In this issue of Blood, Han et al demonstrate that endotoxin-induced mortality in a murine model of acute lung injury (ALI) was associated with increased vascular permeability attributable to loss of the Src family kinase (SFK) Lyn.1
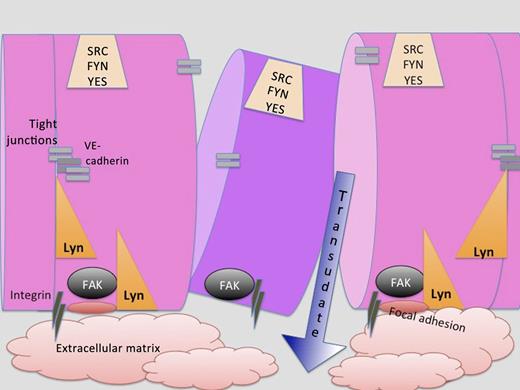
Endothelial cells form a barrier, the integrity of which is mediated by integrins, tight junctions, VE cadherin, FAK, and the Src kinase Lyn. Loss of Lyn affects FAK’s ability to form adhesions and may also contribute to tight junctions. When the integrity of the endothelial barrier is compromised by injury, vascular leakage into the extracellular matrix or surrounding tissues occurs.
Endothelial cells form a barrier, the integrity of which is mediated by integrins, tight junctions, VE cadherin, FAK, and the Src kinase Lyn. Loss of Lyn affects FAK’s ability to form adhesions and may also contribute to tight junctions. When the integrity of the endothelial barrier is compromised by injury, vascular leakage into the extracellular matrix or surrounding tissues occurs.
Endothelial barrier stability required Lyn’s phosphorylation of another tyrosine kinase, focal adhesion kinase (FAK). Could this mechanism also explain the pleural effusions and pulmonary edema observed infrequently and transiently to the SFK inhibitor dasatinib? Could promoting the Lyn-FAK pathway lead to decreased vascular leakiness and decreased mortality in acute respiratory distress syndrome (ARDS)?
The vascular-endothelial (VE) barrier is critical for tissue homeostasis, regulating normal fluid balance, inflammatory cell infiltration, vascular tone, angiogenesis, and thrombogenic responses. Injury to the endothelial barrier results in tissue edema, inappropriate inflammatory cell infiltration, coagulopathy, and exudation of serum proteins, which can lead to shock and death. Catastrophic damage to the pulmonary capillary barrier function, as occurs in ALI and ARDS, can be induced by lung infection or sepsis. ARDS is frequently associated with the failure of other organ systems, where their endothelial barriers may also be compromised. ALI and ARDS have been postulated to have an important immune cell component, and a variety of inflammatory cells have been implicated for the production of inflammatory mediators that affect subsequent vascular leakage. However, it remains unclear which inflammatory cells and intracellular signaling pathways are critical for inflammation-related endothelial dysfunction.2
Efforts to identify the essential cellular activities underlying vascular leakage have focused on altered signaling in the endothelial cells that comprise the vascular barrier. The endothelial cell barrier function is maintained by cell–cell contacts and by cellular adhesion to the extracellular matrix and the basement membrane. These attachments can be affected by alterations in cytoskeleton, gap junctions, tight junctions, and adherens junctions, as well as changes in the extracellular matrix.2 Interestingly, alteration of the endothelial barrier function involves cross talk between multiple signaling pathways and a balance between opposing signals. For instance, the interplay between the second messengers cyclic guanosine monophosphate and cyclic adenosine monophosphate (cAMP) regulates cell adhesions through the activation/inhibition of the serine/threonine protein kinase A. The subcellular compartmentalization of cAMP generation further complicates these signals because different locations of protein kinase A activation have opposing effects on barrier function. Similar complexity may be found in tyrosine kinases and their regulation of the endothelium. Early studies of SFKs suggested that activation of c-Src and Yes induced endothelial barrier permeability. However, evidence from recent clinical trials suggested that this may be a simplified picture of tyrosine kinases in barrier function. Fluid retention and pleural effusions, involving exudates with lymphocytic accumulations, were demonstrated to be an adverse effect of dasatinib, which inhibits SFKs, BCR-ABL, platelet-derived growth factor receptor β, and c-Kit. A study of patients receiving dasatinib for the treatment of chronic myeloid leukemia showed that 9 out of 40 patients developed fluid retention and lung abnormalities.3 Interestingly, this side effect dissipates with time, suggesting tolerance or emergence of counterregulatory mechanisms.
Mice or humans express 8 closely related SFKs: Blk, Fgr, Fyn, Hck, Lck, Lyn, c-Src, and Yes.4 The Src kinases are potent, phosphorylating a diverse range of substrates, many of which are involved in cytoskeletal assembly or reorganization.5 They differ in their tissue expression patterns, such as epithelial or neural, lymphoid or myeloid. They also differ in the amino acid sequences at their N terminus, a region that has not been solved structurally or characterized functionally other than serving as an acceptor site for acyl groups that localize the kinases to the plasma membrane. Subtle differences likely exist in their Src homology 3 and Src homology 2 domains, which interact with proline-rich motifs and phosphotyrosine residues, respectively.
Lyn, which is expressed chiefly in myeloid cells, B lymphocytes, and endothelial cells, has been the odd kinase.4 Described as a double-edged kinase,6 Lyn promotes both positive and negative actions. This is best evidenced by its ability to phosphorylate immunoreceptor tyrosine-based activation motifs and immunoreceptor tyrosine-based inhibition motifs in hematopoietic cells. Lyn also differs from other Src kinases in that it undergoes both myristoylation and palmitoylation, which may affect its localization to lipid rafts or other subcellular regions.7
Gene-targeting studies have illuminated different phenotypes for the Src kinases. Still, it was surprising that Han and colleagues found that Lyn differed from c-Src and Yes in affecting endothelial cell integrity, its permeability in response to endotoxin, and, importantly for the mouse challenged by lipopolysaccharide (LPS), its survival. Genetically engineered mice lacking only the SFK Lyn displayed a higher mortality rate when challenged with LPS or VE growth factor. Transplantation of wild-type bone marrow cells into Lyn-deficient mice did not protect the mice against LPS-induced mortality, suggesting that expression of Lyn in endothelial tissues was a critical factor. Opposing Lyn, the other endothelial SFKs (c-Src, Yes, and Fyn) promote LPS-mediated disruption of the human lung endothelial barrier.8
Loss of Lyn did not result in a compensatory increase in c-Src, Fyn, and Yes expression, so how does Lyn differ from these other SFKs expressed in endothelial cells? Han and coworkers found discontinuities in VE cadherin distribution and formation of interendothelial junctional gaps in Lyn-deficient mice. This same effect was observed when FAK levels were reduced by short interfering RNA in human umbilical vein endothelial cells. Deficiency of Lyn also reduced FAK phosphorylation at tyrosine residues 576/577, 861, and 925 in lung endothelial cells. Knockdown of FAK demonstrated that it was downstream of Lyn in a biochemical and cytoskeletal pathway promoting endothelial barrier stability (see figure). Still, the other endothelial SFKs associate with and phosphorylate FAK. What makes Lyn different from them? Could the explanation lie in the dual acylation of Lyn, which localizes it to a distinct region of the plasma membrane where FAK might play a critical role? Might other mediators acting on inflammatory cells provide a “perfect (cytokine) storm” in the Lyn knockout mouse model? Lastly, how much can we translate from this ALI mouse model to the tens of thousands of patients who have ALI and ARDS in our intensive care units for which more effective therapies are desperately needed?9
Conflict-of-interest disclosure: The authors declare no competing financial interests.