Key Points
This study is a retrospective analysis of long-term outcomes of patients with FL treated at Stanford University for 4 decades.
Study results showed significant improvement in OS in patients with FL despite no change in event-free survival after first-line therapy.
Abstract
Recent studies report an improvement in overall survival (OS) of patients with follicular lymphoma (FL). Previously untreated patients with grade 1 to 2 FL treated at Stanford University from 1960-2003 were identified. Four eras were considered: era 1, pre-anthracycline (1960-1975, n = 180); era 2, anthracycline (1976-1986, n = 426); era 3, aggressive chemotherapy/purine analogs (1987-1996, n = 471); and era 4, rituximab (1997-2003, n = 257). Clinical characteristics, patterns of care, and survival were assessed. Observed OS was compared with the expected OS calculated from Berkeley Mortality Database life tables derived from population matched by gender and age at the time of diagnosis. The median OS was 13.6 years. Age, gender, and stage did not differ across the eras. Although primary treatment varied, event-free survival after the first treatment did not differ between eras (P = .17). Median OS improved from 11 years in eras 1 and 2 to 18.4 years in era 3 and has not yet been reached for era 4 (P < .001), with no suggestion of a plateau in any era. These improvements in OS exceeded improvements in survival in the general population during the same period. Several factors, including better supportive care and effective therapies for relapsed disease, are likely responsible for this improvement.
Introduction
Follicular lymphoma (FL) is the second most common subtype of non-Hodgkin lymphoma.1 It is characterized by an indolent clinical course and a continuous pattern of relapse. In addition, there is a risk for transformation to an aggressive lymphoma of ∼20% at 5 years and 30% at 10 years.2-6
We have previously reported that the natural history of FL was not altered by the use of various management approaches at Stanford University between 1960 and 1992.7 Recent studies have suggested that the overall survival (OS) duration of patients with grade 1 to 2 FL has improved because of progress in treatment and supportive care.8-16 In this retrospective analysis, we have updated our previous results and extended the period of analysis to 2007. We sought to determine if changes in outcome were attributed to frontline treatment or effective salvage strategies, which varied across 4 eras reflecting changes in the treatment of FL.
Patients and methods
Previously untreated patients with all stages of grade 1 to 2 FL who received primary treatment at Stanford University Medical Center between January 1960 and December 2003 were identified from the lymphoma database. All diagnostic specimens were reviewed by pathologists in the Department of Pathology at Stanford and were reclassified according to the World Health Organization classification.17 Patients with grade 3 FL or composite lymphoma were excluded. Disease characteristics, time to first treatment, type of frontline treatment, and outcomes were evaluated retrospectively. Data regarding additional salvage treatment administered at other facilities were recorded when available. “Immediate treatment” was arbitrarily defined as treatment received within 2 months of referral, whereas “no initial therapy” was expectant management continuing for >2 months after referral. For survival analysis, we categorized patients according to 4 eras reflecting changes in treatment of FL: era 1, preanthracycline (1960-1975); era 2, anthracycline (1976-1986); era 3, aggressive chemotherapy/purine analogs (1987-1996); and era 4, rituximab (1997-2003). Data on therapy received were censored in January 2007. The Social Security Administration Database was searched to obtain current vital status and data censored in December 2007. Because the study cohorts spanned a 43-year period, variables to calculate the Groupe d'Etude des Lymphomes Folliculaires18 criteria often were not available. The Follicular Lymphoma International Prognostic Index (FLIPI)19 was calculated for era 4. The study was conducted according to the Stanford University Institutional Review Board and in accordance with the Declaration of Helsinki.
Statistical considerations
For patient characteristics and treatment exposure, P values < .05 were considered to indicate statistical significance. The χ2 tests and the t tests were used for comparisons of categorical and continuous variables, respectively, among the treatment eras. Comparisons of event-free survival (EFS) for frontline treatments were restricted to patients with advanced-stage disease (stages III and IV) because those with limited-stage disease were usually treated with radiation therapy (RT) alone, and the intent of this analysis was to evaluate the impact of changes in systemic therapies. EFS was calculated from the date of initial treatment to the date of first event defined as progression, relapse, next treatment, or death. All patients were included for OS analysis, which was calculated from the date of diagnosis to death related to any cause.
Kaplan-Meier survival curves were estimated for the 4 eras and were compared for statistical differences by use of the log-rank test in the univariate analyses.20,21 Additional analyses were performed for subsets defined as “immediate treatment” vs “no initial therapy,” age ≥60 years vs <60 years, stage I/II vs stage III/IV disease, gender, and exclusion of patients who received vaccine therapies. We also assessed OS for patients who were monitored at Stanford at the time of the first event.
For comparison of the observed OS in our patient cohort with that of the general population, patients were matched by diagnosis date, age, and gender with single calendar year, single year of age, and gender-specific life tables from the Berkeley Mortality Database to determine the expected OS for the general population.22 The latter accounts for other causes of death and provides an estimate of the cause-specific survival through relative survival analysis.23,24
Results
Patient characteristics
A total of 1334 patients who met predefined criteria were identified. Patient demographics and clinical characteristics are depicted in Table 1. Patients were distributed according to 4 eras: era 1 (n = 180), era 2 (n = 426), era 3 (n = 471), and era 4 (n = 257), with a median follow up of 11.1, 8.6, 11.3, and 6.1 years, respectively. During these years, a trend toward an increasing interval from disease diagnosis to Stanford referral was noted. Median age at diagnosis was 50 years (age range, 21-87 years), and 77% of patients were ≤60 years old. Approximately 60% had grade 1 FL and >80% presented with advanced-stage disease. These characteristics did not differ significantly across the eras. FLIPI was available for 234 patients (91%) in era 4: 18.8%, 42.7%, and 38.5% of patients had low-, intermediate-, and high-risk disease, respectively. Histologic transformation of FL to a more aggressive lymphoma proven on subsequent biopsy results was seen in 32% of patients, as reported separately.6
Patient demographics and clinical characteristics
| Characteristic . | Era 1 (1960-1975) N = 180 . | Era 2 (1976-1986) N = 426 . | Era 3 (1987-1996) N = 471 . | Era 4 (1997-2003) N = 257 . | P value . |
|---|---|---|---|---|---|
| Age | |||||
| Median (y) | 52 | 51 | 48 | 50 | .17 |
| ≥60 y (%) | 22 | 24 | 24 | 17 | .09 |
| Male, (%) | 51 | 57 | 55 | 53 | .54 |
| Stage (%) | .84 | ||||
| I | 7 | 9 | 8 | 7 | |
| II | 10 | 12 | 9 | 9 | |
| III | 28 | 27 | 25 | 25 | |
| IV | 56 | 53 | 58 | 58 | |
| Histology, (%) | .01 | ||||
| FL grade 1 | 66 | 66 | 55 | 56 | |
| FL grade 2 | 34 | 24 | 36 | 39 | |
| Other* | 1 | 9 | 9 | 5 | |
| Median time from diagnosis to referral (days) | 16 | 27 | 42 | 49 | .15 |
| Median time from referral to first treatment (days) | 30 | 61 | 163 | 143 | .001 |
| Characteristic . | Era 1 (1960-1975) N = 180 . | Era 2 (1976-1986) N = 426 . | Era 3 (1987-1996) N = 471 . | Era 4 (1997-2003) N = 257 . | P value . |
|---|---|---|---|---|---|
| Age | |||||
| Median (y) | 52 | 51 | 48 | 50 | .17 |
| ≥60 y (%) | 22 | 24 | 24 | 17 | .09 |
| Male, (%) | 51 | 57 | 55 | 53 | .54 |
| Stage (%) | .84 | ||||
| I | 7 | 9 | 8 | 7 | |
| II | 10 | 12 | 9 | 9 | |
| III | 28 | 27 | 25 | 25 | |
| IV | 56 | 53 | 58 | 58 | |
| Histology, (%) | .01 | ||||
| FL grade 1 | 66 | 66 | 55 | 56 | |
| FL grade 2 | 34 | 24 | 36 | 39 | |
| Other* | 1 | 9 | 9 | 5 | |
| Median time from diagnosis to referral (days) | 16 | 27 | 42 | 49 | .15 |
| Median time from referral to first treatment (days) | 30 | 61 | 163 | 143 | .001 |
Follicular and diffuse architecture, grade 1 to 2 FL, follicular lymphoma.
Treatment
The median time from referral to initiation of therapy was longer in eras 3 and 4 compared with earlier eras (P = .001) (Table 1). The proportion of patients who had “no initial therapy” in eras 1 to 4 was 16%, 44%, 59%, and 60%, respectively (P < .001). The median time to first treatment among these patients was 2.9 years (supplemental Figure 1). For era 4, an estimated 11% of patients in whom therapy was deferred had high-risk FLIPI compared with 30% in those who received immediate treatment. Frontline therapy of patients with advanced disease was variable (Table 2). Although primary RT was an important modality of treatment of advanced-stage disease in the earlier eras, in the later 2 eras, it was used largely for local control or palliation. Cyclophosphamide, vincristine, and prednisone, was the most frequent frontline alkylator-based chemotherapy, whereas CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) was used in a minority of patients, mainly in era 3. Upfront rituximab use, either as a single agent or in combination with cyclophosphamide, vincristine, and prednisone or CHOP, was introduced in eras 3 and 4. Approximately one quarter of the patients were accrued into FL vaccination trials in combination with chemotherapy in eras 3 and 4. Cumulative treatment exposure according to era is also shown in Table 2. At relapse, patients in eras 1 and 2 were more likely to receive repeated courses of RT or alkylator-based treatments, whereas patients in eras 3 and 4 had a wider range of therapeutic and salvage options available. Cumulative exposure to rituximab varied considerably. Only 1% and 11% of patients in eras 1 and 2, respectively, received rituximab at some point during therapy, compared with 27% in era 3 and 46% in era 4. Because of patterns of referral and the retrospective nature of this analysis, data regarding all additional salvage treatments that patients may have received at other facilities are limited.
Initial and cumulative treatment exposure in patients with advanced-stage grade 1 to 2 FL
| . | Era 1 (1960-1975) N = 147 N (%) . | Era 2 (1976-1986) N = 330 N (%) . | Era 3 (1987-1996) N = 380 N (%) . | Era 4 (1997-2003) N = 213 N (%) . |
|---|---|---|---|---|
| Initial therapy | ||||
| Radiotherapy only* | 37 (25) | 46 (14) | 11 (3) | 6 (3) |
| Single-agent alkylator* | 35 (24) | 60 (18) | 68 (18) | 13 (6) |
| CVP-like* | 68 (46) | 150 (45) | 125 (33) | 110 (52) |
| CHOP-like* | 1 (1) | 17 (5) | 49 (13) | 11 (5) |
| Fludarabine-based* | 0 | 0 | 38 (10) | 4 (2) |
| Rituximab-chemotherapy* | 0 | 0 | 7 (2) | 19 (9) |
| Rituximab* | 0 | 0 | 7 (2) | 19 (9) |
| SCT** | 0 | 3 (1) | 19 (5) | 2 (1) |
| Unknown or no treatment*** | 6 (4) | 53 (16) | 57 (15) | 30 (14) |
| Cumulative treatment exposure in advanced-stage patients by era | ||||
| Radiotherapy | 59 (40) | 86 (26) | 46 (12) | 17 (8) |
| Single-agent alkylator | 60 (41) | 110 (35) | 91 (24) | 17 (8) |
| CVP-like | 97 (66) | 205 (62) | 163 (43) | 113 (53) |
| CHOP-like | 37 (25) | 106 (32) | 106 (28) | 32 (15) |
| Fludarabine-based | 2 (1) | 10 (3) | 87 (23) | 17 (8) |
| Rituximab | 1 (1) | 36 (11) | 103 (27) | 98 (46) |
| Vaccine trial | 1 (1) | 10 (3) | 99 (26) | 60 (28) |
| SCT | 0 | 10 (3) | 65 (17) | 19 (9) |
| Salvage regimen without SCT | 0 | 3 (1) | 11 (3) | 6 (3) |
| . | Era 1 (1960-1975) N = 147 N (%) . | Era 2 (1976-1986) N = 330 N (%) . | Era 3 (1987-1996) N = 380 N (%) . | Era 4 (1997-2003) N = 213 N (%) . |
|---|---|---|---|---|
| Initial therapy | ||||
| Radiotherapy only* | 37 (25) | 46 (14) | 11 (3) | 6 (3) |
| Single-agent alkylator* | 35 (24) | 60 (18) | 68 (18) | 13 (6) |
| CVP-like* | 68 (46) | 150 (45) | 125 (33) | 110 (52) |
| CHOP-like* | 1 (1) | 17 (5) | 49 (13) | 11 (5) |
| Fludarabine-based* | 0 | 0 | 38 (10) | 4 (2) |
| Rituximab-chemotherapy* | 0 | 0 | 7 (2) | 19 (9) |
| Rituximab* | 0 | 0 | 7 (2) | 19 (9) |
| SCT** | 0 | 3 (1) | 19 (5) | 2 (1) |
| Unknown or no treatment*** | 6 (4) | 53 (16) | 57 (15) | 30 (14) |
| Cumulative treatment exposure in advanced-stage patients by era | ||||
| Radiotherapy | 59 (40) | 86 (26) | 46 (12) | 17 (8) |
| Single-agent alkylator | 60 (41) | 110 (35) | 91 (24) | 17 (8) |
| CVP-like | 97 (66) | 205 (62) | 163 (43) | 113 (53) |
| CHOP-like | 37 (25) | 106 (32) | 106 (28) | 32 (15) |
| Fludarabine-based | 2 (1) | 10 (3) | 87 (23) | 17 (8) |
| Rituximab | 1 (1) | 36 (11) | 103 (27) | 98 (46) |
| Vaccine trial | 1 (1) | 10 (3) | 99 (26) | 60 (28) |
| SCT | 0 | 10 (3) | 65 (17) | 19 (9) |
| Salvage regimen without SCT | 0 | 3 (1) | 11 (3) | 6 (3) |
CVP, cyclophosphamide, vincristine, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; SCT, stem-cell transplant.
P < .001.
P = .001.
at last follow-up.
EFS
For patients with advanced disease, the median EFS duration across eras 1 to 4 was 2.0 years (range, 1.5-3.3 years; P = .4) with no significant differences despite variable initial treatments (Figure 1A). In a similar fashion, EFS did not differ for patients receiving “immediate treatment” (1.6 years; range, 0.9-1.8 years; P = .2) and for patients receiving “no initial therapy” (2.2 years; range, 1.5-3.5 years; P = .4) (Figures 1B-C).
EFS after first treatment course by era of diagnosis. (A) All patients. (B) Patients with no initial therapy. (C) Patients receiving immediate treatment.
EFS after first treatment course by era of diagnosis. (A) All patients. (B) Patients with no initial therapy. (C) Patients receiving immediate treatment.
OS
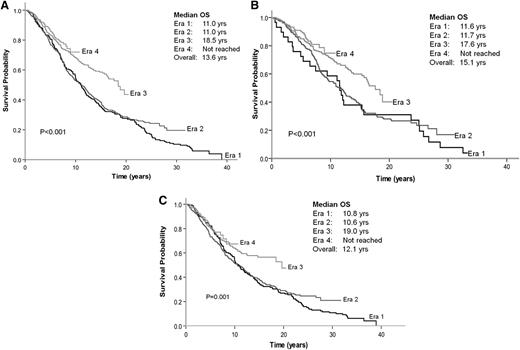
The median OS duration for the 1334 patients was 13.6 years: 11.0 years for era 1, 11.0 years for era 2, 18.5 years for era 3, and an unreached value for era 4 (P < .001) (Figure 2A). The 10-year OS duration for patients in eras 1 to 4 was 54%, 54%, 68%, and 73%, respectively. Gains in OS were seen in both early-stage disease (stages I-II) and advanced-stage disease (stages III-IV) (supplemental Figure 2A-B, respectively). Greater gains in OS were observed in patients <60 years old compared with patients ≥60 years old (supplemental Figure 3A-B, respectively).
OS by era of diagnosis. (A) All patients. (B) Patients with no initial therapy. (C) Patients receiving immediate treatment.
OS by era of diagnosis. (A) All patients. (B) Patients with no initial therapy. (C) Patients receiving immediate treatment.
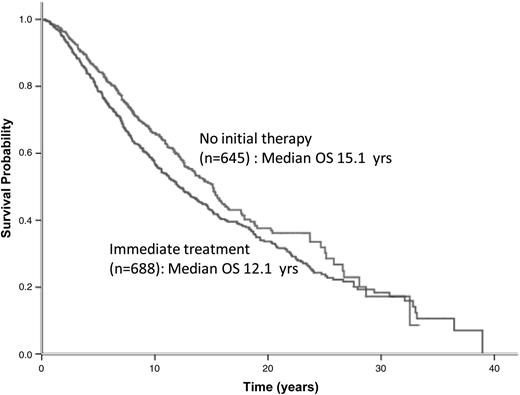
Patients in eras 3 and 4 had significantly better OS than those in eras 1 and 2, regardless of whether they were in the “no initial therapy” or “immediate treatment” group (Figures 2B-C). The median OS duration for patients assigned to “no initial therapy” and “immediate treatment” was 15.1 and 12.1 years, respectively (P = .007) (Figure 3). The noninferior OS of patients assigned to “no initial therapy” was observed across all eras despite changes in frontline therapeutic strategies. Therapy choice (or decision for “no initial therapy”) did not differ by gender, but improvements in OS were greater in women compared with men (P < .0001 for women and P = .03 for men) (supplemental Figure 4A-B, solid lines). A total of 219 patients (∼25%) received vaccine-based therapies in eras 3 and 4. When these patients were excluded from the analysis, the median OS duration for era 1 and 2 was 11 years; for era 3, 16.4 years; and for era 4, unreached value (P = .002) (supplemental Figure 5). An estimated 86.7% of patients with advanced disease were being observed at Stanford at the time of their first event. The median OS after the first event was 4.1 years for era 1, 6 years for era 2, 10.2 years for era 3, and an unreached value for era 4 (P < .001) (supplemental Figure 6). The magnitude of improvements in OS across eras among patients with FL far exceeded the gains in life expectancy and OS of the general population during the same period (supplemental Figure 4C). As expected, however, in all 4 eras, the observed OS of patients with FL was significantly inferior to the expected OS of the general population (P < .0001). The 10-year relative survivals for eras 1 to 4 were 0.61, 0.61, 0.76, and 0.8, respectively (P < .001) (Table 3).
Comparison of expected vs observed survival by era of diagnosis
| . | Age/gender match . | Patients with FL . | |
|---|---|---|---|
| Era | 10-y OS rate expected (%) | 10-y OS rate observed (%) | 10-y relative survival rate in FL patients (%) |
| 1965-1975 (era 1) | 88 | 54 | 61 |
| 1976-1986 (era 2) | 87 | 53 | 61 |
| 1987-1996 (era 3) | 89 | 68 | 76 |
| 1997-2003 (era 4) | 90 | 72 | 80 |
| . | Age/gender match . | Patients with FL . | |
|---|---|---|---|
| Era | 10-y OS rate expected (%) | 10-y OS rate observed (%) | 10-y relative survival rate in FL patients (%) |
| 1965-1975 (era 1) | 88 | 54 | 61 |
| 1976-1986 (era 2) | 87 | 53 | 61 |
| 1987-1996 (era 3) | 89 | 68 | 76 |
| 1997-2003 (era 4) | 90 | 72 | 80 |
Discussion
In this analysis spanning more than 50 years, we noted a marked improvement in OS of patients with FL. The median OS duration was 11 years in the first 2 eras, 18.5 years in era 3, and an unreached value in era 4, with no plateau observed in any era to date. In our series, there was no detrimental effect on OS in patients who had “no initial therapy” compared with those who were treated immediately. The EFS was similar across the eras despite variations in the choice of initial therapy, with no significant differences whether patients had “no initial therapy” or “immediate treatment.” Although it has been the institutional practice at Stanford to defer the use of anthracycline therapy for treatment after relapse and/or aggressive histologic transformation,25 the availability of anthracycline beginning in era 2 did not significantly affect the OS of our patients. In a similar fashion, the availability of the purine analog fludarabine for upfront use or for relapsed disease did not seem to have an impact in era 3.26-28 Rituximab as a component of initial therapy was used in 4% and 18% of patients in cohorts 3 and 4, respectively. The strategy of patient-specific vaccines, designed to provoke a humoral and/or cellular immune response against the clonal surface immunoglobulin (idiotype) unique to each patient with FL, was available in the context of clinical trials initiated in era 3.29 When patients treated with vaccine-based therapies were excluded, the OS gains were similar to the entire cohort. Therefore, the OS seen in eras 3 and 4, which was superior to the OS in eras 1 and 2, cannot be attributed to any single particular regimen.
Because the OS of an indolent malignancy reflects both the efficacy of initial therapy and all subsequent treatments, the improvement in OS may be attributable to the sequential application of effective new therapies for relapsed or progressive disease, as well as improved supportive therapy. Our observation of an OS improvement in the absence of a significant change in the EFS supports this concept. This finding is further supported by the improvements noted in OS across eras after the first event. Increasing exposure to different treatments and lower reliance on repetition of prior therapies are evident in Table 2, which shows increased use of both myeloablative therapy and rituximab-based combination chemotherapy in patients experiencing relapse in era 3 and a declining use of single-agent chlorambucil and RT in eras 3 and 4. Of note is that the survival curves for eras 2 and 3 start to diverge from that of era 1 at approximately the 20-year and 5-to 6-year landmarks, respectively. It is likely that patients from eras 2 and 3 who survived past these landmarks entered the era of effective newer therapies. Treatment with rituximab at relapse was rapidly adopted after approval of the agent in 1997 by the US Food and Drug Administration, as reflected in eras 3 and 4 in which more patients were exposed to rituximab, predominantly in the relapse setting. It is likely that the overall use of rituximab in our study is underestimated because detailed evaluation of all subsequent treatments, which often occurred at outside facilities, could not be performed.
Our findings are similar to observational studies from the Surveillance Epidemiology and End Results database that reported statistically significant improvement in OS among unselected patients across consecutive diagnostic eras, as well as to those from a European center that reported improvement in disease-specific survival in patients with FL.9,30 Some series have suggested that a better response to frontline therapy potentially translates into improved OS for patients with FL in both the pre- and postrituximab eras.31,32 Reports from the Southwest Oncology Group (SWOG) and the MD Anderson Cancer Center attribute the improvement in survival in patients participating in sequential therapeutic trials for FL largely to the incorporation of immunotherapy into frontline regimens.8,10 In the SWOG studies, monoclonal antibody trials included CHOP followed by rituximab (SWOG 9800) and CHOP followed by 131II-tositumomab (SWOG 9911). The progression-free survival and OS rates were significantly superior in the immunotherapy era (61% and 91%, respectively), and superiority was retained after adjustment for differences in prognostic factors between the pre- and postimmunotherapy study group. In the MD Anderson Cancer Center series, an improvement in 5-year OS rates from 64% to 95% was noted during 5 successive clinical trials, in which rituximab was included in later trials.10 Similar observations have been reported in a Cochrane meta-analysis evaluating 1943 patients from 7 randomized clinical trials using rituximab in indolent lymphoma with an improvement in overall response rates and a reduction in the hazard ratio for deaths to 0.63 (95% confidence interval, 0.51-0.79; P < .001).33 Compared with the latter series, in our study only a minority of patients received rituximab in frontline regimens; hence, the potential impact of incorporation of rituximab in EFS could not be determined. Despite this, we observed significant improvements in OS, again supporting the concept that subsequent therapies are just as important as the choice of frontline treatment in defining long-term outcome.34
Although our data were for consecutive patients treated in a single center, a certain degree of inherent selection bias was inevitable because Stanford is a large referral center. This was reflected by a relatively younger median age (50 years) of our patients. Another limitation of our analysis was that staging methods evolved during the study period, and there may have been variations in frequency and type of imaging used for follow-up care. The concept of lead-time bias (ie, patients with FL in the later eras were diagnosed earlier, which resulted in longer observed OS) is difficult to measure. In our study, there was no significant difference in the distribution of stage across eras, and OS improvement was noted both for early-stage as well as for advanced-stage disease (supplemental Figure 2A-B, respectively). We noted that for patients diagnosed in later eras, the median time from diagnosis to referral to Stanford was longer (Table 1); however, this trend did not affect OS results when measured from the time of referral (data not shown). Nonetheless, improvements in the observed OS were notable compared with improved life expectancy in the general population and were more marked in women than in men. The latter finding is provocative within the context of the emerging body of literature suggesting that men with B-cell lymphoma have an inferior outcome, which might be attributed to differences in metabolism of rituximab between genders.35-37
The longer survival times in recent eras reported in our series are likely related to several other factors, including improved supportive care and improved outcome of transformed FL.6 We have recently reported that the OS duration of patients with FL after transformation has increased from 0.8 years to 3.3 years.6 This finding may be related to earlier recognition because of advancement in imaging modalities, such as positron emission tomography, and improved treatment of transformed disease.
In conclusion, in our series, patients with grade 1 to 2 FL experienced an improvement in OS that was greater than improvements in OS of the general population during the same periods. Our data demonstrate that although there has been considerable progress, FL remains an incurable disease. Challenges remain in disease management; future studies that evaluate tailored sequencing of emerging novel therapeutic agents, along with a better understanding of the biology of the disease, may eventually lead to a cure.
Presented in abstract form at the 49th annual meeting of the American Society of Hematology, Atlanta, GA, December 9, 2007; the 44th annual meeting of the American Society of Clinical Oncology, Chicago, IL, June 2, 2008; and the 10th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 6, 2008.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute, National Institutes of Health grant CA 34233.
National Institutes of Health
Authorship
Contribution: D.T., S.J.H., and R.H.A. organized the project, assembled data, and wrote the manuscript; B.M.S., S.S.H., and S.K.P. provided statistical analysis; and D.T., S.J.H., R.T.H., R.L., S.A.R., B.M.S., R.A.W., Y.N., S.S.H., A.Y., S.K.P., and R.H.A. interpreted data and reviewed, edited, and approved the final manuscript.
Conflict-of-interest disclosure: S.J.H. is currently employed by Genentech and owns Roche stock. The remaining authors declare no competing financial interests.
The current affiliation for D.T. is the Raffles Cancer Center/Raffles Hospital, Singapore. The current affiliation for S.J.H. is Genentech Inc, South San Francisco, CA. The current affiliation for B.M.S. is Risk Management Solution, Newark, CA.
Corresponding author: Ranjana H. Advani, 875 Blake Wilbur Dr, Suite CC-2338, Stanford, CA 94305-5821; email: radvani@stanford.edu.