In this issue of Blood, Liapis et al investigate the characteristics of the tumor microenvironment as well as the role of viral components in AIDS-related diffuse large B-cell lymphoma (AR-DLBCL) and compare these findings with sporadic cases of DLBCL.1
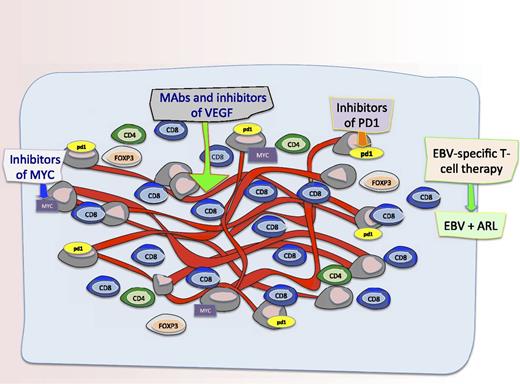
The microenvironment and novel targets in AR-DLBCL. Schematic representation of the microenvironment and potential novel targets in AR-DLBCL. Liapis and colleagues found differences in angiogenesis and the tumor microenvironment in cases of AR-DLBCL compared with sporadic cases. Specifically, they show that AR-DLBCL has PDL1 expression on tumor cells, increased hyperproliferation and c-Myc rearrangements, reduced CD4+ and FOXP3+ T cells, and increased activated CD8+ T cells. Moreover, there was more angiogenesis and higher numbers of CD8+ T cells in patients with AR-DLBCL expressing LMP1 and/or p24 compared with cases lacking viral antigens which has some implications for therapies to augment the immune response and/or targeting the vasculature. Therefore, as shown in this figure, potential therapeutic targets for this disease include T cells targeting EBV proteins LMP1 and LMP2 as well as antibodies and inhibitors of Myc, VEGF, and PD1.
The microenvironment and novel targets in AR-DLBCL. Schematic representation of the microenvironment and potential novel targets in AR-DLBCL. Liapis and colleagues found differences in angiogenesis and the tumor microenvironment in cases of AR-DLBCL compared with sporadic cases. Specifically, they show that AR-DLBCL has PDL1 expression on tumor cells, increased hyperproliferation and c-Myc rearrangements, reduced CD4+ and FOXP3+ T cells, and increased activated CD8+ T cells. Moreover, there was more angiogenesis and higher numbers of CD8+ T cells in patients with AR-DLBCL expressing LMP1 and/or p24 compared with cases lacking viral antigens which has some implications for therapies to augment the immune response and/or targeting the vasculature. Therefore, as shown in this figure, potential therapeutic targets for this disease include T cells targeting EBV proteins LMP1 and LMP2 as well as antibodies and inhibitors of Myc, VEGF, and PD1.
There is now a burgeoning recognition that components of the local environment of tumor cells play important roles in cancer biology. Within the “tumoral microenvironment,” stromal and immune-related cells as well as angiogenic vascular cells are likely to engage in “cross-talk” with tumor cells and this may provide a critically important milieu for tumor growth and invasion. The assorted roles of these components and the communication they engage in within the tumor microenvironment have not been well elucidated, and fresh insights are needed to identify new targets for drug discovery and development.
In DLBCL, gene expression profiling has amply established the existence of molecular subtypes with distinct genetic signatures and clinical outcomes.2,3 As to the role that the tumor microenvironment plays, 1 survival model, in HIV-negative cases, examined 2 signatures derived from the nonmalignant cells in DLBCL and determined that these could independently predict outcome.4 The first of these signatures, termed “stromal-1,” was prognostically favorable in patients treated with both cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and rituximab plus CHOP (R-CHOP) and included genes that are coequally expressed in many normal mesenchymal tissues and mostly associated with the extracellular matrix. Infiltration by myeloid lineage cells was a characteristic attribute. In contrast, DLBCL tumors with the prognostically unfavorable “stromal-2” signature included genes involved in endothelial cell biology and adipocyte function and correlated with increased blood vessel density.
The composition of the nonmalignant tumor environment and the roles of these various components in AIDS-related lymphoma have not been well studied. In this issue, Liapis and colleagues set out to characterize the tumor microenvironment in AR-DLBCL and directly compare its features with those of sporadic DLBCL cases.1 Using paraffin-embedded tissue, their study revealed a number of interesting findings. First, they found that the microenvironment of AR-DLBCL was characterized by much higher angiogenic activity than sporadic cases of DLBCL and this correlated with immunoblastic and plasmablastic morphology as well as with Epstein-Barr virus (EBV) positivity, suggesting an association between viral lymphomagenesis and angiogenesis. The principal EBV oncoprotein, latent membrane protein 1 (LMP1), increases production of vascular endothelial growth factor (VEGF) in nasopharyngeal carcinoma; this is a likely mechanism in AR-DLBCL.5 Additionally, HIV-1 may directly promote angiogenesis.6 Second, they found that a much higher proportion of AR-DLBCL cases (23.5% vs 5.6% of sporadic cases) were characterized by a MYC rearrangement. Third, compared with sporadic cases, they found reduced T-helper (CD4) and T-regulatory (FOXP3+) cells but markedly higher numbers of CD8+ T cells in cases of AR-DLBCL. Cases with viral antigen expression (LMP1 and/or p24) had much higher numbers of CD8+ T cells implicating differences in immunosurveillance in virally driven tumors.
The study provides interesting insights into the pathophysiology of AR-DLBCL thus providing a rationale for the consideration of novel therapeutics targeting the tumor microenvironment and angiogenesis (see figure). For example, future efforts could focus on characterizing the function of the tumor-infiltrating CD8+ T cells, especially with the advent of EBV-directed cytotoxic T-cell therapies targeting LMP antigens as a potential therapeutic for EBV-associated lymphomas.7,8 Additionally, it would be compelling to assess gene-expression signatures from the host microenvironment cells in AR-DLBCL as has been done in sporadic cases. Regulating angiogenesis involves a complicated interplay of cells and cytokines and studies that better elucidate the mechanics of this are needed; inhibiting angiogenesis by monoclonal antibodies to VEGF, or by using small-molecule inhibitors, may be interesting future strategies to investigate in AR-DLBCL.9,10
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal