain, 17%; ADA, 19%; RAG, 15%). In this cohort, 20 (17%) of 117 patients did not have a molecular diagnosis at the time of transplant, although more detailed analysis of this group is now being undertaken using new-generation sequencing strategies.
Different SCID forms categorized on the type of the molecular defect
| Type of defect . | Specific molecular abnormality . |
|---|---|
| Cytokine signaling defects | γc, JAK3, IL-7Rα |
| TCR defects | CD3δ/ε/ζ, ZAP-70, lck, Orai1 |
| VDJ recombination defects | RAG1/2, Artemis, DNA ligase IV, Cernunnos-XLF, DNA PKcs |
| Defects of metabolism | ADA, AK2 (reticular dysgenesis) |
| Other | CD45, Coronin 1A, RMRP |
| Type of defect . | Specific molecular abnormality . |
|---|---|
| Cytokine signaling defects | γc, JAK3, IL-7Rα |
| TCR defects | CD3δ/ε/ζ, ZAP-70, lck, Orai1 |
| VDJ recombination defects | RAG1/2, Artemis, DNA ligase IV, Cernunnos-XLF, DNA PKcs |
| Defects of metabolism | ADA, AK2 (reticular dysgenesis) |
| Other | CD45, Coronin 1A, RMRP |
ADA, adenosine deaminase; AK2, adenylate kinase 2; DNA PKcs, DNA protein kinase catalytic subunit; IL-7Rα, interleukin 7 receptor α; JAK3, janus kinase 3; lck, lymphocyte-specific protein tyrosine kinase; Orai1, ORAI calcium release-activated calcium modulator 1; RAG1/2, recombinase activating gene 1 and 2; RMRP, RNA component of mitochondrial RNA processing endoribonuclease; XLF, X-ray cross-complementing gene 4-like factor; ZAP-70, ζ associated protein-70.
Our growing understanding of these conditions and their molecular basis has also shown that the genes that cause the immunologic and clinical phenotype of SCID can, because of hypomorphic or specific mutations, also result in more variable phenotypes such as Omenn’s syndrome21 and later onset combined immunodeficiency (CID) associated with or without inflammatory manifestations. Omenn’s syndrome is clinically characterized by failure to thrive, opportunistic infections, a severe erythrodermatous rash, and organomegaly and immunologically characterized by the presence of autologous nonfunctional oligoclonal autoreactive T cells and poor humoral immunity. In addition, most patients have high immunoglobulin E (IgE) levels and hypereosinophilia. It was originally thought that Omenn’s syndrome arose solely from hypomorphic mutations in RAG1/2, which led to partial recombinase activity and the emergence of specific autoreactive T-cell clones.22 However, it is now clear that hypomorphic mutations in many different genes can also lead to the clinical and immunologic Omenn’s phenotype.23-25 This article will focus on our practices with regard to SCID and Omenn’s syndrome but will not cover CID (including major histocompatibility complex II deficiency and purine nucleoside phosphorylase deficiency in which T-cell numbers are often much higher than in classic SCID forms (especially in the first year of life), given the diversity that exists within that disease spectrum and the lack of formal data in such patients. FOXN1 defect disorders of thymic development (eg, severe DiGeorge syndrome) have also been excluded since they are not usually treated by HSCT.
Generic issues related to transplantation in SCID
SCETIDE has collected data on nearly 700 patients for more than 30 years, and a number of important publications have documented the outcomes and important risk factors.26,27 The major factors influencing outcome include (1) the type of donor, with matched sibling donors (MSDs) having the best outcome; (2) the type of SCID, with T−B− forms of SCID having a poorer outcome; (3) preceding comorbidity (pneumonitis, septicemia, viral illness) adversely influencing outcome; (4) age at transplant, with patients younger than age 6 months having an improved outcome; (5) undertaking transplants in a protected environment; and (6) the use of septrin/co-trimoxazole prophylaxis.
The initial management of a presenting SCID patient is probably as crucial to the eventual outcome as the choice of conditioning regimen and acute peritransplant management. Until the advent of newborn screening for SCID, and excluding those diagnosed at birth because of a previous family history, all patients with SCID were diagnosed because of recurrent or opportunistic infection and/or other infection-related problems. Even with increasing awareness, a number of patients will die before transplant as a result of infectious complications; since 2000, we have had 17 SCID children die before they could have a definitive procedure. It is therefore imperative that all patients are recognized early, investigated thoroughly for the type and source of infection, and then treated effectively and aggressively. If there is any suspicion of SCID, any blood products required for treatment should be irradiated. It is important to stress that routine vaccinations should not be given; they will be ineffective and, in the case of live-agent vaccines, they are dangerous. Unfortunately, sometimes Bacillus Calmette–Guérin (BCG) vaccination has already been given before diagnosis, resulting in a high rate of disseminated vaccine-related disease.28 Rotavirus vaccine from 2 months of age is due to be introduced to our national immunization schedule in September 2013 and may result in vaccine-related illness as reported from the United States.29 Once the presenting acute infection has resolved, patients are placed on prophylactic medication, including co-trimoxazole (to prevent Pneumocystis jirovecii infection), fluconazole or itraconazole (as antifungal prophylaxis), and Ig replacement therapy via either the intravenous or subcutaneous route. The use of antiviral prophylaxis using aciclovir is used in some centers but is not routinely used in our practice unless there is a history of herpetic infection. For infants who received BCG immunization prior to diagnosis, we will begin treatment with two antimycobacterial agents, usually rifampicin and isoniazid, but substituting the latter with another agent if the BCG used is resistant. All patients are given only cytomegalovirus (CMV)-negative and irradiated blood products to prevent viral transmission and transfusion-associated graft-versus-host disease (GVHD), respectively. It has been shown that 20% of CMV seropositive mothers excrete CMV in their breast milk during lactation.30 In an earlier survey (unpublished) in our unit, we showed in 59 SCID patients that CMV infection prior to HSCT was exclusively found in those infants who were breast-fed. We have therefore, in recent years, strongly discouraged breast-feeding unless the mother is CMV antibody negative.
Attention must also be devoted to nutrition, because many patients will be malnourished as a result of recurrent infection and diarrhea. Patients may require nasogastric or, in more severe cases, parenteral feeding to correct weight and nutritional status. Skincare may also be important, especially in patients with an Omenn’s phenotype or maternal GVHD in which the inflammatory effects of autologous or maternal T cells may necessitate treatment with topical or, in severe cases, systemic immunosuppression. In severe Omenn’s syndrome, our practice has been to wait for effective control of skin or gastrointestinal problems, usually requiring cyclosporin and/or systemic corticosteroids, before proceeding to HSCT because ongoing inflammation may cause activation of donor T cells and exacerbate the inflammatory process resulting in GVHD.
Another generic issue of importance is the isolation of patients prior to transplant. In some centers, SCID patients are routinely hospitalized in cubicles following diagnosis and will remain hospitalized until after transplant. In our practice, partly because of the availability of beds for transplant and partly because of concern for and risk of hospital-acquired infections, patients who are clinically stable are sometimes discharged home prior to transplant with strict instructions for isolation and hygiene measures. Such a practice is used only after a careful risk assessment based on the home circumstances, including the presence of other young children in the household, and is possible only with very close nursing and medical communication with families and local health care personnel and regular follow-up in our daycare unit. Whether these very different practices have an impact on the outcome has not been formally assessed.
Choice of donor for transplantation in SCID
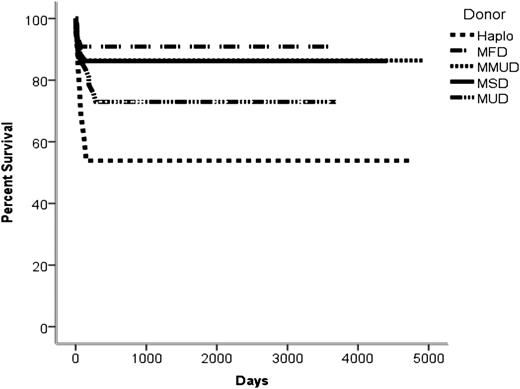
Once the diagnosis of SCID has been made, there is an urgent need to identify a suitable donor. Evidence from a large number of retrospective studies demonstrates that the choice of donor has a significant impact on OS. The best outcomes are seen following MSD transplants, with OS figures now approaching 90%, and MSDs remain the donor of choice for nearly all centers including our own. Phenotypically matched family donor (MFD) transplants from family members other than siblings (usually from consanguineous pedigrees) is our second donor choice. At our center and within the SCETIDE registry for the most recent time period (2000-2005),26 there is now equivalence in outcome between MSDs and MFDs at ∼85%. Our increased use of MFDs may reflect the SCID population in the United Kingdom, and our series shows that only 38% of 117 SCID transplants since 2000 were from white families. Of the remaining patients, 35% were from an Asian background in which there was a high degree of consanguinity. Our survival data since 2000 show OS of 86% for MSD and 91% for MFD transplants (Table 2 and Figure 2). When considering infusion of these donor sources without cytoreductive conditioning, we often include cyclosporin with MFDs since, in our series, all MFD transplants developed some form of GVHD (10/11 acute GVHD; 1/11 chronic GVHD) in contrast to an incidence of 65% in the MSD transplant series (15/29 acute GVHD; 4/29 chronic GVHD) (Table 3).
No. of transplants for SCID with donor source and OS since 2000 (difference from MSD shown)
| Donor source . | No. . | OS (%) . | 95% CI . | P . |
|---|---|---|---|---|
| MSD | 29 | 86 | 3266.26-4361.81 | — |
| MFD | 11 | 91 | 2755.87-4002.86 | .676 |
| MMUD | 22 | 86 | 3561.87-4970.58 | .987 |
| Haplo | 13 | 54 | 1324.1-3856.81 | .028* |
| MUD | 37 | 73 | 2214.59-3235.03 | .225 |
| Other† | 5 | 60 | 440.00-2146.81 | .201 |
| Donor source . | No. . | OS (%) . | 95% CI . | P . |
|---|---|---|---|---|
| MSD | 29 | 86 | 3266.26-4361.81 | — |
| MFD | 11 | 91 | 2755.87-4002.86 | .676 |
| MMUD | 22 | 86 | 3561.87-4970.58 | .987 |
| Haplo | 13 | 54 | 1324.1-3856.81 | .028* |
| MUD | 37 | 73 | 2214.59-3235.03 | .225 |
| Other† | 5 | 60 | 440.00-2146.81 | .201 |
MUD includes 5 fully matched (10/10) UCB. MMUD includes 17 UCB (15 are 9/10 matches and 2 are 8/10 UCB matches). Confidence intervals expressed as survival days posttransplant.
P > .05.
MSD, MFD, MSD + Haplo.
Incidence of GVHD following different donor sources
| Donor source . | No. . | aGVHD . | cGVHD . |
|---|---|---|---|
| MSD | 29 | 15 | 4 |
| MFD | 11 | 10 | 1 |
| MMUD | 22 | 17 | 8 |
| Haplo | 13 | 7 | 0 |
| MUD | 37 | 21 | 4 |
| Other | 5 | 1 | 0 |
| Donor source . | No. . | aGVHD . | cGVHD . |
|---|---|---|---|
| MSD | 29 | 15 | 4 |
| MFD | 11 | 10 | 1 |
| MMUD | 22 | 17 | 8 |
| Haplo | 13 | 7 | 0 |
| MUD | 37 | 21 | 4 |
| Other | 5 | 1 | 0 |
aGVHD, acute GVHD; cGVHD, chronic GVHD.
The increasing availability of unrelated donors (now 15 million worldwide) and umbilical cord blood (UCB) donations (now 0.5 million worldwide) offers further donor choices. In the absence of an MSD or MFD, our third choice would be a 10/10 matched unrelated donor (MUD) or 10/10 UCB unit (UCBs are typed to 10 rather than 6 loci). Of the patients for whom an unrelated donor search was undertaken, we were able to find a 10/10 UCB in 8 individuals (5 in white, 2 in Asian, and 1 in Middle Eastern patients), 5 of which were subsequently used for transplant. Our experience since 2000 shows that we have used MUDs more than any other donor choice (32%) of all transplants with an OS of 73%, which is similar to the overall registry data. For the vast majority of cases, we have opted to use cytoreductive chemotherapy in this setting.27 In the absence of a fully matched MUD or UCB, we will consider a single-antigen adult mismatched unrelated donor (1Ag MMUD) or an 8/10 or 9/10 UCB match. The final choice between a 1Ag MMUD and UCB is dependent on (1) availability of adult donor for donation within an appropriate time period and willingness to donate peripheral blood stem cells (PBSCs) (since in this setting, our experience suggests a higher rate of engraftment with PBSCs), (2) virus status of adult donor, and (3) cell dose of UCB and whether this is sufficient for the patient weight (although in most SCID cases, this is usually appropriate). As seen in Table 2 and Figure 2, MMUD transplants have been associated with a high degree of success, and in this series, there is no significant difference in survival outcome between MUD and MMUD transplants (73% vs 86% OS).
With these donor choices, a suitable fully matched or partially mismatched donor is usually available for the vast majority of patients, and it is only rarely that a mismatched parental/haploidentical donor (haplo) transplant is undertaken at our center. Although a large retrospective study has shown that there is no difference in OS between UCB and haplo transplants,31 our preference has been to use mismatched UCB since immune reconstitution is more rapid, especially because we often undertake cord blood procedures without the use of serotherapy.32 Since 2000, we have undertaken 117 transplants for SCID and only 13 (11%) were haplo transplants. This is in stark contrast to overall registry data, which show that of 181 transplants undertaken from 2000 to 2005, 96 (53%) were from haplo donors. This may in part reflect our growing experience and confidence in using unrelated donor sources. It is probably also due to our willingness to wait for a suitable donor to be identified through donor registries or through extended family searches, even if this may take longer than the currently recommended 6 to 8 weeks for the diagnosis-to-transplant time period. Indeed, the median time in our series while waiting for a transplant was 129 days. This can be achieved only if there is very close communication and liaison with families to ensure that patients are stable and clinically well while a donor search is being undertaken.
In centers where such resources or close contact with families is not available, it may be appropriate to move more swiftly to a haplo procedure. However haplo transplants still carry the poorest outcomes. Even though haplo transplant outcomes have improved with time, the 3-year OS for the 2000 to 2005 time period is 66% (n = 96) from the SCETIDE registry27 and 53% (n = 40) in a Canadian-Italian retrospective survey;33 these are consistent with our haplo survival figures since 2000 (Table 2). Better outcomes for haplo transplants have been seen at other centers where procedures where undertaken without any cytoreductive conditioning34 or when patients were transplanted soon after birth because of early diagnosis through a previous family history.35,36
Unconditioned transplants in SCID patients
Case 1
A 1-year-old boy presented with bronchiolitis, and adenovirus, enterovirus, and influenza B were isolated from nasopharyngeal aspirate. Adenovirus and herpes simplex 1 were detected in his blood. On investigation, he was found to be lymphopenic with an absolute lymphocyte count of 920 cells per milliliter, with total T cells of 810 cells per milliliter, CD4 count of 220 cells per milliliter, B cells of 40 cells per milliliter, and natural killer (NK) cells of 20 cells per milliliter. The response to mitogenic stimulation with phytohemagglutinin was absent. The low number of nonfunctional T cells with virtually absent B and NK cells led us to suspect ADA deficiency. He was found to have a raised deoxyadenosine triphosphate level with negligible intracellular erythrocyte ADA activity, thereby confirming the diagnosis. Tissue typing revealed that an MSD was available. Given the excellent result of unconditioned MSD transplants in ADA SCID and despite the presence of some autologous T cells, he underwent an unconditioned procedure with unmanipulated sibling donor marrow. Nine months after the procedure, he has normal T, B, and NK cell numbers and distribution, is making Ig, has cleared all viral infections, and is clinically well.
The high success rates following MSD or MFD transplants for SCID are often attributed to the ability to undertake a transplant without the use of cytoreductive chemotherapy. This avoids the short- and long-term toxicities associated with the use of standard chemotherapeutic agents and is a significant factor in the improved outcome. SCID is the only hematopoietic condition for which an unconditioned HSCT can be performed because the significant lack of host adaptive immunity prevents graft rejection and allows engraftment of donor cells, which in the MSD/MFD setting, have a low incidence of significant GVHD. In these situations, unmanipulated bone marrow can be infused without the need for chemotherapy or serotherapy. In the MSD setting, transplants are given without the need for GVHD prophylaxis, although in MFD transplants, we often use cyclosporin prophylaxis because there is a higher incidence of GVHD, possibly due to greater minor antigen mismatch. The transfer of mature antigen–experienced T cells allows swift clearance of host viral infections and improves clinical status, and together with the avoidance of chemotherapy-related toxicity, are the most important factors for improved outcome. It should be noted that in the majority of cases, there is a lack of true stem cell engraftment.
The data on unconditioned transplants in large retrospective studies are presented for all forms of SCID, and again it is important to determine which particular forms of SCID benefit most from this approach. Certainly for T−B+ forms (predominantly IL2RG defects [SCID-X1] and interleukin-7 receptor α [IL-7Rα] defects)26 and the ADA-deficient form of SCID,37 disease-specific evidence suggests that there is excellent OS outcome following unconditioned MSD/MFD HSCT. For T−B− forms (predominantly RAG1/2 and Artemis defects), there is no disease-specific study, but the large numbers of T−B− patient data embedded within the overall SCID outcome reports suggests that these patients also respond well to an unconditioned procedure in terms of survival. However, our own recent center-specific data also suggest that while survival in T−B−NK+ SCID is good, immune reconstitution is poorer, and the need for a second procedure is higher in this group. As can be seen in Case 1, the confirmation of ADA SCID and the availability of data from large-scale, retrospective analysis37 gave us the confidence to transplant the child without any conditioning and an excellent outcome was achieved.
Case 2
A 5-month-old boy of consanguineous parents presented with recurrent infections and was found to be severely lymphopenic. Immunologic and molecular analysis showed a T−B−NK+ form of SCID due to a homozygous mutation in RAG1. A MSD was identified. Given the unavailability of data on outcome for specific SCID types at the time and also because of the reluctance of the parents to have the child undergo conditioning, an unconditioned MSD transplant was performed. Although he engrafted donor T cells, the total number of T cells remained <300 cells per milliliter). A second procedure was discussed, and the parents remained reluctant to proceed with conditioning, so a second MSD transplant without conditioning was performed. Eight years after the first transplant and 6 years after the second, he remains lymphopenic, with T cells of 260 cell/mm3 (all of donor origin), no B-cell engraftment, and on Ig replacement. He has chronic lung changes and poor growth. He is now being assessed for a third procedure but this time with conditioning.
The more difficult question is which forms of SCID do not respond to an unconditioned MSD/MFD HSCT. Data now being collected suggest that patients with reticular dysgenesis do not respond to unconditioned procedures (Manfred Hoenig, personal communication). Our own personal experience also suggests that in patients such as case 2 in whom there is some autologous T-cell development, perhaps as a result of a hypomorphic mutation or in SCID forms with an intact number of NK cells (such as the VDJ recombination group including RAG1/2), HSCT without conditioning leads to limited and suboptimal engraftment. In such individuals, despite clinical well-being, the poor levels of T- and B-cell recovery have necessitated re-transplantation from the same donor but this time with the use of cytoreductive conditioning. The most definitive data for an unconditioned MSD/MFD infusion exists for T−B+NK− and ADA forms of SCID in which there is no autologous T-cell development, and here the strongest recommendations can be made.
It is also important to understand the quality of immune reconstitution that can be achieved following an unconditioned transplant from an MSD or MFD donor. This is again related to the underlying defect which determines the block in lymphoid development and thereby which niches are empty and will permit engraftment by donor cells. In SCID-X1, Janus kinase 3 (JAK3), IL-7Rα, and specific T-cell activation defects, the thymic niche is empty and can be occupied by donor T-cell precursors and so allow excellent T-cell recovery including the reconstitution of naïve T-cell populations.38 By contrast, in VDJ recombination defects, early thymic progenitors are still present, although full thymic development cannot proceed. Thus, in an unconditioned HSCT, donor cells still face competition for thymic engraftment, which may explain why, in our experience, a large number of patients with VDJ recombination defects have poor numbers of T cells and naïve T cells after unconditioned procedures. It is likely that in these cases, full T-cell recovery can only be achieved following cytoreduction. Our practice now, unlike the situation in Case 2, would be to condition all VDJ recombination defective patients, even if a fully MSD was available.
ADA SCID patients achieve normal T-cell numbers and subpopulations with normal T-cell responses to mitogens, but thymic output is low, suggesting that engraftment of predominantly late thymic progenitors or mature postthymic donor T cells has been achieved.37 To date, there have been no long-term detrimental clinical consequences, but clearly patients need to be followed carefully to ensure there is no T-cell exhaustion. In ADA SCID as in adenylate kinase 2 (AK2) reticular dysgenesis, the metabolic defect may also be important in determining progenitor cell engraftment.
Case 3
A male baby was diagnosed at birth with SCID-X1 (no T cells, normal B-cell numbers, no NK cells, and a confirmed IL2RG mutation) because of a previous family history. No sibling donor was available and a decision was made to undertake a paternal haploidentical transplant without conditioning in the first month of life. PBSCs were mobilized from the haploidentical father, CD34+ cells were isolated by magnetic bead selection (cell dose >10 × 106 CD34 cells per kilogram) and infused without conditioning. Twelve years later, he is clinically well and has normal T-cell numbers and function but has remained on Ig replacement therapy. There is 100% donor engraftment in the T-cell lineage but no engraftment of donor B cells.
Humoral recovery following unconditioned HSCT is dependent on the underlying defect and the need for engraftment of functional donor B cells.39 In IL-7Rα and CD3 subunit deficiencies in which the B-cell compartment is functionally intact, one would expect humoral correction once T-cell function is restored. However, in SCID-X1 and JAK3 deficiency, host B-cell function is defective predominantly because of the lack of IL-21 signaling, which is critical for long-lived humoral immunity and antibody secretion and responses.40 As in Case 3, it is to be expected that humoral function will not be restored, and the parents of this child were counseled that he would require lifelong Ig replacement. Recovery of humoral function should be achieved only after donor B-cell engraftment, but a proportion of patients will achieve normal Ig levels and vaccine-specific responses without donor B-cell engraftment. This suggests either that there is donor B-cell engraftment (but at levels below the sensitivity of current chimerism assays [microchimerism]) or that wild-type T-cell help can indeed restore IL2RG- or JAK3-deficient B-cell function. Further detailed studies are required to answer this question, but overall data from a number of different studies (reviewed by Haddad et al41 ) suggest that the degree of B-cell engraftment is increased if a conditioning regimen is used and that there is improved B-cell function following conditioning. For ADA SCID, it is clear that engraftment of donor T cells and metabolic detoxification are sufficient to restore humoral function, even without B-cell engraftment, suggesting that ADA-deficient B cells are not significantly intrinsically abnormal in terms of Ig production.37 In the case of VDJ recombination defects, engraftment of donor B cells is an absolute requirement for humoral recovery, and this is very unlikely in the context of an unconditioned transplant. Patients undergoing such procedures are therefore advised that ongoing Ig replacement will be necessary after transplant.
Another major issue is whether unconditioned transplants can be performed when faced with other donor sources, namely unrelated or parental haplo donors. We have attempted unconditioned HSCT in 3 children with SCID-X1 using well-matched UCB donations (11/12 or 12/12 matched). Although all 3 patients engrafted, all experienced severe grade 4 GVHD of skin and gut, despite the use of prophylactic cyclosporin in 2 patients. The additional use of in vivo T-cell depletion with serotherapy may be useful in preventing GVHD but may be counterproductive in individuals with ongoing infective problems. For ADA SCID, there is limited experience, but certainly rejection following unconditioned MUD HSCT has been reported. There are no formal reports of unconditioned MUD/UCB transplants in other SCID forms, but it is likely that these forms will be even more difficult to engraft. Given our experience and lack of evidence for efficacy in the literature, our own preference is not to perform unconditioned MUD/UCB HSCT transplants for SCID.
For haplo donors, there has been strong advocacy from specific centers for the use of unconditioned T-depleted haplo HSCT. The rationale for this is that SCID is a medical emergency, and rather than waiting for a MUD to be found, a parent is readily available and willing to donate. Overall results suggest that following such procedures, there is a high rate of survival and T-cell recovery. However, more detailed analysis of this data suggests that only the T−B+ forms of SCID (predominantly SCID-X1, JAK3, and IL-7Rα defects) respond well to this approach.34,42 Immune reconstitution following unconditioned haplo procedures is again dependent on molecular type. Only in IL-7Rα defects is there convincing evidence for both T- and B-cell reconstitution. In SCID-X1 and JAK3 deficiency, there is long-term functional T-cell recovery, but the majority of patients do not recover humoral immunity as previously discussed. However, for other forms of SCID, although survival is good, there is high rate of nonengraftment, especially in ADA deficiency, and T-cell reconstitution is limited in RAG deficiencies with predictable lack of B-cell recovery. The best outcomes are also seen when patients are younger than age 3.5 months. For these reasons, we have confined the use of unconditioned haplo transplants to SCID-X1, JAK3, and IL-7Rα patients who are younger than age 3 to 4 months and for whom no well-matched MSD/MFD donors are available. Our practice for the use of unconditioned transplants in SCID is summarized in Table 4.
Unconditioned HSCT recommendations and outcomes for different SCID types
| Type of SCID . | MSD/MRD . | Comment . | MUD/MMUD/UCB . | Haplo(T depleted) . | Comment . |
|---|---|---|---|---|---|
| SCID-X1/JAK3 | ✔ | T-cell engraftment; variable recovery of humoral immunity | X | ✔ | For patients younger than 3.5 months, T-cell engraftment; variable recovery of humoral immunity |
| T-B+NK+ | ✔ | T-cell engraftment; recovery of humoral immunity | X | ✔ | For patients younger than 3.5 months, T-cell engraftment; recovery of humoral immunity |
| ADA SCID | ✔ | T-cell engraftment; recovery of humoral immunity | X | X | |
| T-B-NK + (VDJ recombination defects) | X | Poor T-cell recovery; absent humoral recovery; may require repeat HSCT | X | X | |
| Other SCID forms | X | X | X |
| Type of SCID . | MSD/MRD . | Comment . | MUD/MMUD/UCB . | Haplo(T depleted) . | Comment . |
|---|---|---|---|---|---|
| SCID-X1/JAK3 | ✔ | T-cell engraftment; variable recovery of humoral immunity | X | ✔ | For patients younger than 3.5 months, T-cell engraftment; variable recovery of humoral immunity |
| T-B+NK+ | ✔ | T-cell engraftment; recovery of humoral immunity | X | ✔ | For patients younger than 3.5 months, T-cell engraftment; recovery of humoral immunity |
| ADA SCID | ✔ | T-cell engraftment; recovery of humoral immunity | X | X | |
| T-B-NK + (VDJ recombination defects) | X | Poor T-cell recovery; absent humoral recovery; may require repeat HSCT | X | X | |
| Other SCID forms | X | X | X |
Conditioning regimens in SCID
Although we have discussed in detail the specific indications for unconditioned transplant, the majority of transplants for SCID undertaken at our institution involve cytoreductive chemotherapy conditioning. This includes MSD/MFD transplants in SCIDs in which there is some residual T-cell function, nearly all MUD/UCB transplants in SCID (the majority of patients treated at our center), and any haplo transplants other than those stated above. Numerous different conditioning regimens have been used, and different centers have particular preferences based on their own experience. Importantly, there have been no formal comparative studies to guide the choice of regimen and the evidence available is predominantly from single-center retrospective reports. We have tended to adhere to the guidelines generated by the European Society for Immunodeficiencies/European Blood and Marrow Transplantation Society (ESID/EBMT) working party,43 which recommends 3 specific conditioning regimens for the treatment of SCID: (1) intravenous busulfan/fludarabine plus serotherapy (busulfan dosing to area under the curve [AUC] of 90 ± 5 mg⋅h/L), (2) intravenous busulfan/fludarabine plus serotherapy (busulfan dosing to AUC of 60 ± 5 mg⋅h/L), and (3) treosulfan/fludarabine with or without serotherapy. In general, the choice of chemotherapy has been dependent on the donor available. For MUD/MMUD, we have used a treosulfan/fludarabine with or without serotherapy protocol.44 This has also been used in UCB transplants and more recently without the use of serotherapy, which has resulted in rapid T-cell reconstitution posttransplant.32 This strategy has been very effective in clearing viral infections, although there is a higher incidence of acute GVHD. In these cases, all patients are given dual GVHD prophylaxis with ciclosporin and mycophenolate mofetil. For MSD in which there is a higher incidence of mixed chimerism when using reduced-intensity conditioning regimens, to date we have generally used myeloablative conditioning with intravenous busulfan/fludarabine (busulfan dosing to AUC of 90 ± 5 mg⋅h/L) without serotherapy. However, emerging evidence suggests that a reduced intensity regimen consisting of intravenous busulfan/fludarabine (busulfan dosing to AUC of 60 ± 5 mg⋅h/L) together with serotherapy using antithymocyte globulin may be sufficient to achieve curative engraftment without the need for myeloablation. Although the fludarabine/melphalan/alemtuzumab regimen may improve outcomes and potentially reduce late effects in older patients with CID, the use of this regimen in patients younger than age 1 year has been associated with a high risk of pulmonary complications, with 17 (57%) of 30 patients needing intensive care. As a result, we have avoided this regimen in SCID patients younger than age 1 year, although it remains a useful for option for older patients presenting with a leaky phenotype.45
The overall re-transplant rate in our cohort was 8.5% (total of 10 procedures: 8 re-transplants and 2 top-ups), which compares favorably with a much higher rate of 29% reported by Teigland et al.46 The major difference is that in the Teigland study, all patients had received unconditioned procedures and, indeed, 7 of the 10 re-transplants/top-ups in our series were also in patients who had initially received an unconditioned procedure.
Conditioning in radiosensitive SCID forms
Case 4
A 3-year-old boy presented with recurrent severe infections, which had started at age 1 year. With further investigation, it was found that he had a CID with very low T (170 cells per milliliter) and B (20 cells per milliliter) cells, low CD4 (40 cells per milliliter) and an impaired phytohemagglutinin response. NK cells were relatively normal (100 cells per milliliter). A fibroblast line showed significant radiosensitivity, suggesting a defect of double-strand break repair, and a molecular diagnosis of DNA ligase IV deficiency (due to mutations in LIG4) was subsequently established. A fully MUD was available for HSCT. On the basis of the fibroblast radiosensitivity, a conditioning regimen designed to avoid high doses of alkylating agents was used. The regimen used was that described in the ESID/EBMT guidelines for radiosensitivity disorders but with the addition of anti-CD52 and anti-CD45 antibodies to further deplete progenitor cells. He tolerated conditioning well and has normal immune recovery. As expected in DNA ligase IV deficiency, he has learning difficulties but is physically very well.
Radiosensitive disorders such as Artemis, DNA ligase IV deficiency, Cernunnos-XLF deficiency, and DNA protein kinase catalytic subunit (DNA PKcs) are increasingly being identified and, although some present at a later stage (as in Case 4) with CID, a number still present early with an SCID immunophenotype and require early transplantation. In these cases, the presence of NK cells suggests that cytoreductive conditioning is required for successful engraftment. Because many of the conditioning regimens use alkylating agents that are particularly damaging to DNA, less toxic regimens may be required to successfully treat these patients. The largest study to date has compared the outcome in RAG1/2 defects (non-radiosensitive) and Artemis (radiosensitive) defects. Early outcomes in terms of survival or immune reconstitution between the two groups are similar but late outcomes related to growth and puberty are significantly worse in patients with Artemis deficiency (Neven, personal communication). Whether these problems are related to the use of alkylating agents or to constitutional abnormalities related to the Artemis deficiency is not clear. Certainly for the more radiosensitive disorders such as DNA ligase IV deficiency, Cernunnos-XLF deficiency, and DNA PKcs deficiency, the recommendation has been to use a less intensive cytoreductive regimen and avoid the use of alkylating agents altogether. One such regimen is detailed in the ESID/EBMT guidelines and involves the use of fludarabine and low-dose cyclophosphamide in combination with alemtuzumab. Further prospective data are required to determine whether such a regimen improves the outcome in these patients.
Transplantation in newborn SCID patients
Newborn screening for SCID through analysis of T-cell receptor-rearrangement excision circle levels in DNA extracted from routine heel-prick dried blood spots is a major advance in the management of SCID.47 Programs of universal screening are already in place in a number of states in the United States, and it is likely that regional or national programs will soon be implemented in Europe.48-50 Detection of SCID at birth allows immediate protection with prophylactic Ig substitution and antibiotics, thereby keeping children free from infection until a definitive procedure can be undertaken. Comparative data shows that detection at birth significantly improves transplant outcome, regardless of the type of SCID, donor source, or type of transplant undertaken.36 Early detection, however, does present the problem of how to transplant very young babies. Clearly, if an MSD/MFD is available then, as in older individuals and in permissive genotypes, an unconditioned transplant can be performed. However, as we and others have seen, in NK+ or partially T-cell–deficient forms, the barriers to engraftment remain even at very early ages. The use of very high doses of CD34+ stem cells without conditioning has not necessarily improved engraftment. Thus, in SCID forms, where cytoreduction is necessary, the choice of which chemotherapeutic regimen to use in a 1- to 2-month-old or younger baby is extremely difficult, given the potential for toxicity in organs at early stages of development. The issue of which conditioning protocol is best for these young babies has generated considerable debate among many members of the SCID transplant community. One potential way forward is to determine the lowest level of alkylating agent necessary to allow donor cell engraftment by using a dose escalation protocol and involving multiple centers to enhance patient recruitment. Such a trial is currently being planned by the North American Primary Immunodeficiency Treatment Consortium (PIDTC). The results of such a study will be extremely important, but until then, our approach will be to use no conditioning whenever possible in newborn SCIDs, even if this may require performing a second conditioned transplant at a later stage or using a low-dose regimen (intravenous busulfan/fludarabine plus serotherapy [busulfan dosing to AUC of 60 ± 5 mg⋅h/L) when the need for conditioning is clearly evident. Other regimens such as treosulfan/fludarabine or fludarabine/low-dose cyclophosphamide may also be good alternatives.
Another option is to use antibody-targeted conditioning. At our center, 8 of 8 SCID patients with significant comorbidity pre-HSCT survived after a minimal-intensity conditioning protocol consisting of fludarabine, low-dose cyclophosphamide, and anti-CD52 and anti-CD45 antibodies.51 Although survival was excellent, stem cell engraftment was not achieved in some patients receiving bone marrow as the stem cell source. This was corrected by the use of PBSCs but at the price of increased rates of GVHD. However, at the present time, anti-CD45 antibodies are not readily available.
Enzyme replacement therapy and gene therapy for SCID
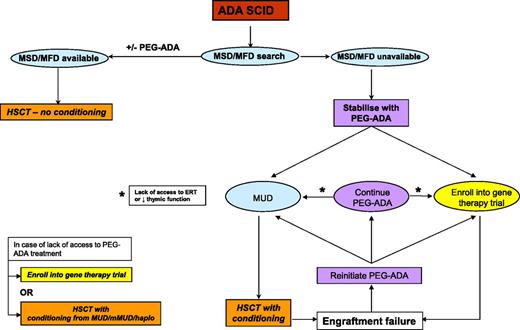
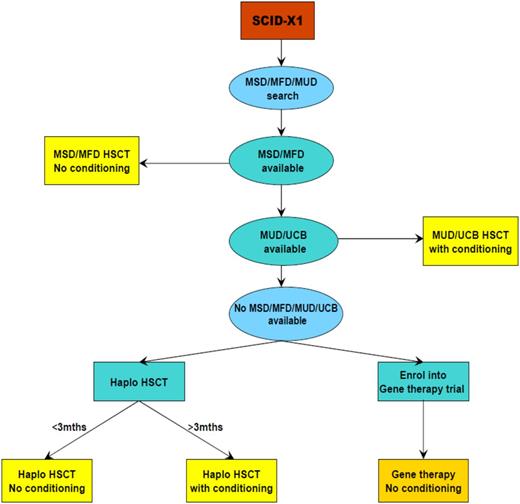
The other option in patients with SCID-X1 and ADA SCID is the use of gene therapy and for ADA SCID enzyme replacement with pegadamase bovine ADA (PEG-ADA). We have previously discussed the use of both enzyme replacement and gene therapy in the context of ADA SCID.52 Since then, the experience with gene therapy in the 3 trials worldwide has extended to more than 40 patients with 100% survival and 72% (29/40) showing immune recovery and being able to discontinue enzyme replacement therapy long term.53-56 These encouraging results have led us to offer ADA gene therapy in preference to MMUD or haplo HSCT and as an alternative to MUD HSCT; patients are counseled with regard to the risks and benefits of both procedures. In view of the improving outcomes with definitive therapies, our tendency has been to use PEG-ADA as a stabilizing measure before HSCT or gene therapy rather than to continue with treatment long term. This differs from previously published guidelines.42 Our current approach to the treatment of ADA-deficient SCID is illustrated in Figure 3. For SCID-X1, the incidence of T-cell leukemia in 5 patients in 2 trials of gammaretroviral vector–mediated gene therapy has altered the risk/benefit ratio.57,58 In a new clinical trial using a self-inactivating vector design,59 which has a potentially better safety profile, patients are offered gene therapy only when no MSD/MFD/MUD is available and as an alternative to a haplo procedure. The success or otherwise of ongoing trials for both ADA SCID and SCID-X1 will determine the role of gene therapy in the therapeutic option hierarchy. Current guidelines for the management of SCID-X1 were agreed upon by the Inborn Errors Working Party of the ESID/EBMT and are shown in Figure 4.43 Preclinical studies in murine models have shown the success of gene therapy in correcting T- and B-cell development defects in the RAG1/260,61 and Artemis forms of SCID,62 and it is likely that clinical trials for these forms will start in the next few years.
Other issues related to the specific form of SCID
Several other important issues arise from understanding the molecular basis of these defects and the resulting phenotype. The VDJ recombination defects (giving rise to T−B−NK+ SCID) can result from mutations in the lymphoid-specific RAG1/2 or in the constitutively expressed genes: Artemis, Cernunnos-XLF, DNA ligase IV, and DNA-PKcs. These latter defects also lead to nonimmunologic abnormalities, including microcephaly and growth retardation. Similarly, defects in the ubiquitously expressed gene ADA also lead to nonimmunologic systemic abnormalities, including neurologic, audiologic, and other organ-specific defects. These abnormalities are not corrected by HSCT and in the case of ADA SCID, not by gene therapy, so families need to be counseled appropriately63,64 (H.B.G., unpublished data).
Follow-up
Long-term follow-up of SCID children after HSCT or gene therapy is of paramount importance. We follow up with all patients whenever possible, the longest follow-up time being 33 years post-HSCT. This is important not only for monitoring the durability of the immune reconstitution achieved but also for screening for nonhematopoietic complications. Among the latter, we monitor for hearing deficit, which may be part of the original disorder, as in ADA SCID,65 or a consequence of the HSCT procedure; dental defects as previously described66 ; endocrine and fertility deficits in those receiving conditioning chemotherapy; and, importantly, neurobehavioral disorders, which we have shown to be potential problems in this group of patients.64,67 Most families will want genetic and family planning counseling. Related to these, we will help facilitate preimplantation, prenatal, and neonatal diagnostic testing, and cord blood storage for potential future-directed cord blood transplants.
Summary
Transplantation for SCID has shown major advances over the last 3 to 4 decades. Where once patients with SCID were far more likely to die than survive, the converse is now true. The survival rates especially from well-matched donor sources are now high, and patients with successful transplants can expect sustained long-term immune recovery. With the advances in gene sequencing technologies, we can increasingly adapt our transplant strategies to the underlying gene defect with the aim that this will further improve outcome. The advent of newborn screening for SCID and the protection of affected babies from birth will also improve survival, although we still need to develop less toxic yet efficacious conditioning regimens for this very young cohort. Gene therapy offers another treatment option to improve outcome, and the challenge remains to determine where best to use HSCT or gene therapy in specific SCID forms. Our own experience and practice outlined here is the result of a highly integrated and multidisciplinary approach to patient care within our institution. In addition, because of the rarity of these conditions and the multiple different problems that patients face, the collaborative nature of the international immunodeficiency transplant community in sharing data and performing multicenter outcome studies has been invaluable in guiding management choices.
Acknowledgments
We acknowledge the contributions of other members of the clinical immunology and transplant teams, including Dr Amel Hassan, Dr Robert Chiesa, Dr Austen Worth, Dr Cathy Cale, and Dr Alison Jones. We also acknowledge Ashley Waterman and especially Zoe Allwood for statistical help. We are also extremely thankful to the clinical nurse specialists, ward nursing staff, and laboratory staff from immunology, hematology, and bone marrow transplantation and would like to acknowledge their enormously important role in our SCID transplant program.
Authorship
Contribution: H.B.G. wrote the initial draft; and W.Q., E.G.D., K.R., P.J.A., and P.V. reviewed the manuscript, suggested changes, and approved the final version.
Conflict-of-interest disclosure: H.B.G. has been an occasional consultant for Enzon Inc., former manufacturers of PEG-ADA. The remaining authors declare no competing financial interests.
Correspondence: H. Bobby Gaspar, Molecular Immunology Unit, UCL Institute of Child Health, 30 Guilford St, London WC1N 1EH; e-mail: h.gaspar@ucl.ac.uk.