Key Points
CX3CR1 mediates monocyte retention in the bone marrow.
Myelorestoration after chemotherapy is controlled by chemokine receptors.
Abstract
The chemokine receptor CCR2 controls the release of Ly6Chigh monocytes from the bone marrow and their recruitment to sites of inflammation. A second chemokine receptor, CX3CR1, is differentially expressed on monocyte subsets. We examined the role of CX3CR1 in monocyte trafficking during the recovery phase after cyclophosphamide (CP)-induced myeloablation and observed that, in the absence of CCR2, Ly6Chigh monocytes accumulated in the bone marrow and peripheral reconstitution was severely impaired compared with wild-type (WT) mice. In contrast, in the absence of CX3CR1, Ly6Chigh monocytes accumulated less rapidly in the marrow but recovered faster in the blood and were more recruited into the spleen, suggesting an opposite action between CCR2 and CX3CR1 in myelorestoration. During the recovery phase, marrow medullar monocytes displayed lower CX3CR1 expression and reduced their adherence to coated CX3CL1. Intravital imaging of the bone marrow showed that CP treatment impacts monocyte trafficking between the parenchyma and the vasculature. Medullar monocytes in CX3CR1−/− mice and mice treated with a specific antagonist of CX3CR1 displayed increased mean velocity and displacement and a reduced arrest coefficient compared with WT mice. This study indicates that CX3CR1 reduces the motility of Ly6Chigh monocytes in the bone marrow and thereby controls their release.
Introduction
Monocytes are myeloid-derived cells that belong to the mononuclear phagocyte system.1 They arise from hematopoietic stem cells in the bone marrow, are released into the bloodstream, and colonize peripheral organs in normal and inflammatory conditions,2 where they differentiate into macrophages or dendritic cells with multiple functions in homeostasis and innate and acquired immunity.3-5 The alkylating agent cyclophosphamide (CP) is a common chemotherapeutic drug known to adversely cause transient ablation of mature myeloid cell populations.6-8 The recovery phase following CP treatment provides a model of clinical relevance in which to study the processes that control monocyte trafficking.
The migration of myeloid cells is controlled by specific chemoattractants called chemokines. Subsets of monocytes in the mouse, defined by the expression of the hematopoietic cell differentiation antigen Ly6C, also differ in their expression of the chemokine receptors CCR2 and CX3CR1.5,9 Monocytes accumulate in the bone marrow of mice lacking CCR2 and fewer circulating monocytes and monocyte-derived populations are detected in peripheral organs.10-13 These findings implicate CCR2 in the egress of monocytes from the bone marrow. CCR2 is also required for the early phase of monocyte recruitment to an inflammatory site,11 although this recruitment later becomes CCR2 independent.10
The level of CX3CR1 on monocytes increases with maturation in the marrow and correlates inversely with the Ly6C marker and with CCR2 in the blood. The ligand of CX3CR1, CX3CL1, can be presented either in a soluble form with potent chemoattractive activity14 or in a membrane-anchored form giving rise to a very strong adhesive activity.15-18 CX3CR1 may contribute to cell recruitment during inflammation through either chemotactism1,19 or adhesion.20,21 In addition, CX3CR1 is required for monocyte crawling or “patrolling” in the lumen of the blood vessels.22
In the current study, we examined the expression and function of CX3CR1 during monocyte recovery after CP-induced myeloablation. We further developed an intravital imaging approach of calvaria bone tissues to examine the role of CX3CR1 in cell motility within the bone marrow. The results indicate a complex interplay between CCR2 and CX3CR1 in the control of monocyte release from the bone marrow.
Materials and methods
Mice
Balb/c mice were purchased from Elevage Janvier (Le Genest, Saint Isle, France). CCR2−/− and CX3CR1−/− Balb/c mice and CX3CR1-GFP-Kin (CX3CR1gfp/+ and CX3CR1gfp/gfp)23 and Csf1r-Gal4VP16/UAS-ECFP (MacBlue)24 mice were bred in the animal facility Nouvelle Animalerie Commune, Pitié-Salpétrière. All mice were used between 8 and 12 weeks old. All experiment protocols were approved by the local animal experimentation and ethics committee and followed its guidelines.
Chemotherapeutic treatment
For all experiments, mice were treated or not (NT or day 0) with a single intraperitoneal injection of CP (Sigma-Aldrich, Saint-Quentin Fallavier, France) at 175 mg/kg reconstituted in 0.9% saline.
Flow cytometry
Flow cytometry was performed using an LSRII (BD, Franklin Lakes, NJ) for acquisition and analysis was performed using FlowJo software (Tree Star, Ashland, OR). Blood was drawn and directly stained with antibodies. Bone marrow cells were harvested by flushing out thighbones or mashing skull bones in phosphate-buffered saline (PBS) with 0.5% of bovine serum albumin (BSA). Spleen cells were filtered using a 70-μm cell strainer (BD Biosciences, San Jose, CA). Surface staining was performed by incubating 50 μL of cell suspension (1/10th of the total organ) with 1 μg/mL purified anti-CD16/32 (2.4G2, BD Biosciences) for 10 minutes at 4°C to block Fc-mediated binding and for an additional 20 minutes with the appropriate dilution of specific antibodies (see “Flow cytometry” in supplemental Methods, available on the Blood Web site). After incubation, cell suspensions were washed once in PBS 0.5% BSA. For cell-cycle staining, after surface staining, cells were fixed using Lyse/fix buffer kit (BD Biosciences) for 10 minutes, washed twice, and incubated for 20 minutes in perm/wash solution. Cells were washed once in PBS. Subsequent DNA staining was performed by incubating the cells with 2 µM Topro-3 iodide (Life Technologies, Saint Aubin, France) and 50 μg/mL of ribonuclease A in PBS. After 1 hour, cell suspensions were directly analyzed by flow cytometry.
Adoptive transfer experiment
Bone marrow monocytes were isolated from CX3CR1gfp/+ and CX3CR1gfp/gfp mice 5 days after CP treatment and labeled in PBS with Hoechst 33342 (3 μM) and CMTMR (10 μM), respectively. Cells were then cotransferred into C57Bl6 recipient mice treated with CP 2 days before. Bone marrow (1 thighbone), blood, and spleen of recipient mice were harvested 20 hours after adoptive transfer and the percentage of transferred monocytes was calculated by counting the absolute number of green fluorescent protein (GFP)+Hoechst+ and GFP+CMTMR+ cells recovered in each organ and per mL of blood.
CX3CR1 staining
Bone marrow cells from CX3CR1gfp/+ and CX3CR1gfp/gfp mice were harvested and 1/10th of the cell suspensions were incubated in 96 round-bottom well plates in RPMI (Gibco, Invitrogen, Cergy Pontoise, France) supplemented with antibiotics and 10% fetal calf serum in the presence of 100 nM human CX3CL1–Alexa 647 (Almac Group, Craigavon, United Kingdom) for 1 hour at 37°C. Cells were washed twice in PBS-BSA 0.5% and directly analyzed by flow cytometry. Results are expressed in mean fluorescence intensity (MFI) of Alexa 647 gated on the GFPlow population as previously performed with CX3CL1-Fc.25
Cell adhesion assays
A total of 25 nM full-length CX3CL1-His (R&D Systems, Lille, France) was adsorbed overnight to flat-bottom 96-well microtiter plates (Nunc A/S, Roskilde, Denmark) at 4°C in 50 μL of 25 mM Tris (pH 8) and 150 mM NaCl. Well surfaces were then blocked for 2 hours at room temperature with 1% nonfat milk in the same buffer. For adhesion assays, bone marrow cells from CX3CR1gfp/+ or CX3CR1gfp/gfp mice were resuspended in Ca/Mg-free PBS and 5 × 105 total cells were added per well and incubated at room temperature. For blocking experiments, cells were treated before adhesion for 15 minutes at room temperature with 500 nM CX3CL1, CCL2 (Peprotech-Levalloi-Perret, France), or CX3CL1 antagonist protein (F1) (kindly provided by A. Proudfoot, Merck-Serono). After 45 minutes, wells were washed to remove nonadherent cells as described previously.26 A wide-field picture of each well was captured using a Nikon AZ100 macroscope with an FITC filter set. The percentage of GFP+ adherent cells was calculated as a ratio of the total number of GFP+ adherent cells to the total number of GFP+ cells loaded in each well, determined by flow cytometric analysis for each condition.
Results
CX3CR1 and CCR2 have opposite actions in Ly6Chigh monocyte recovery after myeloablation
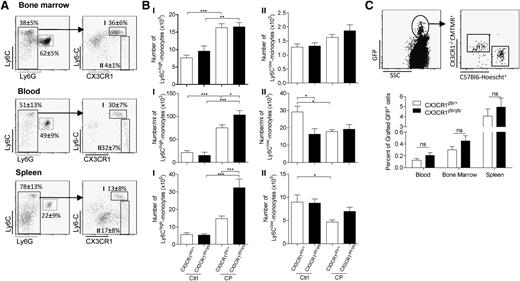
In order to investigate the mechanism of myelorestoration, wild-type (WT), CCR2−/−, and CX3CR1−/− Balb/c mice were treated with a single dose of CP (175 mg/kg). The number of cells of the different myeloid subsets was monitored in the bone marrow (Figure 1A; supplemental Figure 1), blood (Figure 1B; supplemental Figure 1), and spleen (Figure 1C; supplemental Figure 1) of Balb/c mice at different time points before and after CP treatment. The CD11b+ Ly6ChighLy6G− populations represented the immature or inflammatory monocytes (Figure 1). This population expressed CCR2, CD115, and F4/80 (data not shown). The CD11b+ Ly6ClowLy6G− population was more heterogeneous (supplemental Figure 1). The levels of all surface markers varied in different tissues, reflecting variation among mature monocytes and subsets of macrophages. Finally, the CD11b+ Ly6ClowLy6G+ population represented neutrophils27 (supplemental Figure 1). Within 24 hours, treatment with CP almost completely ablated the Ly6Chigh monocyte subset in the bone marrow (Figure 1A), blood (Figure 1B), and spleen (Figure 1C) in all 3 mouse strains. In the bone marrow of WT mice, the monocyte number recovered by day 5, with an apparent overshoot of the number at steady state (24.8 ± 7 × 104 at day 0 vs 45 ± 22 × 104 cells per thighbone at day 5; P < .05), which was maintained until day 12 (Figure 1A). Consistent with the role of CCR2 in inflammatory monocyte egress, Ly6Chigh monocyte accumulation was somewhat greater by day 7 in the CCR2−/− mice (49.8 ± 3.6 × 104 vs 67.6 ± 6.1 × 104 cells per thighbone in WT and CCR2−/−, respectively) (Figure 1A). By contrast, in CX3CR1−/− mice, there was significant reduction in the number of Ly6Chigh monocytes in the bone marrow at day 5 after CP treatment (45 ± 22 × 104 compared with 25 ± 4.7 × 104 cells per thighbone in WT and CX3CR1−/−, respectively) (Figure 1A). The recovery phase in the bone marrow was associated with a rebound in the blood (Figure 1B) and the spleen (Figure 1C). Consistently, CCR2 deficiency resulted in a substantial defect in the number of Ly6Chigh monocytes in the blood and the spleen.10,11 Conversely, CX3CR1 deficiency led to an increased accumulation of Ly6Chigh monocytes at day 5 in the blood (Figure 1B) and at day 7 in the spleen (Figure 1C).
CX3CR1 and CCR2 have opposing actions in monocyte recovery after myeloablation. Representative dot plots previously gated on CD11b+ cells indicate the gating strategy for a Ly6Chigh monocyte subset from the bone marrow (A), blood (B), and spleen (C) (percent ± standard deviation [SD] of the gated subset from untreated mice are indicated). Kinetic of Ly6Chigh monocyte recovery after CP treatment (175 mg/kg) in Balb/c (empty), CCR2−/− (gray), and CX3CR1−/− (black) mice was monitored. D0 corresponds to untreated mice. Graph represents the absolute number ± standard error of the mean (SEM) of Ly6Chigh monocytes after CP treatment in bone marrow, blood, and spleen (n = 6-15 mice for each time point out of at least 3 independent experiments). A 2-way analysis of variance (ANOVA) with Bonferroni comparison post-test was performed. Statistical significance between WT and knockout strains is indicated.
CX3CR1 and CCR2 have opposing actions in monocyte recovery after myeloablation. Representative dot plots previously gated on CD11b+ cells indicate the gating strategy for a Ly6Chigh monocyte subset from the bone marrow (A), blood (B), and spleen (C) (percent ± standard deviation [SD] of the gated subset from untreated mice are indicated). Kinetic of Ly6Chigh monocyte recovery after CP treatment (175 mg/kg) in Balb/c (empty), CCR2−/− (gray), and CX3CR1−/− (black) mice was monitored. D0 corresponds to untreated mice. Graph represents the absolute number ± standard error of the mean (SEM) of Ly6Chigh monocytes after CP treatment in bone marrow, blood, and spleen (n = 6-15 mice for each time point out of at least 3 independent experiments). A 2-way analysis of variance (ANOVA) with Bonferroni comparison post-test was performed. Statistical significance between WT and knockout strains is indicated.
The Ly6Clow populations were also ablated by CP treatment and recovered in the bone marrow and the spleen by day 7 and accumulated by day 12 (supplemental Figure 1A). The recovery was similar in the absence of CCR2 or CX3CR1, except that the accumulation in spleen was reduced in CCR2−/− mice, which may reflect a role for Ly6Chigh cells as progenitors28 (supplemental Figure 1A, right). Finally, neutrophil reconstitution was also similar in all mouse strains (supplemental Figure 1B), showing that the effects of CCR2 and CX3CR1 deficiency were restricted to Ly6Chigh monocytes.
The difference observed in the recovery phase of the Ly6Chigh monocytes could be related to differential proliferation rates between mutant mice. Nevertheless, the number of cycling Ly6Chigh monocytes was similar in the bone marrow of mutant and WT mice (supplemental Figure 2A). By contrast, the proportion of cycling cells at day 1 was considerably lower in the CCR2−/− mice (2% ± 2% in CCR2−/− and 61% ± 2.4% in WT mice), which can be attributed to the impaired egress of noncycling monocytes (supplemental Figure 2A). The proportion of cycling Ly6Chigh monocytes in the spleen was very low compared with the bone marrow during the recovery phase (1.3% at day 5 and 3.6% at day 7) and increased at a later time point (23% at day 12) but was the same in the CX3CR1−/− mice (supplemental Figure 2B).
In conclusion, CP treatment induces a systemic ablation of myeloid subsets because of the rapid turnover of these populations.29 This depletion is followed by an increased rate of Ly6Chigh monocyte proliferation, egress from the marrow, and peripheral replenishment. Our data confirm the known role of CCR2 in this process but indicate an unexpected opposing function of CX3CR1.
CX3CR1 reduced medullar Ly6Chigh monocyte accumulation into the bloodstream
Splenic recruitment of Ly6C+(Gr1+) monocytes into the spleen is partially CX3CR1 dependent after irradiation or infection but CX3CR1 independent in the steady state,1,25 suggesting that Ly6Chigh monocyte accumulation was linked to a higher frequency of blood monocytes released from the marrow in CX3CR1−/− mice. To address this possibility, we first used CX3CR1gfp/+ and CX3CR1gfp/gfp reporter lines23 and monitored the 2 monocyte subsets based on GFP expression (Figure 2A). Consistent with the results above, CX3CR1gfp/gfp mice had a greater recovery of CX3CR1lowLy6Chigh monocytes in blood and spleen than CX3CR1gfp/+ mice after CP ablation and the CX3CR1highLy6Clow subset was similarly affected (Figure 2B).
CX3CR1 reduced medullar Ly6Chigh monocyte release into the bloodstream. (A) Representative dot plots previously gated on CD11b+ cells indicate the gating strategy in the bone marrow, blood, and spleen for Ly6Chigh- and Ly6Clow-myeloid subsets based on CX3CR1 expression using CX3CR1gfp/+ mice. (B) Bars represent absolute number ± SEM of the 2 monocyte subsets before and 5 days after CP treatment in bone marrow, blood, and spleen of CX3CR1gfp/+ and CX3CR1gfp/gfp mice (n = at least 12 mice for each time point pooled from 5 independent experiments; a 1-way ANOVA with Bonferroni comparison post-test was performed). (C) Bone marrow cells from CX3CR1gfp/+ and CX3CR1gfp/gfp mice were harvested 5 days after CP treatment, labeled with CMTMR and Hoechst, respectively, and grafted into C57Bl6 recipient mice 2 days after CP treatment. The day after, bone marrow, blood, and spleen were harvested and the percent of transferred cells recovered was calculated for each organ and per mL of blood. Dot plots indicate the gating strategy of transferred cell recovery. Ctrl, control; D, day; NT, not treated; SSC, saline sodium citrate.
CX3CR1 reduced medullar Ly6Chigh monocyte release into the bloodstream. (A) Representative dot plots previously gated on CD11b+ cells indicate the gating strategy in the bone marrow, blood, and spleen for Ly6Chigh- and Ly6Clow-myeloid subsets based on CX3CR1 expression using CX3CR1gfp/+ mice. (B) Bars represent absolute number ± SEM of the 2 monocyte subsets before and 5 days after CP treatment in bone marrow, blood, and spleen of CX3CR1gfp/+ and CX3CR1gfp/gfp mice (n = at least 12 mice for each time point pooled from 5 independent experiments; a 1-way ANOVA with Bonferroni comparison post-test was performed). (C) Bone marrow cells from CX3CR1gfp/+ and CX3CR1gfp/gfp mice were harvested 5 days after CP treatment, labeled with CMTMR and Hoechst, respectively, and grafted into C57Bl6 recipient mice 2 days after CP treatment. The day after, bone marrow, blood, and spleen were harvested and the percent of transferred cells recovered was calculated for each organ and per mL of blood. Dot plots indicate the gating strategy of transferred cell recovery. Ctrl, control; D, day; NT, not treated; SSC, saline sodium citrate.
We next transferred CX3CR1gfp/+ labeled with Hoechst and CX3CR1gfp/gfp bone marrow cells labeled with CMTMR intravenously into CP-treated mice and looked at the proportion of monocytes infiltrating the bone marrow and the spleen (Figure 2C). CX3CR1gfp/+ and CX3CR1gfp/gfp monocytes from the blood colonized the bone marrow and the spleen equally. Accordingly, we hypothesized that CX3CR1 expression constrains the release of medullar Ly6Chigh monocytes into the bloodstream after CP treatment.
CP treatment reduced the CX3CR1-dependent adherence of marrow monocytes
CX3CR1 activity in the marrow would depend upon the presence of its ligand. The presence of the membrane-bound form of the ligand CX3CL1 was examined with a goat antibody against rat CX3CL1 using flow cytometry of isolated bone marrow cells. Specific staining was detected on both CD45−CD31− stromal cell populations and CD45−CD31+ endothelial cell populations (supplemental Figure 3A). The number and frequency of CX3CL1-expressing cells decreased transiently following CP administration and recovered by day 5 (supplemental Figure 3B). In contrast, soluble CX3CL1 in the serum was not modified by CP treatment (supplemental Figure 3C).
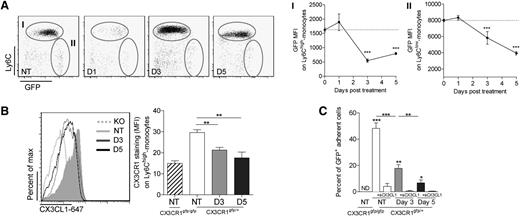
CX3CR1 expression in bone marrow monocytes was also transiently modified after CP treatment. GFP MFI in both subsets of monocytes was reduced at day 3 and started to recover by day 5 in Ly6Chigh monocytes (Figure 3A). To confirm the loss of CX3CR1 function in response to CP, we used CX3CL1 conjugated with Alexa 647 to stain the receptor (Figure 3B) as previously performed.25 Membrane CX3CR1 staining was lower at days 3 and 5 after CP treatment compared with untreated mice. Specificity of the detection was confirmed by the absence of signal in CX3CR1gfp/gfp mice. The adherence of monocytes to CX3CL1 was also strongly reduced following CP treatment (Figure 3C). We conclude that CP treatment induces a strong local modulation of both the receptor- and membrane-anchored forms of ligand within the CX3CR1 axis in the bone marrow.
CP treatment reduced the CX3CR1-dependent adherence of marrow monocytes. (A) Dot plots represent Ly6C and GFP expression gated on CD11b+Ly6G− cells on both bone marrow monocyte subsets at different time points after CP treatment of CX3CR1gfp/+ mice. Mean GFP MFI ± SD is indicated (n = 5-10 mice per time point out of 3 independent experiments; a 1-way ANOVA with Bonferroni comparison post-test was performed). (B) Representative histogram plot overlay of CX3CL1–Alexa 647 expression gated on bone marrow GFP+ cells in each indicated conditions. GFPhigh-expressing cells corresponding to Ly6Clow monocytes have been excluded from the gating. Graph represents mean ± SEM of the CX3CL1–Alexa 647 MFI in each condition (n = 5-9 different mice out of 4 independent experiments; 1-way ANOVA with Bonferroni comparison post-test was performed). (C) Graph represents mean ± SEM of the percentage of adherent GFP+ bone marrow cells on CX3CL1-coated wells from CP-treated or untreated CX3CR1gfp/+ and CX3CR1gfp/gfp mice. To evaluate adherence specificity to coated CX3CL1, cells have been previously incubated or not with soluble CX3CL1. GFP+ adherent cells on uncoated wells have been subtracted for each condition. Data are collected from triplicate out of 2 independent experiments; a 1-way ANOVA with Bonferroni comparison post-test was performed. D, day; KO, knockout; ND, not determined; NT, not treated.
CP treatment reduced the CX3CR1-dependent adherence of marrow monocytes. (A) Dot plots represent Ly6C and GFP expression gated on CD11b+Ly6G− cells on both bone marrow monocyte subsets at different time points after CP treatment of CX3CR1gfp/+ mice. Mean GFP MFI ± SD is indicated (n = 5-10 mice per time point out of 3 independent experiments; a 1-way ANOVA with Bonferroni comparison post-test was performed). (B) Representative histogram plot overlay of CX3CL1–Alexa 647 expression gated on bone marrow GFP+ cells in each indicated conditions. GFPhigh-expressing cells corresponding to Ly6Clow monocytes have been excluded from the gating. Graph represents mean ± SEM of the CX3CL1–Alexa 647 MFI in each condition (n = 5-9 different mice out of 4 independent experiments; 1-way ANOVA with Bonferroni comparison post-test was performed). (C) Graph represents mean ± SEM of the percentage of adherent GFP+ bone marrow cells on CX3CL1-coated wells from CP-treated or untreated CX3CR1gfp/+ and CX3CR1gfp/gfp mice. To evaluate adherence specificity to coated CX3CL1, cells have been previously incubated or not with soluble CX3CL1. GFP+ adherent cells on uncoated wells have been subtracted for each condition. Data are collected from triplicate out of 2 independent experiments; a 1-way ANOVA with Bonferroni comparison post-test was performed. D, day; KO, knockout; ND, not determined; NT, not treated.
Comparative imaging of CX3CR1gfp/+ and MacBlue mice
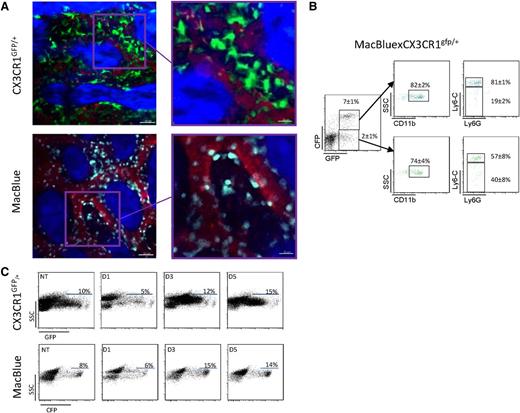
To understand the function of CX3CR1 in monocyte trafficking in the marrow, we developed intravital imaging of the skull bone marrow using 2 different mouse strains: the CX3CR1gfp/+ mouse23 and the MacBlue transgenic mouse.24,30 GFP+ and cyan fluorescent protein (CFP)+ cells were located in similar areas; the GFP+ cells in CX3CR1gfp/+ mice were larger with numerous dendritic-like structures, whereas CFP+ cells of MacBlue mice were mainly rounded (Figure 4A). GFP+ cells in the CX3CR1gfp/+ line include RANK-positive cells able to differentiate into mature osteoclasts.31 On fluorescence-activated cell sorter profiling, all CFP+ marrow cells from a MacBlue-CX3CR1gfp/+ mouse were GFP+ but around 22% of GFP+ cells were CFP−, confirming that cell populations are not fully overlapping (Figure 4B). Ly6Chigh monocytes were CFP+/GFP+, as expected from the profile on circulating monocytes,22 but the level of GFP was considerably less intense than the CFP, which is amplified in the MacBlue mouse (Figure 4B). In addition, the level of CFP was not downregulated after CP treatment (Figure 4C). Hence, the MacBlue mice provide a better model than the CX3CR1gfp/+ mice with which to monitor the behavior of medullar monocytes.
Comparative imaging of CX3CR1gfp/+ and MacBlue mice. (A) Representative 2-photon laser scanning microscopy (TPLSM) images of mouse skull bone tissues from CX3CR1gfp/+ and MacBlue mice. GFP+ cells appear in green, CFP+ cells appear in cyan, and the microvasculature was visualized using rhodamine-dextran (red). The bone matrix is visualized by second harmonic generation (SHG; blue). (B) Representative dot plots of GFP and CFP coexpression and CD11b, Ly6C, and Ly6G expression gated on the indicated populations from MacBlue-CX3CR1gfp/+ mouse (mean percentage ± SD from n = 6 different mice pooled from 2 independent experiments are indicated). (C) Impact of CP treatment on GFP and CFP expression on skull bone marrow total cell from CX3CR1gfp/+ and MacBlue mice.
Comparative imaging of CX3CR1gfp/+ and MacBlue mice. (A) Representative 2-photon laser scanning microscopy (TPLSM) images of mouse skull bone tissues from CX3CR1gfp/+ and MacBlue mice. GFP+ cells appear in green, CFP+ cells appear in cyan, and the microvasculature was visualized using rhodamine-dextran (red). The bone matrix is visualized by second harmonic generation (SHG; blue). (B) Representative dot plots of GFP and CFP coexpression and CD11b, Ly6C, and Ly6G expression gated on the indicated populations from MacBlue-CX3CR1gfp/+ mouse (mean percentage ± SD from n = 6 different mice pooled from 2 independent experiments are indicated). (C) Impact of CP treatment on GFP and CFP expression on skull bone marrow total cell from CX3CR1gfp/+ and MacBlue mice.
CP treatment induced monocyte redistribution in the bone marrow
Different bone marrow compartments were initially distinguished through microvasculature staining by intravenous injection of rhodamine-dextran. Areas outside the microvasculature were representative of bone marrow parenchyma.32 We thus analyzed CFP+ cell dynamics in these distinct regions of the bone marrow tissue.
Representative wide fields of skull bone marrow tissue illustrate the cell density in the parenchymal region before and after CP treatment (Figure 5A; supplemental Video 1). The proportion of Ly6Chigh monocytes among CFP+ cells is indicated in the associated dot plot. Following CP treatment, the massive CP-mediated ablation of CFP+ monocytes was evident. From day 1 to day 3, the parenchyma was denuded of cells; however, numerous nonmotile blastic cells became detectable (Figure 5A; supplemental Video 1). Ly6Chigh monocytes represented a lower proportion of CFP+ cells at these time points, confirming their preferential elimination. By day 5, the proportion of Ly6Chigh monocytes increased and cells reappeared in clusters (Figure 5A; supplemental Video 1). Time-lapse imaging showed that at day 7, consistent with the accumulation observed by flow cytometry, the density of cells was so high that distinction between cells was difficult. To follow the migration of the monocyte compartment after ablation and during the recovery phase, we performed automatic 3-dimensional tracking of individual CFP+ cells and compared the distribution of cell velocity as a function of track straightness at different time points after CP treatment. Quadrant defined the median value of cell velocity (2 μm/min) and the median value of track straightness (0.25) at steady state in parenchyma. In the steady state, most CFP+ cells were relatively sessile: 58% with a slow mean velocity (<2 µm/min) and 48% with reduced track straightness (<0.25) (Figure 5A). The proportions of these motility classes changed only marginally following CP administration, despite the large changes in cell numbers.
CP treatment induced monocytes redistribution in the bone marrow. TPLSM images of monocyte cells (cyan) in parenchymal zones (A) and bone marrow vasculature (B) of skull bone tissue of MacBlue mice. The vasculature is in red (rhodamine-dextran) and the bone matrix is in blue (SHG). White bars represent 50 µm. Dot plots (upper right corner) indicate the percentage of Ly6-Chigh monocytes among CFP+ cells. Graphs represent the distribution of track straightness as a function of cell mean velocity after CP treatment. Quadrants separate cells according to track straightness (value is 0.25 for parenchyma and 0.5 for vasculature) and mean velocity (2 μm/min for parenchyma and 4 μm/min for vasculature) (corresponding to the median values for each compartments in untreated mice). Quadrant statistics are indicated for each condition (parenchyma: not treated [NT] n = 687, day [D] 1 n = 327, D3 n = 219, D5 n = 448; vasculature: NT n = 583, D1 n = 899, D3 n = 566, D5 n = 498; from at least 3 different mice in each condition).
CP treatment induced monocytes redistribution in the bone marrow. TPLSM images of monocyte cells (cyan) in parenchymal zones (A) and bone marrow vasculature (B) of skull bone tissue of MacBlue mice. The vasculature is in red (rhodamine-dextran) and the bone matrix is in blue (SHG). White bars represent 50 µm. Dot plots (upper right corner) indicate the percentage of Ly6-Chigh monocytes among CFP+ cells. Graphs represent the distribution of track straightness as a function of cell mean velocity after CP treatment. Quadrants separate cells according to track straightness (value is 0.25 for parenchyma and 0.5 for vasculature) and mean velocity (2 μm/min for parenchyma and 4 μm/min for vasculature) (corresponding to the median values for each compartments in untreated mice). Quadrant statistics are indicated for each condition (parenchyma: not treated [NT] n = 687, day [D] 1 n = 327, D3 n = 219, D5 n = 448; vasculature: NT n = 583, D1 n = 899, D3 n = 566, D5 n = 498; from at least 3 different mice in each condition).
Representative fields of skull bone marrow vessels illustrate the cell density in the vasculature before and after CP treatment (Figure 5B and supplemental Video 2). Monocytes in vascular areas of untreated mice displayed higher median velocities (4 μm/min) and more directed migration (median track straightness = 0.5) from those in the parenchyma (Figure 5B). Cells could be arrested, slowly motile, or crawling on the endothelium (supplemental Video 3). After CP treatment, the proportion of very motile monocytes (53% with a mean velocity > 4 μm/min) diminished by day 1 (40%) and further by day 3 (29%), consistent with the clearance of monocytes from the bloodstream. Very motile monocytes in the vasculature started to reappear by day 5 (41%), when cell egress became evident. In addition, cells accumulated in the vicinity of microvasculature (supplemental Video 2), apparently adhered for several minutes to the abluminal side of the vessels, and then, following transendothelial migration, rapidly disappeared in the blood flow (supplemental Video 4). In summary, after CP treatment, monocytes were mostly depleted in the parenchymal area of the bone marrow and the vasculature. During the recovery phase, monocytes redistributed in the whole parenchyma, formed proliferating clusters, and reconstituted the medullar compartment. They subsequently accumulated in the vicinity of blood vessels and egressed toward the bloodstream.
CX3CR1 reduced monocyte motility in the bone marrow
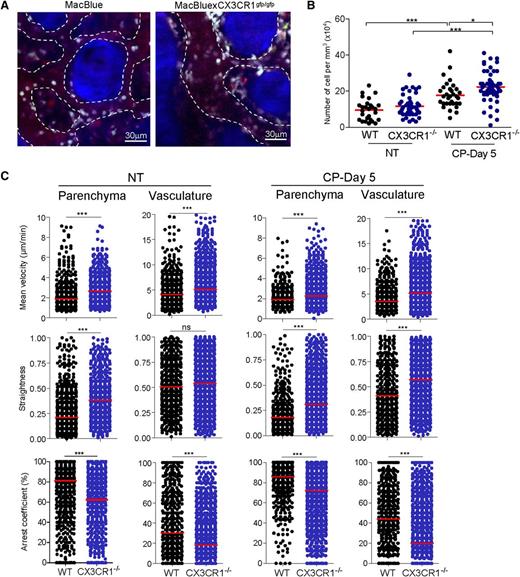
We quantified the number of CFP+ cells localized in the close vicinity of vasculature in MacBlue CX3CR1gfp/gfp and MacBlue mice in the steady state and during the recovery phase (Figure 6A). CP treatment induced a significant accumulation of cells per mm3 of vasculature, which further increased in the absence of CX3CR1 (Figure 6B).
CX3CR1 reduced monocytes motility in the bone marrow. (A) TPLSM images of CFP+ cells (cyan) in blood vessels (red) of skull bone tissue 5 days after CP treatment of MacBlue (left) and MacBluexCX3CR1gfp/gfp mice (right). Dashed lines indicate the vasculature areas and the bone matrix is in blue (SHG). (B) Quantification from the 3-dimensional videos of CFP+ cell numbers normalized to the vasculature volume (red bar represents the mean of different vasculature areas; a 1-way ANOVA with Bonferroni comparison post-test was performed). (C) Summary of CFP+ cell velocity, track straightness, and arrest coefficient in bone marrow parenchyma and vasculature of untreated and CP-treated MacBlue (WT, black) and MacBluexCX3CR1gfp/gfp (CX3CR1−/−, blue) mice. Red bars represent median (data points represent individual cells compiled from at least 3 different experiments; a Mann-Whitney sum test was performed). D, day; NT, not treated.
CX3CR1 reduced monocytes motility in the bone marrow. (A) TPLSM images of CFP+ cells (cyan) in blood vessels (red) of skull bone tissue 5 days after CP treatment of MacBlue (left) and MacBluexCX3CR1gfp/gfp mice (right). Dashed lines indicate the vasculature areas and the bone matrix is in blue (SHG). (B) Quantification from the 3-dimensional videos of CFP+ cell numbers normalized to the vasculature volume (red bar represents the mean of different vasculature areas; a 1-way ANOVA with Bonferroni comparison post-test was performed). (C) Summary of CFP+ cell velocity, track straightness, and arrest coefficient in bone marrow parenchyma and vasculature of untreated and CP-treated MacBlue (WT, black) and MacBluexCX3CR1gfp/gfp (CX3CR1−/−, blue) mice. Red bars represent median (data points represent individual cells compiled from at least 3 different experiments; a Mann-Whitney sum test was performed). D, day; NT, not treated.
We next compared the motility of monocytes in MacBlue CX3CR1gfp/gfp and MacBlue mice in the different compartments (Figure 6C; supplemental Video 5). Prior to CP administration, in both the parenchyma and the vasculature, mean velocity and track straightness were higher and the arrest coefficient was lower in the absence of CX3CR1, consistent with lower cell adherence (Figure 6C). The differences were retained following CP administration. Mean velocity and track straightness were significantly higher and the arrest coefficient was lower in the absence of CX3CR1 in both the parenchymal areas and the vasculature (Figure 6C).
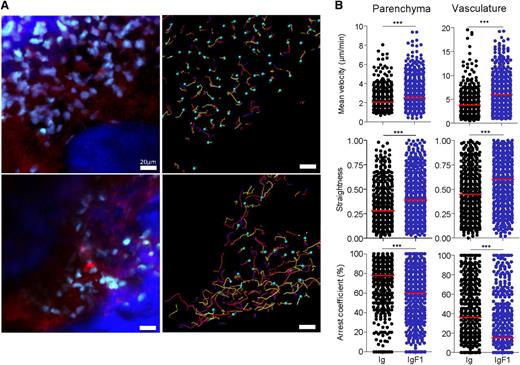
To confirm the function of CX3CR1 in marrow monocytes, we used the polypeptide antagonist of CX3CR1 (F1)33 in combination with live imaging. An in vitro cell adhesion assay (as described in Figure 3C) showed that soluble F1 impaired cell adhesion of CX3CR1+ cells to fixed CX3CL1 in vitro (supplemental Figure 4A). To study the impact of the antagonist in vivo, we employed the hydrodynamic-based in vivo transfection procedure described by Liu et al34 to deliver a plasmid encoding F1 conjugated with the mouse immunoglobulin (Ig) G2aFc domain,33 which increases in vivo stability of the chemokine. In this experimental system, systemic expression of IgF1 was detected for at least 3 days after transfection (supplemental Figure 4B). Transfection of the IgF1 or Ig control plasmid was performed 3 days after CP treatment and the effect was examined 2 days later in the MacBlue mouse (Figure 7A; supplemental Video 6). Consistent with the results obtained in the absence of CX3CR1, CFP+ cells increased their mean velocity and track straightness and decreased their arrest coefficient in both the parenchyma and the vasculature of the bone marrow, consistent with reduced adhesion to CX3CL1+ stromal and endothelial cells (Figure 7B).
CX3CR1 blockade changed monocyte behavior in the marrow. (A) TPLSM images of CFP+ cells (cyan) in blood vessels (red) of skull bone tissue 5 days after CP treatment of MacBlue mice and 2 days after in vivo transfection of an Ig-encoding plasmid (top) or IgF1-encoding plasmid (bottom). Associated track paths of cells are represented by colored lines (right panels). (B) Summary of CFP+ cell velocity, track straightness, and arrest coefficient after in vivo transfection with Ig-encoding plasmid (black round) or IgF1-encoding plasmid (blue round) in both parenchyma (left) and vasculature (right). Red bars represent medians (data points represent individual cells compiled from a minimum of 3 different experiments; a Mann-Whitney sum test was performed).
CX3CR1 blockade changed monocyte behavior in the marrow. (A) TPLSM images of CFP+ cells (cyan) in blood vessels (red) of skull bone tissue 5 days after CP treatment of MacBlue mice and 2 days after in vivo transfection of an Ig-encoding plasmid (top) or IgF1-encoding plasmid (bottom). Associated track paths of cells are represented by colored lines (right panels). (B) Summary of CFP+ cell velocity, track straightness, and arrest coefficient after in vivo transfection with Ig-encoding plasmid (black round) or IgF1-encoding plasmid (blue round) in both parenchyma (left) and vasculature (right). Red bars represent medians (data points represent individual cells compiled from a minimum of 3 different experiments; a Mann-Whitney sum test was performed).
We conclude that CX3CR1 slows down monocyte trafficking within the parenchyma and reduces adherence to endothelial cells and thereby limits their release into the bloodstream.
Discussion
Ly6C+ monocytes are a relatively short-lived population in mouse peripheral blood. Previous studies using monocyte ablation with toxic liposomes35 suggested that ly6C+ cells are immature and the marker is downregulated as they mature in the circulation. As reviewed by Geissman et al,5 adoptive transfer experiments suggest that these cells can also traffic back into marrow. A recent study demonstrated that the maturation of Ly6C+ monocytes requires CSF-1 receptor signaling.36 Monocyte production, egress from the marrow, circulating half-life, entry into tissues, local proliferation, and eventual death are all regulated processes that contribute to their many functions in health and disease.2,30,37-39 Although monocytic cell recovery and mobilization after CP treatment is not physiological, it is clearly relevant to recovery after chemotherapy in human patients.
The novel feature of this study was the demonstration of the function of CX3CR1/CX3CL1 axis in the control of release of Ly6Chigh monocytes. In CX3CR1−/− mice, Ly6Chigh monocyte release was increased, resulting in monocyte accumulation into the spleen, whereas accumulation of Ly6Clow subsets was similar in WT and CX3CR1−/− mice. By contrast, neutrophil release following CP administration was unaffected in CCR2−/− and CX3CR1−/− mice compared with WT, so the function of CX3CR1 is specific to monocytic cells that express low levels of the receptor. Recently, Yona et al provided strong evidence that the LyC6low subset in the blood arises from Ly6Chigh monocyte differentiation rather than a specific release from the bone marrow.28 Ly6Clow subset accumulation in the spleen after CP treatment correlated with a reduction in the number of Ly6Chigh monocytes, reflecting a role for this last subset as progenitors. We cannot exclude that CX3CR1 deficiency affects Ly6Clow cell survival and reduces the accumulation of this subset.21 CP treatment induced a disruption of the CX3CR1/CX3CL1 axis through both CX3CL1-expressing cell elimination and CX3CR1 downregulation. Interestingly, we did not detect any variation in concentration of serum CX3CL1, suggesting that increased release is linked to reduced anchored CX3CL1-mediated retention or recapture in the marrow rather than soluble CX3CL1-mediated chemotaxis. Indirectly, this disruption could impact the survival of macrophages21,40 that participate in the retention of hematopoietic stem cell retention through the CXCR4 axis.41 The relative importance of the survival, adhesive, and chemotactic properties of CX3CR1 may be determined through further investigation. CX3CR1 has been shown to be involved in the recruitment of monocytes into the spleen during Listeria infection and after irradiation but not in steady state.1 Cotransfer of CX3CR1gfp/+ and CX3CR1gfp/gfp bone marrow monocytes in the context of CP treatment showed no differences in splenic homing. The first conclusion is that CX3CR1 is not required to enter the spleen after CP treatment as well as in steady state. As a consequence, the increased accumulation observed in CX3CR1−/− mice is due to increased released from the bone marrow toward the blood. Local proliferation of Ly6Chigh monocytes could contribute also to their accumulation within the spleen to reconstitute the secondary reservoir.13,29,42,43 However, cell-cycle analysis in CX3CR1−/− mice was similar compared with WT mice, which confirmed that the increased accumulation observed between days 5 and 7 could not be attributable to an increased rate of cycling in CX3CR1−/− mice. Previous studies have shown that CCR2 is essential for egress of monocytes from bone marrow and their recruitment to an inflammatory site after treatment with intraperitoneal thioglycollate11 or lipopolysaccharides.44 By contrast, we found no effect of CX3CR1 deficiency on the depletion of ly6Chigh monocytes from the marrow or blood in response to thioglycollate (data not shown), suggesting that CCR2-mediated recruitment overrides the function of CX3CR1 in retention. Because inflammatory-mediated mobilization of medullar monocytes is very rapid (within hours) and monocytopenia can be observed after the emigration toward the inflamed tissue, the mechanisms involved between this process and the recovery phase after chemotherapy might not be comparable.
In vivo imaging using 2-photon microscopy has provided new insights into the behavior of these highly motile cells, but the marrow compartment has been difficult to access. In the present study, the use of MacBlue mice permitted effective imaging of the behavior of monocytes within different marrow compartments and showed that CX3CR1 reduced monocyte motility within the parenchyma and adherence to endothelial cells. It is unclear whether the absence of CX3CR1 affects the migration from the parenchyma toward the vasculature. It is likely that CCR2 axis mainly governs this directed migration44 and that CX3CR1, through its adhesive property, slowed down monocyte migration and reduced the release from and favored retention to the luminal side of the bone marrow endothelium. Slowing down monocyte egress might have an important role in the quality control of monocytes and might participate in the delivery of survival signals through CX3CR121 before egress.
In conclusion, we have used a novel approach to demonstrate the function of the CX3CR1/CX3CL1 axis in the mobilization of monocytes after a chemotherapeutic treatment. Modulating the rate of cell mobilization during the myelorestorative phase by combining therapeutic regimens targeting the CX3CR1 axis could contribute to recovery from a range of insults and to effective host defense.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank William Avery Hudson for editorial assistance, Dr Behazine Combadière for helpful discussions, the Plateforme Imagerie Pitié-Salpétrière for assistance with the 2-photon microscope, and the animal Facility “NAC” for mice breeding assistance.
This work was supported by the European Community’s Seventh Framework Programme (FP7/2007-2013 under grant agreements 304810-RAIDs and 241440-Endostem), INSERM, Université Pierre et Marie Curie Emergence, la Ligue Contre le Cancer, and Agence Nationale de la Recherche” (ANR-09-PIRI-0003). F.L. was supported by the Fondation pour la Recherche Medicale. C.C. is recipient of a contract from Assistance Publique-Hopitaux de Paris. P.D. is a scientist of the Centre National de la Recherche Scientifique.
Authorship
Contribution: S.J. performed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; L.F. and P.H. performed research and analyzed data; K.D. performed research, analyzed data, and produced vital reagents; L.P. and E.G. performed research and contributed vital reagents; P.D. designed research and wrote the manuscript; D.A.H. provided vital reagents and wrote the manuscript; C.C. designed research, contributed vital reagents, and wrote the manuscript; A.B. designed research, performed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexandre Boissonnas, INSERM UMRS 945 Immunité et Infection, CHU Pitié-Salpêtrière, 91 Boulevard de L’Hopital, Paris 75013, France; e-mail: alexandre.boissonnas@upmc.fr.

![Figure 1. CX3CR1 and CCR2 have opposing actions in monocyte recovery after myeloablation. Representative dot plots previously gated on CD11b+ cells indicate the gating strategy for a Ly6Chigh monocyte subset from the bone marrow (A), blood (B), and spleen (C) (percent ± standard deviation [SD] of the gated subset from untreated mice are indicated). Kinetic of Ly6Chigh monocyte recovery after CP treatment (175 mg/kg) in Balb/c (empty), CCR2−/− (gray), and CX3CR1−/− (black) mice was monitored. D0 corresponds to untreated mice. Graph represents the absolute number ± standard error of the mean (SEM) of Ly6Chigh monocytes after CP treatment in bone marrow, blood, and spleen (n = 6-15 mice for each time point out of at least 3 independent experiments). A 2-way analysis of variance (ANOVA) with Bonferroni comparison post-test was performed. Statistical significance between WT and knockout strains is indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/5/10.1182_blood-2013-01-480749/4/m_674f1.jpeg?Expires=1769110924&Signature=ISx75CiPtrDx17KcKe0cHo3KVxwaUCLJU1pKiKvhWS5pK9Qg0aKiFebKeikxYqx-IPNLR2E7hwJqpqaQ1fUlww2jnIiP3PD8x0AMwa8-JkQRUYQi3m9coDJxU9sG-8c1eGmaythZ~IFZ0Xo2WPh-mEyYwnoSHrSq~l1fRuT6vbX4DsE-ANPnUoxaGJrbfjgdKHZB59vGaAmv1sPjsuPVf8JPSakso76RI7g1Mu9POFqdlo6WO-QAL06cnmF3WxBlDvAGPbTSes67jbYNjFzmw2cJsya9OG8~kr7x0SDrjQHtrh6RjmUFeWIp4wshVtdcHsbg3bLVZZbpg-bB2X02Pg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. CP treatment induced monocytes redistribution in the bone marrow. TPLSM images of monocyte cells (cyan) in parenchymal zones (A) and bone marrow vasculature (B) of skull bone tissue of MacBlue mice. The vasculature is in red (rhodamine-dextran) and the bone matrix is in blue (SHG). White bars represent 50 µm. Dot plots (upper right corner) indicate the percentage of Ly6-Chigh monocytes among CFP+ cells. Graphs represent the distribution of track straightness as a function of cell mean velocity after CP treatment. Quadrants separate cells according to track straightness (value is 0.25 for parenchyma and 0.5 for vasculature) and mean velocity (2 μm/min for parenchyma and 4 μm/min for vasculature) (corresponding to the median values for each compartments in untreated mice). Quadrant statistics are indicated for each condition (parenchyma: not treated [NT] n = 687, day [D] 1 n = 327, D3 n = 219, D5 n = 448; vasculature: NT n = 583, D1 n = 899, D3 n = 566, D5 n = 498; from at least 3 different mice in each condition).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/5/10.1182_blood-2013-01-480749/4/m_674f5.jpeg?Expires=1769110924&Signature=ZjOLEN~p1HJB~Fn5Du18nMfAUk2vqII7b~fY3Q8k1u4hSZQBr4wemCt5NLz7OR~QzOKYCyKMTN25yiV6Km8X91QoJatXWrGkv7OWF5itE88bJmrguD2bO0Zgo6-M01ae3bjvolFeWFTbdRxGMMDIa-enb7ZErA87TnE2MzPiTVnUvnlQjRZ3aybsvXllnk8SAf6R7uuMl4hchr1owwmX1k9g7O99BMuIXgVO5vHiMqAXdcxc8RF74xtrGLlgVKpb3zQa~DPnuFBAR-novYjdPTkBG~z4y8YiNc~SSY05onuLCSOmY83iAgAb~rMbnAuAQ~x9VkK1CbyG8~BnJH5OCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal