In this issue of Blood, Cader et al show that tumor microenvironment promotes Epstein-Barr virus (EBV)-driven lymphomagenesis in Hodgkin lymphoma by a novel pathway involving latent membrane protein 1 (LMP1) and discoidin domain receptor 1 (DDR1), which is activated by collagen(s) and contributes to the survival of Reed-Sternberg (RS) cells.1
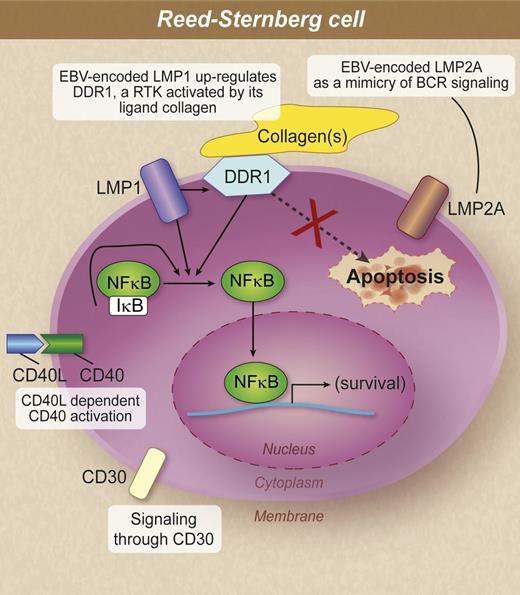
Molecules capable of promoting proliferation and survival and antiapoptotic mechanisms in RS cells. According to the study of Cader et al, in EBV-driven lymphomagenesis, the DDR1 pathway is likely to be an alternative or additional pathway to CD40 or CD30 signaling in the pathogenesis of Hodgkin lymphoma. IκB, inhibitory NF-κB. Professional illustration by Debra T. Dartez.
Molecules capable of promoting proliferation and survival and antiapoptotic mechanisms in RS cells. According to the study of Cader et al, in EBV-driven lymphomagenesis, the DDR1 pathway is likely to be an alternative or additional pathway to CD40 or CD30 signaling in the pathogenesis of Hodgkin lymphoma. IκB, inhibitory NF-κB. Professional illustration by Debra T. Dartez.
DDR1 is a receptor tyrosine kinase (RTK) that regulates cell differentiation, proliferation, migration, and remodeling of the extracellular matrix (ECM).2 The collagen matrix constitutes the primary ECM portion of connective tissues in which the interaction of the cell and the surrounding collagen fibers has an impact on cell and tissue physiology, including motility. DDR1 and DDR2 have been identified as the RTKs that are activated upon collagen binding.3 Collagen(s) are the only known ligand for DDR1. While both DDRs exhibit the same specificity for binding fibrillar collagens, the two receptors display distinct preferences for certain nonfibrillar collagens, with the basement membrane collagen IV being exclusively recognized by DDR1.4 Following binding to collagen, DDR1 phosphorylation triggers the activation of downstream signaling pathways, including nuclear factor κB (NF-κB).5 Activation of these pathways by DDR1 induces the expression of matrix-degrading enzymes and is implicated in the development of inflammatory disorders and oncogenesis.
In their study, Cader et al1 show that (1) expression of the EBV-encoded LMP1 in germinal center B cells, the presumed progenitors of RS cells, upregulates DDR1; (2) RS cells strictly associated with collagen overexpress DDR1; and (3) activated DDR1 significantly increases lymphoma cell survival in vitro. Overall, the results of the study identify a function for collagen in protecting Hodgkin lymphoma cells from apoptosis.1 Therefore, tumor microenvironment contributes to EBV-driven oncogenesis by collagen, an external ligand-activating DDR1, that in turn promotes the oncogenic effect of EBV.
According to the observed pathway1 in EBV-associated Hodgkin lymphoma, LMP1 not only upregulates DDR1 but also modulates NF-κB in association with activated DDR1 and probably in addition to CD40 signaling in RS cells.6,7 It is worth noting that activation of DDR1 and CD40 is mediated by external ligands expressed on environmental collagen and on T cells, respectively. However, LMP1 could also interact with the microenvironment. Recent evidence indicates that EBV can manipulate the tumor microenvironment through the secretion of specific viral and cellular components into exosomes, small endocytically derived vesicles that are released from cells.8,9 Exosomes produced by tumor cells from EBV-infected nasopharyngeal carcinoma contain LMP1, which can activate critical signaling pathways in uninfected neighboring cells, suggesting messenger functions of virus-modified exosomes.8 Moreover, in B-cell lines, EBV-modified exosomes could activate cellular signaling mediated through integrins, actin, interferon, and NF-κB.9 Further insights into these mechanisms are emerging from current knowledge about the capability of EBV to modulate (tumor-like) microenvironment.
Taken together, the results from Cader et al1 advance our understanding of EBV-driven lymphomagenesis. The observed pathway involving LMP1 and DDR1 and its ligand collagen(s) provides evidence that the pathogenesis of EBV-associated Hodgkin lymphoma is likely distinct from that of the disease observed in the absence of EBV. However, in the study of Cader et al,1 the DDR1 overexpression was not restricted to EBV-positive cases; in fact, DDR1 expression was detected in some EBV-negative Hodgkin lymphoma cell lines and in EBV-negative primary RS cells.1 These observations are consistent with the existence of nonviral mechanisms capable of promoting DDR1 expression in the absence of EBV. Therefore, the DDR1 pathway described by Cader et al1 is likely to be an alternative or additional pathway to already known signaling pathways in the pathogenesis of Hodgkin lymphoma. The supposed existence of EBV-unrelated mechanisms upregulating DDR1 expression envisages that DDR1 activation by its ligand collagen(s) plays a significant pathogenetic role in EBV-related and unrelated Hodgkin lymphoma (see figure).
Besides the relationship of EBV-driven oncogenesis with the tumor microenvironment, the article by Cader et al1 focuses the reader’s attention on interesting, but still poorly known, microenvironmental factors in Hodgkin lymphoma such as ECM and fibroblasts. The Hodgkin lymphoma microenvironment, in fact, also contains a collagen-rich ECM, mesenchymal stem cells, and a large number of fibroblasts defined as Hodgkin lymphoma-associated fibroblasts. There is a wide range of interactions between RS cells and Hodgkin lymphoma-associated fibroblasts,7,10 which produce collagen-rich reticular fibers composed of collagen I, III, and IV covered by the ECM. Interestingly, collagen IV is recognized by DDR1.4 The study of the interactions among DDR1, fibroblasts, and ECM might be an interesting field of research because of their potential impact on DDR1 upregulation and activation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal