In this issue of Blood, Lavender et al take an important step forward in the development of humanized mouse models and particularly for the analysis of human immunity.1
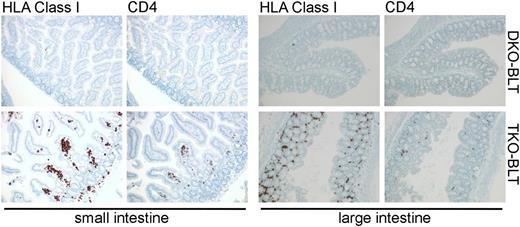
Immunostained sections of intestine taken from DKO- and TKO-BLT mice that show substantial repopulation of the lamina propria by human cells, including CD4+ T cells, in the TKO-BLT model.
Immunostained sections of intestine taken from DKO- and TKO-BLT mice that show substantial repopulation of the lamina propria by human cells, including CD4+ T cells, in the TKO-BLT model.
There have been intensive efforts to model the human hematolymphoid system in immunodeficient mice. Such efforts were catalyzed by the identification of the spontaneous severe combined immunodeficiency (SCID) mutation that eliminates murine adaptive immunity.2 The SCID mouse and other mutations generated by knockout technology (eg, RAG2) enabled the study of human hematopoiesis and immunity in vivo, but also other tissues (eg, liver).3 The identification of multipotent and self-renewing human hematopoietic stem cells (HSCs), as well as leukemic stem cells, has benefited from the development of these models.4 Introduction of other mutations (eg, NOD, γc) onto SCID or RAG2 mutant backgrounds further impaired adaptive and natural killer cell–mediated immunity in murine hosts and thus increased the degree of human hematolymphoid engraftment.5 However, despite these advances, the full elaboration of a robust human hematolymphoid system in mice has eluded the field due to incompatibilities between mouse and humans in cytokines and cell trafficking molecules, murine complement defects, and human xenogeneic graft-versus-host disease (GVHD) responses, as well as the ability of the murine macrophages, unaffected by the SCID or RAG2 mutation, to devour human cells.6 The latter issue hampered the study of human hematopoiesis, but proved especially limiting for the development of a functional human immune system because trafficking of immune cells between sites of infection at epithelial surfaces and immune structures is an essential requirement for the development of effective immune responses. Resolution of this impasse would have a significant impact on the study of human immunity, but also for the development of vaccines and therapies for human pathogens such as HIV.
Lavender et al capitalized on previous findings that showed murine macrophages are able to recognize CD47 null cells as “non-self” via signal recognition protein α (SIRPα), the CD47 receptor.7,8 The absence of the “Don’t eat me!” SIRPα ligand CD47 on cells leads to their phagocytosis and elimination from circulation. Evidence that genetic modulation of SIRPα-CD47 signaling might enable improved human engraftment was provided by Takenaka et al when they found that NOD-encoded SIRPα, which binds human CD47, enhances human HSC engraftment,9 thus tricking murine macrophages into tolerance of primary human hematolymphoid cells. Unfortunately C57BL6 mice do not possess a SIRPα allele with similar human binding capacity. The use of immunodeficient mice on a C57BL6 background offers some advantages such as tolerance of higher radiation doses (to increase engraftment of human stem cells), an intact complement system, and the ability to incorporate murine mutations and human transgenes already present on the C57BL6 background. Here Lavender et al noted that CD47−/− hosts are tolerant of CD47−/− null hematopoietic grafts.10 This surprising tolerizing effect on murine macrophages is mediated by nonhematopoietic CD47−/− cells, but interestingly does not appear to apply to red blood cells (RBCs), as CD47−/− RBCs are still engulfed by macrophages.10 Lavender et al capitalized on this finding by creating Rag2−/−γc−/−CD47−/− triple knockout (TKO) mice on a C57BL6 background. Both Rag2−/−γc−/− double knockout (DKO) and TKO hosts received Thy/Liv grafts under the renal capsule and human fetal liver CD34+ cells by intravenous injection. The authors show that TKO-bone marrow, liver, thymus (BLT) hosts have significantly improved multilineage reconstitution and circulation of human immune cells that includes T cells, B cells, and dendritic cell populations. Importantly, circulating mature T cells have a CD4:CD8 ratio comparable to that of normal humans. Increased numbers of human cells with a primitive human hematopoietic stem/progenitor phenotype are also noted in the bone marrow of TKO-BLT vs DKO-BLT controls suggesting that human HS/PC are protected from engulfment by bone marrow macrophages. A further advance is that a significant number of all major human immune cell types are found in lymph nodes and spleens of TKO-BLT mice. Moreover, human T cells and B cells appear to assemble into rudimentary follicular structures in the spleen. There also appears to be significantly improved seeding of the gut lamina propria by human immune cells in TKO-BLT mice (see figure). Importantly, the improved human engraftment comes with little or no xenogeneic GVHD. Xenogeneic GVHD in humanized mice, and the resulting inflammatory milieu, limits the utility of such models. Finally, Lavender et al show that the TKO-BLT mice can be infected with HIV via the rectal administration of virus. Viremia is sustained, and the human T-cell compartment shows depletion of CD4+ T cells. Moreover, they document B- and T-cell responses to the HIV pathogen with the latter consisting of responses to peptide, suggesting there is significant human antigen presentation to T cells in the TKO-BLT model. This is a feature whose absence has limited the ability to study vaccination in other humanized models.
The TKO-BLT model represents a significant step forward in the development of immunodeficient models of human hematopoiesis and immunity. Further advances likely await as this model is extended to other immunology, pathogenesis, and stem cell questions. Of particular priority will be the application of the TKO-BLT model to the development and testing of vaccines for HIV and other pathogens. One further improvement the Rag2−/−γc−/−CD47−/− model may promote is the analysis of human Peyer’s patch generation and the analysis of human lymphoid tissue inducer cell function. Interestingly, Peyer's patch structures containing human immune cells were not noted in TKO-BLT mice. Perhaps this could be induced by introduction of human receptor transgenes onto the Rag2−/−γc−/−CD47−/− background that enable murine stromal cells to receive inductive signals from human lymphoid tissue inducer. Such an advance would improve the human mucosal immunity observed in Rag2−/−γc−/−CD47−/− mice and its infection by human mucosal pathogens. Perhaps the Rag2−/−γc−/−CD47−/− mouse, having lost its appetite for human cells, can now be persuaded to host an even larger array of human hematopoietic, mesenchymal, and neuro-epithelial stem/progenitor cells.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal